Back to Journals » Drug Design, Development and Therapy » Volume 12
Fasudil inhibits actin polymerization and collagen synthesis and induces apoptosis in human urethral scar fibroblasts via the Rho/ROCK pathway
Authors Li XD, Wu YP, Chen SH, Liang YC, Lin TT, Lin T, Wei Y , Xue XY , Zheng QS, Xu N
Received 4 November 2017
Accepted for publication 2 July 2018
Published 3 September 2018 Volume 2018:12 Pages 2707—2713
DOI https://doi.org/10.2147/DDDT.S156095
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Manfred Ogris
Xiao-Dong Li,* Yu-Peng Wu,* Shao-Hao Chen,* Ying-Chun Liang, Ting-Ting Lin, Tian Lin, Yong Wei, Xue-Yi Xue, Qing-Shui Zheng, Ning Xu
Departments of Urology, First Affiliated Hospital of Fujian Medical University, Fuzhou, People’s Republic of China
*These authors contributed equally to this work
Purpose: To examine the effects and mechanism of action of fasudil on cytoskeletal polymerization, collagen synthesis, and apoptosis in fibroblasts derived from human urethral scar tissue.
Materials and methods: Fibroblasts treated with or without transforming growth factor β1 (TGF-β1, 10 ng/mL) were incubated with fasudil (12.5, 25, 50 µmol/L) for 24 hours. Quantitative real-time polymerase chain reaction and Western blotting were used to determine the expression of Arp2, Arp3, WASP, and WAVE2. Collagen I and III protein levels were also evaluated by Western blotting. The filamentous actin cytoskeleton was examined by immunofluorescence and epifluorescence microscopy. An Annexin V-FITC/PI staining assay was used to investigate apoptosis.
Results: TGF-β1-dependent induction of actin polymerization and collagen synthesis and promotion of apoptosis were dose dependent. When compared with untreated controls, fasudil significantly decreased the expression of Arp2, Arp3, WASP, WAVE2, Collagen I, and Collagen III in cells treated with or without TGF-β1. Fasudil also promoted apoptosis in cells, irrespective of TGF-β1 treatment.
Conclusion: Irrespective of TGF-β1 activation status, fasudil suppressed actin polymerization and collagen synthesis and induced apoptosis in human urethral scar fibroblasts via the Rho/ROCK signaling pathway.
Keywords: fasudil, polymerization, cytoskeleton, Rho/ROCK
Introduction
Urethral stricture is a challenging and common problem in urology.1–3 The mechanism of this fibrotic disease is unclear, and both its surgical and medical management remain unsatisfactory. Quality of life is significantly affected in patients with urethral stricture.4
Chemical agents and extracellular signaling factors can induce changes in the morphology, proliferation, and migratory potential of urethral fibroblasts. Previous studies have shown that excessive fibroblast proliferation and migration, collagen synthesis and secretion, and extracellular matrix deposition and contraction are involved in the process of urethral stricture.5,6 Transforming growth factor-β1 (TGF-β1) plays an important role in the formation of urethral scar tissue.7
Arp2/3 complex promotes the assembly of new actin filaments (F-actin) to mediate cytoskeletal changes in response to extracellular signals, as well as to monitor and regulate actin polymerization.8,9 The Arp2/3 complex is intrinsically inactive but is activated through interactions with nucleation-promoting factors (NPFs).8,10 WASP and WAVE2 are two members of the NPF family that are required for Arp2/3 complex-dependent actin nucleation.
ROCK-dependent signaling pathway has been implicated in scar formation.11,12 Fasudil is an inhibitor of ROCK and suppresses fibrosis in various diseases.13–15 However, the effects of fasudil on the cytoskeleton, collagen synthesis, and apoptosis of urethral fibroblasts have not been evaluated. In this study, we examined the effects and mechanism of action of fasudil on these aspects of cellular behavior in fibroblasts derived from human urethral scar tissue.
Materials and methods
Ethical statement
This study was approved by the Ethics Committee of the First Affiliated Hospital of Fujian Medical University, and all patients provided written informed consent.
