Back to Journals » Cancer Management and Research » Volume 11
Familial association of lung cancer with liver cancer in first-degree relatives
Authors Xu CR , Lin H, Su J, Zhang XC, Huang YS, Yang XN , Zhou Q , Yang JJ, Zhong WZ, Wu YL
Received 26 December 2018
Accepted for publication 3 April 2019
Published 28 June 2019 Volume 2019:11 Pages 5813—5819
DOI https://doi.org/10.2147/CMAR.S199462
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chien-Feng Li
Chong-Rui Xu,1* Huan Lin,1,2* Jian Su,1 Xu-Chao Zhang,1 Yi-Sheng Huang,3 Xue-Ning Yang,1 Qing Zhou,1 Jin-Ji Yang,1 Wen-Zhao Zhong,1 Yi-Long Wu1
1Guangdong Lung Cancer Institute, Guangdong General Hospital & Guangdong Academy of Medical Sciences, Guangzhou, Guangdong, People’s Republic of China; 2The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, The Second Clinical College of Guangzhou University of Chinese Medicine, Guangzhou, Guangdong, People’s Republic of China; 3Affiliated Zhongshan Hospital of Guangdong Medical University, Zhongshan, Guangdong, People’s Republic of China
*These authors contributed equally to this work
Purpose: Besides the smoking and occupational exposures, heritable factors have been proven to be a risk factor for lung cancer by several population-based studies, which would misestimate the risk of lung cancer.
Patients and methods: To quantify the magnitude of the high risk of lung cancer with family history, we performed a case-based study with 1373 enrolled individuals, which may be more accurate than a population-based study.
Results: Risk of lung cancer was higher in people with lung cancer family history than in the control group (OR 2.50, p<0.001). Individuals with family history of liver cancer also had a higher risk of lung cancer than the control group (OR 1.78, p=0.038) while there was no significant difference within the individuals with family history of colorectal cancer, esophageal cancer, nasopharyngeal cancer or breast cancer. Furthermore, the risk of lung cancer in the subjects with early-onset cancers (age <50 years) was higher than the later-onset cancers (age ≥50 years), especially in individuals with family history of liver cancer (OR 9.24 vs 1.39). Risk of lung cancer in females with family history of lung cancer or liver cancer was higher than in males.
Conclusion: The results of this study proved that the familial aggregation of lung cancer and liver cancer manifests higher risks of lung cancer, supporting the hypothesis that lung cancer and liver cancer are attributable to common familial predisposition.
Keywords: lung cancer, liver cancer, family history
Introduction
Lung cancer is the leading cause of cancer-related deaths and the most commonly diagnosed cancer worldwide,1–3 while most patients were advanced disease when diagnosed and lost the chance to cure. Early diagnoses are crucial to improve survival of lung cancer. Recently, low dose CT screening for high-risk individuals is effective for the early diagnoses of lung cancer and has been recommended by several guidelines.4 How to define the high-risk patients is differred in several clinical trials. Smoking and occupational exposures are related to lung cancer while heritable factors may also play an important role during the lung cancer occurs. Studying the familial aggregation not only including lung cancer but also with other malignancies can provide information on cancer risk assessment in course of clinical genetic counseling and figure out hints of tumor etiology to improve overall survival.
The familial clustering of different type of cancers has frequently been observed in previous studies, implying that cancers might be regarded as hereditary diseases with multiple phenotypes and shared genetic factors.5–8 However, these conclusions were drawn from population-based studies which might cause various confounding factors and higher over-reporting rate.9 We performed a large-scale clinic-based study to assess and calculate lung cancer risks among individuals with family histories of lung cancer and other malignancies.
Patients and methods
The study protocol and informed consents were approved by the Clinical Ethics Committee of Guangdong General Hospital and the institutional review board. The study was conducted in accordance with the Declaration of Helsinki. All participants were enrolled after signing the informed consents.
Patients
Probands were defined as the patients diagnosed with lung cancer in Guangdong General Hospital between October 2009 and August 2012. First-degree relatives (FDRs) and spouses of the probands were defined as case group and control group respectively. Spouses and their FDRs were excluded if spouses had ever been diagnosed with malignant tumors, divorced from probands or death for more than three years. Offspring of probands and spouses were ruled out for the considering that they acted as common relatives to both groups.