Patients, tissue samples, and cell lines
Surgical specimens of urethral stricture obtained from five patients aged 25–31 years who underwent surgery at the First Affiliated Hospital of Fujian Medical University were included in this study. None of the patients had received chemotherapy or radiotherapy before surgery. Primary urethral scar fibroblasts were cultured as previously described.7 Finally, only one patient-derived fibroblast culture was used in this study. Cells at passage 4–8 were used in all experiments. Each experiment was performed in triplicate. Analysis of vimentin staining by immunofluorescence confirmed that cultured cells were fibroblasts.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from passage 4 primary human urethral stricture fibroblasts using Trizol Reagent (Invitrogen, Carlsbad, CA, USA). SuperScript II Reverse Transcriptase and oligo-dT primers (Invitrogen) were used to generate single-stranded cDNA. iCycler (Bio-Rad, Hercules, CA, USA) and SYBR Green PCR master-mix reagents (Abgene, Portsmouth, NH, USA) were then used to perform qRT-PCR. The following primers were used for the amplification of specific target genes: human Arp2 (F: 5′-CACCTGTGGGACTACACATTTG-3′; R: 5′-TGGTTGGGTTCATAGGAGGTTC-3′); human Arp3 (F: 5′-TTCAACCATGTTC-AGGGAC-3′; R: 5′-GGCTTCAATCTACCACCA-3′); human WASP (F: 5′-GATGCT-TGGACGAAAATGCTTG-3′; R: 5′-CCCCACAATGCTCCTTGGT-3′); human WAVE2 (F: 5′-CTCGGGTAAGCTCCCTTGC-3′; R: 5′-GCTTTTCGGGTGTTGATT-CCTT-3′). Fold changes in expression were calculated using the 2−ΔΔCt method on data acquired from three experiments. β-actin was used as reference genes and gave comparable results.
Western blot analysis
SDS-PAGE and Western blotting were performed as previously described.7 Primary antibodies against Arp2 (MD6566), Arp3 (MD6567), WASP (MD6568), WAVE2 (MD6565), Collagen I (MD6569), and Collagen III (MD6570), and secondary horseradish peroxidase-conjugated goat anti-mouse (MD2142) and goat anti-rabbit IgG antibodies (MD2141) were all purchased from MDL Biotechnology (Beijing, China). All immunoblotting experiments were repeated at least three times. The OD of protein bands was determined using a gel imaging analysis system (GelDoc-It310; UVP, Upland, CA, USA).
Immunofluorescence analysis
Cells on coverslips were processed for immunofluorescence analysis as described previously.16 Briefly, cells were washed once in ice-cold phosphate-buffered saline (PBS) before lysis with actin stabilization buffer (0.1 M PIPES, pH 6.9, 30% glycerol, 5% DMSO, 1 mM MgSO4, 1 mM EGTA, 1% TX-100, 1 mM ATP, and protease inhibitor) on ice for 10 minutes.17 The cells were then washed with PBS, fixed in 4% paraformaldehyde for 10 minutes, and permeabilized in 0.25% Triton X-100/PBS for 5 minutes. To label the actin cytoskeleton, cells were incubated with Alexa-488-conjugated phalloidin for 1 hour at room temperature (Invitrogen). DAPI (Invitrogen) was used as a counterstain. Cells on coverslips were imaged on an epifluorescence microscope (Leica DM3000; Leica, Wetzlar, Germany). The same microscope and acquisition settings were used for all samples, enabling intergroup comparisons to be made following the quantitative analysis of image data. Captured fluorescence images were analyzed using MetaMorph software (Molecular Devices, Inc., San Jose, CA, USA).
Apoptosis assay
Annexin V binding and propidium iodide staining were used to evaluate the effects of fasudil on apoptosis in urethral scar fibroblasts treated with or without TGF-β1. The apoptosis assay was performed using the Apoptosis Detection Kit (Haige Biotechnology Co. Ltd., Fuzhou, Fujian, People’s Republic of China) as described previously.18 The control group was treated with additional binding buffer. Flow cytometry was used to acquire data on cells in the early, late, and final (postapoptotic) stages of apoptosis.
Statistical analysis
All statistical analyses were performed using SPSS software (Version 19.0; IBM Corporation, Armonk, NY, USA). Each experiment was performed in triplicate. Data are presented as mean ± SD. The Mann–Whitney U-test or Kruskal–Wallis test was used to analyze differences between paired groups. P-values <0.05 were regarded as statistically significant.
Results
Identification of urethral scar fibroblast
The presence of urethral scar fibroblasts in day 2–3 urethral stricture tissue explant cultures was determined by immunofluorescence staining for vimentin. Immunofluorescence analysis demonstrated that cultured cells were positive for vimentin (Figure 1A and B).
Effects of fasudil on Arp2, WAVE2, Arp3, and WASP mRNA expression
TGF-β1 significantly increased the mRNA expression levels of Arp2, WAVE2, Arp3, and WASP in primary human urethral scar fibroblasts (P<0.05; Figure 2A–D). Fasudil significantly decreased the mRNA expression levels of these genes in a dose-dependent manner in both the TGF-β1-treated and -untreated groups (Figure 2A–D).
Effects of fasudil on Arp2, Arp3, WASP, WAVE2, Collagen I, and Collagen III protein levels
TGF-β1 significantly increased the expression of Arp2, Arp3, WASP, WAVE2, Collagen I, and Collagen III protein in human urethral scar fibroblasts, when compared with the untreated control group (P<0.05; Figure 3A). Fasudil significantly suppressed the expression of all these proteins in both TGF-β1-treated and -untreated cells in a dose-dependent manner (Figure 3B).