Probands and their spouses were interviewed by the same investigator with a unified and standardized lifestyle questionnaire applied to collect detailed information on age, gender, geographic and socioeconomic characteristics, languages, smoking status, histories of diseases, occupational exposures, pathological diagnoses and TNM stage of lung cancer. To minimize potential recall bias, probands and spouses were asked to make verification on telephone when confronted with uncertain answers.
Statistical analysis
The statistical software package, Statistical Program for Social Sciences (SPSS, SPSS Inc., Chcago, IL, USA) 17.0, was applied to analyze collected data. Demographic characteristics in both groups were compared and balanced via descriptive statistical analyses. Then a multiple logistic regression was performed to evaluate the magnitude of increased risks of lung cancer among patients with family histories of lung cancer or other malignancies. Odds ratios (ORs) and 95% confidence intervals (95% CIs) were calculated for statistically significant predictors. When familial aggregation of different types of cancers co-existed, stratification analyses were performed by gender and age respectively. All tests were two-sided and P-values less than 0.05 were considered statistically significant. Qualitative variables were tested with chi-square or Fisher Exact test for independence.
Results
After screening, more than 1300 individuals from 25 provinces of China, 726 probands pedigrees and 647 spouse pedigrees were enrolled. The epidemiologic characteristics of both groups are listed in Table 1. There are more young objects (<50 years old) in the control group (p=0.041) and more male objects in case group (p<0.001). In the case group smoker, history of pulmonary diseases and living environment were more prevalent than the control group. Family sizes involved in both groups were balanced in the FDRs (p=0.054) and siblings (p=0.491) (Table 2). We aim to quantize the magnitude of high risks related to family histories of lung cancer.
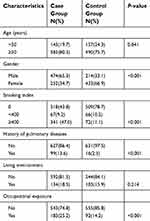 |
Table 1 Epidemiologic characteristics of the two groups |
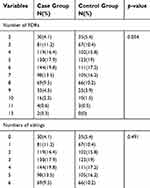 |
Table 2 Comparison of numbers in pedigrees |
Lung cancer risks of the individual whose FDRs with any cancers
Although there was a larger proportion of relatives with any cancers in case group than control group (32.4% vs 21.9%, p<0.001) (Table 3), risk of the any cancer of the probands in case group was higher than the control group (OR 1.70, 95% CI 1.34–2.17, p<0.001). Further analyses illustrated that the degree of elevated risks of any cancers was associated with the number of FDRs with cancers. For example, the OR of the risks of cancer was 1.47 (OR 1.47, 95%CI 1.13–1.91, p=0.004) with one FDR diagnosed with any cancers while and the OR increased to 2.89 (95% CI 1.75–4.78, p<0.001) with more than two FDRs.
 |
Table 3 Risks of first-degree relatives acquiring cancers |
Lung cancer risks of the individual whose FDRs with lung cancers
Relatives with lung cancer in the case group were more than the control group (11.8% vs 5.1%, p<0.001) and OR was 2.50 (95% CI, 1.65–3.79, p<0.001) (Table 3). The quantified correlation between risks of lung cancer and the number of relatives with lung cancer was significant. The lung cancer risk of the individual with one lung cancer patient in the FDRs was higher than the individual without lung cancer in the FDRs (OR 2.22, 95% CI, 1.44–3.41, p<0.001). The OR was 11.51 if more than two FDRs were diagnosed with lung cancer (95%CI, 1.49–88.80, p=0.019).
Lung cancer risks of the individual whose FDRs with liver cancers
Besides the lung cancer, risks related to family history of other specific cancer was described in Table 4. Risks of lung cancer were higher than the control while the family history of liver cancer existed (OR 1.78, 95% CI 1.03–3.08, p=0.038). There was no difference in the risk of lung cancer between the two groups with the family history of colorectal cancer, esophageal cancer, nasopharyngeal cancer or breast cancer. (p=0.714, 0.146, 0.502 and 0.201, respectively).
 |
Table 4 Risks of first-degree relatives developing different types of cancers |
Discussion
The smoking status and occupational exposure have been proved to be the major risk factors of lung cancer, while they cannot entirely explain how lung cancer emerge and progress. Several studies from different sites reported the familial clustering of cancers10–14 and indicated that cancer may bear a broad phenotype with shared genetic factors, but specific associations among different cancer sites remain unclear. It is necessary to explore genetic predisposition among families with histories of lung cancer or other malignancies.