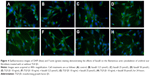
Effects of fasudil on the actin cytoskeleton
The effects of fasudil on the filamentous actin cytoskeleton of human urethral scar fibroblasts treated with or without TGF-β1 were determined by immunofluorescence analysis (Figure 4). The immunofluorescence analysis demonstrated that the F-actin bundles were seen throughout the cell, as can be seen in Figure 4A (control group) and Figure 4E (treated with TGF-β1 10 ng/mL only), there was no significant difference in terms of the F-actin staining between two groups. When compared with controls (Figure 4A, control group), a decrease in F-actin bundles and a concomitant increase in F-actin clumps/globular structures were observed with the increase of fasudil concentration (Figure 4B–D). When compared with controls (Figure 4E, treated with TGF-β1 10 ng/mL only), a decrease in F-actin bundles and a concomitant increase in F-actin clumps/globular structures were also observed with an increase in fasudil concentration (Figure 4F–H; all treated with TGF-β1 10 ng/mL, respectively). Thus, fasudil treatment resulted in a dose-dependent decrease in F-actin bundles and a concomitant increase in F-actin globular structures in cells of both the TGF-β1-treated and -untreated groups (Figure 4B–D and F–H).
Effects of fasudil on apoptosis
Annexin V-FITC/PI assay was used for the effects of fasudil on the apoptosis of fibroblast (Figure 5). The percentage of apoptotic cells after 24 hours was significantly decreased in TGF-β1 group when compared with the control group, demonstrating the prosurvival effects of this growth factor on human urethral scar fibroblasts (P<0.05; Figure 5A and E). Fasudil significantly increased the percentage of apoptotic cells in both the TGF-β1-treated and -untreated groups (Figure 5B–D and F–H). Fasudil therefore has a proapoptotic effect on human urethral scar fibroblasts, irrespective of the effects of TGF-β1.
Discussion
Urethral stricture can result from trauma, idiopathic, or iatrogenic etiologies and inflammation. As a consequence, urethral stricture may result in recurrent infection, bladder calculi, and chronic renal insufficiency.
This study demonstrated a dose-dependent suppressive effect of fasudil (12.5–50 μmol/L) on actin polymerization and collagen synthesis in human urethral fibroblasts treated with or without TGF-β1. Fasudil also significantly increased the apoptosis of human urethral fibroblasts treated with or without TGF-β1, again in a dose-dependent manner. Moreover, the expression of Arp2, Arp3, WASP, WAVE2, Collagen I, and Collagen III was attenuated by the effects of fasudil. Fasudil targets the Rho/ROCK signaling pathway, and these results therefore suggest that suppression of Rho/ROCK signaling may in part contribute to the inhibitory effects of fasudil on the F-actin cytoskeleton and collagen synthesis in human urethral fibroblasts.
Cell growth is regulated by apoptosis. In this study, apoptosis was evaluated by Annexin V-FITC/PI staining and flow cytometry to further elucidate the therapeutic potential of fasudil. This assay showed that fasudil induced apoptosis in a dose-dependent manner in both TGF-β1-treated and TGF-β1-untreated cells.
The major pathological changes in human urethral stricture occur in the extracellular matrix of the urethral spongiosa. The extracellular matrix mainly consists of Collagen I and Collagen III. The urethral stricture spongiosum has been reported to comprise 83.9% Collagen I and 16.1% Collagen III.19 This study demonstrated that Collagen I and Collagen III expression in urethral fibroblasts could be enhanced by TGF-β1 treatment and that the expression of these extracellular matrix proteins in both TGF-β1-treated and -untreated cells could be significantly decreased in a dose-dependent manner by fasudil.
F-actin is the major component of the microfilament cytoskeleton. The Arp2/3 complex regulates cell migration by influencing the polymerization and depolymerization of actin. Thus, the Arp2/3 complex is viewed as a major component in cell migration. Arp2 and Arp3 are members of the Arp2/3 family. Together, they provide nucleation sites for actin and have also been implicated in the migration of cells.8,9 NPFs play an important role in activing the Arp2/3 complex. WASP and WAVE2 are two important NPFs associated with nucleation induced by the Arp2/3 complex.8,10 In this study, Arp2, Arp3, WASP, and WAVE2 expression was significantly decreased in a dose-dependent manner by fasudil in both TGF-β1-treated and TGF-β1-untreated cells. These inhibitory effects of fasudil have a key role in suppressing the polymerization of the actin cytoskeleton. To further evaluate the molecular mechanisms of urethral stricture formation, the F-actin cytoskeleton of fibroblasts was investigated by immunofluorescence analysis. In this study, the inhibitory effects of fasudil on the polymerization of the actin cytoskeleton were reconfirmed; fasudil attenuated the fluorescence intensity of F-actin staining in a dose-dependent manner in both TGF-β1-treated and -untreated cells.