Some population-based case-control studies reported that family history of lung cancer is one of the risk factors for individuals. The accuracy of these studies relied on the relationship degree of information provider to probands. Clinic-based studies were more reliable to avoid the over-report incidence of cancers and more accurate in reporting information on FDRs than population-based studies.15 This large-scale clinic-based case-control study among probands’ FDRs showed that individuals exhibited higher risks of lung cancer if they had a family history of lung cancer (OR=2.50; 95% CI 1.65–3.79; p<0.001) or liver cancer (OR=1.78; 95%CI 1.03–3.08, p=0.038) than the control group. The risk was especially remarkable among female and young probands, contributing more genetic susceptibility.
A familial correlation of liver cancer among FDRs predicts increasing risks of liver cancer with an addition of genetic variance functioning.16–18 Adjusting for common confounding factors like hepatitis B virus infection and alcohol intake, the familial aggregation remains significant (cross ratio [CR]=2.94, 95% CI 1.59–5.45, p=0.001).17 Another recent study conducted in America16 showed that risks exhibited high with a family history of liver cancer (OR=4.1; 95%CI, 1.30–12.9) when potential confounding factors were controlled including age, gender, race, educational level, tobacco smoking, diabetes mellitus, alcohol consumption and HBV/HCV infection. Therefore, the genetic predisposition of liver cancer plays a crucial role in procedure of cancer incidence, which is as the same situation on the hereditary susceptibility of lung cancer. We cannot completely rule out the possibility of occasional founding, although it is intriguing to figure out that subjects in families with the cluster of liver cancer obtain greater risks of lung cancer. There are almost 383,000 patients dying from liver cancer per year in China,19 consisting of approximately 51% of deaths from liver cancer throughout the whole world.3 Considering lung cancer and liver cancer contribute large proportions to cancer burden globally especially in China, there are potential chances of overlapping prevalence between them. However, underlying occasional founding should impossibly account for all the co-existence of liver cancer and lung cancer clustering within families.
Our study showed that the risk of lung cancer (OR=3.06, 95% CI 1.69–5.54, p<0.001) and liver cancer (OR=2.22, 95% CI 1.02–4.82, p=0.039) of female was higher than those of male probands (OR=1.88, 95% CI 1.02–3.45, p=0.041 and OR=1.37, 95% CI 0.61–3.11, p=0.445, respectively), which were consistent with published literatures.13,20–22 However, there were studies illustrating that females with a family history of lung cancer in FDRs suffered from less elevated risks of lung cancer than male population.11,23 For instance, Lo et al23 reported that lung cancer risk of male and female subjects were similar. (OR=2.77, 95% CI 1.53–5.01 and OR=2.44, 95% CI 1.79–3.32, respectively). These studies were all population-based study and the sample size was not enough to illustrate substantial reasons with inevitably inflated false-negative rates.
We found early-onset cancer patients (<50 years old) exhibited higher risks than late-onset ones whose FDRs with any cancer (OR 2.28 vs 1.56, respectively) or lung cancer (OR 3.28 vs.2.32, respectively) or liver cancer (OR, 9.24 vs.1.39, respectively) (Table 5), consistent with several studies about lung cancer or liver cancer.24–26 Some study27,28 discovered that most lung cancer occurred among population with predisposing genes and that lung cancer at an earlier age of onset to some extent greatly manifested consistence with Mendelian dominant inheritance of an allele. While there is no evidence supporting that early-onset liver cancer corresponds to the same phenomenon. Our study has appropriately proved that lung cancer patients at an early onset of diagnosis tend to demonstrate a familial susceptibility of liver cancer, which would appeal for further study to validate.
 |
Table 5 Cancer risks of first-degree relatives stratified by Probands’ age |
It is convinced that cancers are regarded to be associated with multiple phenotypes initiated by environmental factors, while individuals’ susceptibility to cancers are determined by genetic characteristics. Therefore, phenomena of familial clustering of liver cancer with lung cancer can be explained by synergistic effects of shared genetic predisposition.
Several methodological advantages enhance the strength of evidences of our study. Clinic-ascertained sources could provide more accurate information on FDRs with lower false-negative and false-positive rates and the sample size of this study is large enough to provide comprehensive information. To minimize potential selective bias and recall bias, the same investigator was involved to collect data by using unified questionnaire.
However, there are several limitations with our research. The baseline characteristics including age, gender, smoking history, history of pulmonary diseases, and occupational exposure were not well balanced between the two groups. The imbalance may associate the other risk factors of lung cancer. Furthermore, individuals of control group with family history of esophageal, nasopharyngeal, and breast cancer were 16, 11, and 5. The sample size is not enough to compare the risk of lung cancer with family history of these cancers.
There are still some problems need to be solved. First, shared environmental factors are extremely related to liver cancer, such as intensity and duration of alcohol consumption and HBV/HCV infection, but were not taken into consideration. Exclusion of individuals with missing data might lead to conservative conclusions. The questionnaire was limited to most common types of solid tumors which might not completely include all malignancies, resulting in misleading conclusion of interpreting data. Finally, there were inherent limitations in accordance to our methods. Histories of malignant cancers among relatives were not verified neither in-person nor medically, resulting in underestimation of cancer risks.
Conclusion
Present data from the study provide strong evidences that familial aggregation of lung cancer and liver cancer manifests higher risks of lung cancer, supporting the hypothesis that lung cancer and liver cancer are attributable to common familial predisposition. Current data are not enough to verify potential mechanisms, it functions as a reminder that further research and cautious interpretation are imperative and warranted.
Acknowledgments
We thank all the individuals and families who participated in our research and all the doctors who provided fantastic advice, supported us by medical access and consulted with questionnaire design. We appreciate that all of them have contributed a lot to make this study possible. The abstract of this paper was presented at the American Society of Clinical Oncology Annual Meeting 2018 as an ePoster presentation with interim findings https://meetinglibrary.asco.org/record/163850/abstract.
This work was supported by the funding listed below:
- National Key R&D Program of China: 2016YFC1303800
- Special Fund of Public Interest by National Health and Family Control Committee: 201402031
- National Natural Science Foundation of China: 81502439
- Medical Scientific Research Foundation of Guangdong Province of China: A201501
- Science and Technology Plan of Guangzhou: 201510010214
- National Natural Science Foundation of China: 81871891.
Disclosure
YLW reports grants and personal fees from AstraZeneca, Roche, and Boehringer Ingeiheim; he also received personal fees from Eli Lilly, Sanofi, Pfizer, and BMS. The other authors report no conflicts of interest in this work.
References
1. Cancer statistics. JAMA. 2013;310(9):982. doi:10.1001/jama.2013.5289
2. Dyzmann-Sroka A, Malicki J. Cancer incidence and mortality in the greater Poland region-analysis of the year 2010 and future trends. Rep Pract Oncol Radiother. 2014;19(5):296–300. doi:10.1016/j.rpor.2014.04.001
3. Ott JJ, Ullrich A, Mascarenhas M, Stevens GA. Global cancer incidence and mortality caused by behavior and infection. J Public Health (Oxf). 2011;33(2):223–233. doi:10.1093/pubmed/fdq076
4. Horeweg N, van Rosmalen J, Heuvelmans MA, et al. Lung cancer probability in patients with CT-detected pulmonary nodules: a prespecified analysis of data from the NELSON trial of low-dose CT screening. Lancet Oncol. 2014;15(12):1332–1341. doi:10.1016/S1470-2045(14)70389-4
5. Bermejo JL, Sundquist J, Hemminki K. Sex-specific familial risks of urinary bladder cancer and associated neoplasms in Sweden. Int J Cancer. 2009;124(9):2166–2171. doi:10.1002/ijc.24178
6. Hiripi E, Lorenzo Bermejo J, Li X, Sundquist J, Hemminki K. Familial association of pancreatic cancer with other malignancies in Swedish families. Br J Cancer. 2009;101(10):1792–1797. doi:10.1038/sj.bjc.6605363
7. Jacobs EJ, Rodriguez C, Newton CC, et al. Family history of various cancers and pancreatic cancer mortality in a large cohort. Cancer Causes Control. 2009;20(8):1261–1269. doi:10.1007/s10552-009-9339-6
8. Moghimi-Dehkordi B, Safaee A, Vahedi M, Pourhoseingholi MA, Pourhoseingholi A, Zali MR. Population prevalence of first- and second-degree family history of breast and ovarian cancer. East Afr J Public Health. 2011;8(4):275–277.
9. Ziogas A, Anton-Culver H. Validation of family history data in cancer family registries. Am J Prev Med. 2003;24(2):190–198.