Conclusion
This study revealed that fasudil inhibits cytoskeletal polymerization, collagen synthesis, and apoptosis in fibroblasts derived from human urethral scar tissue. Fasudil may therefore have therapeutic potential in urethral stricture disease.
Acknowledgment
This study was supported by the young and middle-aged scholars program of Health and Family Planning Commission of Fujian Province, China (Grant No 2015-ZQN-JC-20).
Disclosure
The authors report no conflicts of interest in this work.
References
Hoag N, Chee J. Surgical management of female urethral strictures. Transl Androl Urol. 2017;6(Suppl 2):S76–S80. | ||
Hampson LA, Lin TK, Wilson L, Allen IE, Gaither TW, Breyer BN. Understanding patients’ preferences for surgical management of urethral stricture disease. World J Urol. 2017;35(11):1799–1805. | ||
Elshout PJ, Veskimae E, MacLennan S, et al. Outcomes of early endoscopic realignment versus suprapubic cystostomy and delayed urethroplasty for pelvic fracture-related posterior urethral injuries: a systematic review. Eur Urol Focus. 2017;3(6):545–553. | ||
Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65(2):467–479. | ||
Yang Y, Yu B, Sun D, Wu Y, Xiao Y. The dose-dependence biological effect of laser fluence on rabbit fibroblasts derived from urethral scar. Lasers Med Sci. 2015;30(3):1019–1029. | ||
Cavalcanti AG, Costa WS, Baskin LS, Mcaninch JA, Sampaio FJ. A morphometric analysis of bulbar urethral strictures. BJU Int. 2007;100(2):397–402. | ||
Xu N, Chen SH, Qu GY, et al. Fasudil inhibits proliferation and collagen synthesis and induces apoptosis of human fibroblasts derived from urethral scar via the Rho/ROCK signaling pathway. Am J Transl Res. 2017;9(3):1317. | ||
Nolen BJ, Pollard TD. Structure and biochemical properties of fission yeast Arp2/3 complex lacking the Arp2 subunit. J Biol Chem. 2008;283(39):26490–26498. | ||
Campellone KG, Welch MD. A nucleator arms race: cellular control of actin assembly. Nat Rev Mol Cell Biol. 2010;11(4):237–251. | ||
Pollard TD. Regulation of actin filament assembly by Arp2/3 complex and formins. Annu Rev Biophys Biomol Struct. 2007;36:451–477. | ||
Jiang HS, Zhu LL, Zhang Z, Chen H, Chen Y, Dai YT. Estradiol attenuates the TGF-β1-induced conversion of primary TAFs into myofibroblasts and inhibits collagen production and myofibroblast contraction by modulating the Smad and Rho/ROCK signaling pathways. Int J Mol Med. 2015;36(3):801–807. | ||
Zhang XH, Sun NX, Feng ZH, Wang C, Zhang Y, Wang JM. Interference of Y-27632 on the signal transduction of transforming growth factor beta type 1 in ocular Tenon capsule fibroblasts. Int J Ophthalmol. 2012;5(5):576–581. | ||
Baba I, Egi Y, Utsumi H, Kakimoto T, Suzuki K. Inhibitory effects of fasudil on renal interstitial fibrosis induced by unilateral ureteral obstruction. Mol Med Rep. 2015;12(6):8010–8020. | ||
Qi XJ, Ning W, Xu F, Dang HX, Fang F, Li J. Fasudil, an inhibitor of Rho-associated coiled-coil kinase, attenuates hyperoxia-induced pulmonary fibrosis in neonatal rats. Int J Clin Exp Pathol. 2015;8(10):12140–12150. | ||
Zhou Y, Huang X, Hecker L, et al. Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J Clin Invest. 2013;123(3):1096–1108. | ||
Zou J, Luo H, Zeng Q, Dong Z, Wu D, Liu L. Protein kinase CK2α is overexpressed in colorectal cancer and modulates cell proliferation and invasion via regulating EMT-related genes. J Transl Med. 2011;9:97. | ||
Rasmussen I, Pedersen LH, Byg L, Suzuki K, Sumimoto H, Vilhardt F. Effects of F/G-actin ratio and actin turn-over rate on NADPH oxidase activity in microglia. BMC Immunol. 2010;11:44. | ||
Li XF, Zhang Z, Chen ZK, Cui ZW, Zhang HN. Piezo1 protein induces the apoptosis of human osteoarthritis-derived chondrocytes by activating caspase-12, the signaling marker of ER stress. Int J Mol Med. 2017;40(3):845–853. | ||
Gallegos MA, Santucci RA. Advances in urethral stricture management. F1000Res. 2016;5:2913. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.