10. Cassidy A, Balsan J, Vesin A, et al. Cancer diagnosis in first-degree relatives and non-small cell lung cancer risk: results from a multi-centre case-control study in Europe. Eur J Cancer. 2009;45(17):3047–3053. doi:10.1016/j.ejca.2009.05.006
11. Cote ML, Liu M, Bonassi S, et al. Increased risk of lung cancer in individuals with a family history of the disease: a pooled analysis from the International lung cancer consortium. Eur J Cancer. 2012;48(13):1957–1968. doi:10.1016/j.ejca.2012.01.038
12. Hemminki K, Bevier M, Sundquist J, Hemminki A. Cancer of unknown primary (CUP): does cause of death and family history implicate hidden phenotypically changed primaries? Ann Oncol. 2012;23(10):2720–2724. doi:10.1093/annonc/mds063
13. Jin YT, Xu YC, Yang RD, Huang CF, Xu CW, He XZ. Familial aggregation of lung cancer in a high incidence area in China. Br J Cancer. 2005;92(7):1321–1325. doi:10.1038/sj.bjc.6602465
14. Young RP, Hopkins RJ, Hay BA, et al. A gene-based risk score for lung cancer susceptibility in smokers and ex-smokers. Postgrad Med J. 2009;85(1008):515–524. doi:10.1136/pgmj.2008.077107
15. Lin H, Zhong WZ, Yang XN, et al. A clinical model to estimate the pretest probability of lung cancer, based on 1198 pedigrees in China. J Thorac Oncol. 2012;7(10):1534–1540. doi:10.1097/JTO.0b013e3182641b82
16. Hassan MM, Spitz MR, Thomas MB, et al. The association of family history of liver cancer with hepatocellular carcinoma: a case-control study in the United States. J Hepatol. 2009;50(2):334–341. doi:10.1016/j.jhep.2008.08.016
17. Liu L, Li L, Zhou S, et al. Familial correlations of onset age of hepatocellular carcinoma: a population-based case-control family study. PLoS One. 2014;9(9):e108391. doi:10.1371/journal.pone.0108391
18. Yang Y, Wu QJ, Xie L, et al. Prospective cohort studies of association between family history of liver cancer and risk of liver cancer. Int J Cancer. 2014;135(7):1605–1614. doi:10.1002/ijc.28792
19. Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatology. 2014;60(6):2099–2108. doi:10.1002/hep.27406
20. Li X, Mutanen P, Hemminki K. Gender-specific incidence trends in lung cancer by histological type in Sweden, 1958–1996. Eur J Cancer Prev. 2001;10(3):227–235.
21. Gu J, Hua F, Zhong D, Chen J, Liu H, Zhou Q. [Systematic review of the relationship between family history of lung cancer and lung cancer risk]. Zhongguo Fei Ai Za Zhi. 2010;13(3):224–229. doi:10.3779/j.issn.1009-3419.2010.03.07
22. Matakidou A, Eisen T, Houlston RS. Systematic review of the relationship between family history and lung cancer risk. Br J Cancer. 2005;93(7):825–833. doi:10.1038/sj.bjc.6602769
23. Lo YL, Hsiao CF, Chang GC, et al. Risk factors for primary lung cancer among never smokers by gender in a matched case-control study. Cancer Causes Control. 2013;24(3):567–576. doi:10.1007/s10552-012-9994-x
24. Lessel D, Vaz B, Halder S, et al. Mutations in SPRTN cause early onset hepatocellular carcinoma, genomic instability and progeroid features. Nat Genet. 2014;46(11):1239–1244. doi:10.1038/ng.3103
25. Park CH, Jeong SH, Yim HW, et al. Family history influences the early onset of hepatocellular carcinoma. World J Gastroenterol. 2012;18(21):2661–2667. doi:10.3748/wjg.v18.i21.2661
26. Turati F, Edefonti V, Talamini R, et al. Family history of liver cancer and hepatocellular carcinoma. Hepatology. 2012;55(5):1416–1425. doi:10.1002/hep.24794
27. Sellers TA, Chen PL, Potter JD, Bailey-Wilson JE, Rothschild H, Elston RC. Segregation analysis of smoking-associated malignancies: evidence for Mendelian inheritance. Am J Med Genet. 1994;52(3):308–314. doi:10.1002/ajmg.1320520311
28. Wu Y, Wang YY, Nakamoto Y, et al. Accelerated hepatocellular carcinoma development in mice expressing the Pim-3 transgene selectively in the liver. Oncogene. 2010;29(15):2228–2237. doi:10.1038/onc.2009.504
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
