Back to Journals » Clinical Interventions in Aging » Volume 15
Falls Risk, Circadian Rhythms and Melatonin: Current Perspectives
Authors Goswami N , Abulafia C, Vigo D, Moser M, Cornelissen G , Cardinali D
Received 1 October 2020
Accepted for publication 3 November 2020
Published 11 November 2020 Volume 2020:15 Pages 2165—2174
DOI https://doi.org/10.2147/CIA.S283342
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Walker
Nandu Goswami,1 Carolina Abulafia,2 Daniel Vigo,2 Maximilian Moser,1 Germaine Cornelissen,3 Daniel Cardinali2
1Physiology Division, Otto Loewi Research Center for Vascular Biology, Immunology and Inflammation, Medical University of Graz, Graz, Austria; 2Institute for Biomedical Research (BIOMED), Catholic University of Argentina (UCA) and National Scientific and Technical Research Council (CONICET), Autonomous City of Buenos Aires, Argentina; 3Halberg Chronobiology Center, University of Minnesota, Minneapolis, MN, USA
Correspondence: Nandu Goswami
Physiology Division, Otto Loewi Research Center for Vascular Biology, Immunology and Inflammation, Medical University of Graz, Neue Stiftingtalstrasse 6, D-5, Graz A 8036, Austria
Tel + 43 316 38573852
Fax + 43 316 385 79005
Email [email protected]
Abstract: Aging is associated with weakening of the circadian system. The circadian amplitude of most physiological variables is reduced, while the circadian phase becomes more labile and tends to occur earlier with advancing age. As the incidence of falls in older persons could follow circadian variations, a better understanding of conditions in which falls occur can lead to the implementation of countermeasures (such as adjusting the scheduling of hospital staff, or changing the timing of anti-hypertensive medication if falls are related to undesirable circadian patterns of blood pressure and/or heart rate). This includes knowing the times of the day, days of the week, and times of the year when falls are more likely to occur at home or in the hospital. Additionally, the links between aging processes and factors associated with an increased risk of developing autonomic dysfunction are well established. A strong association between heart rate variability indexes and aging has been shown. Circadian rhythms of autonomous nervous system activity may play important role for maintenance of orthostatic tolerance. Whether one is concerned with disease prediction and prevention or maintenance of healthy aging, the study of circadian rhythms and the broader time structure underlying physiopathology is helpful in terms of screening, early diagnosis and prognosis, as well as the timely institution of prophylactic and/or palliative/curative treatment. Timing the administration of such treatment as a function of circadian (and other) rhythms also could lead to reduction of falls in older persons. Finally, a prominent circadian rhythm characterizes melatonin, which peaks during the night. The circadian amplitude of melatonin decreases as a function of age, raising the questions whether such a decrease in the circadian amplitude of melatonin relates to a higher risk of falls and, if so, whether melatonin supplementation may be an effective countermeasure. This narrative review assesses the relationships between fall risk and the potential role circadian rhythms and melatonin play in mitigating this risk. We aim to provide healthcare workers adequate information about fall risk in older persons, including the potential role of the circadian rhythms and/or melatonin, as well as to lay foundations for future fall prevention interventional studies.
Keywords: falls, aging, orthostatic intolerance, autonomic, vagus
Introduction
Population ageing has become a global phenomenon. In Europe, 13% of the population is aged 65 or more but by 2030, it is expected to almost double to 24%.1 This means that the average life expectancy is increasing – largely attributed to the development of medical and technological advances – but so are the challenges in geriatric care such as medical aspects of care, rehabilitation, nursing, psychology, sociology, pharmacy, social aspects, amongst others. According to epidemiological data, about 28–35% of people aged 65 or more fall each year and in individuals over 70 years of age, the numbers increase to 32–42%.2 This indicates that falls in older people must be addressed by health-care professionals both in acute and chronic care settings.
Falls are considered alarming adverse events due to their consequences such as lacerations, bruises, loss of function, hip and/or skull fractures or even death.3–6 In addition, World Health Organization, WHO (2018) classified falls as the second highest cause of accidental injuries in older persons, and highlighted that programs to avoid falls and research in underlying causes of falls should be prioritized.7 Falls are adverse events that affect older people not only physically but also psychologically and socially, impacting on their overall quality of life.5,6 Consequently, falls and falls-related injuries are common problems of Public Health as they are associated with high morbidity and mortality in older persons. Besides these aspects, falls and fall-related injuries increase costs in residential homes, nursing homes, public health services, hospitals and to older individuals that have no health insurance. Therefore, it is relevant to contribute for patient safety by preventing falls in old people.
The etiology of falls is multifactorial and could be attributed to the following types of causes: intrinsic (due mainly to medical conditions) and extrinsic (largely arising due to environmental factors). Intrinsic factors are related to physical and psychological state of the person (eg, lack of vision and/or hearing, reaction time, musculoskeletal disorders, vestibular, proprioceptive and musculoskeletal disorders, gait, prolonged sedentary state, among others); to diseases (eg, cardiovascular, neurologic, osteoarticular, genitourinary, among others); and to drugs (eg, those that lead to diminishing of motor functions, dizziness, hypotension, weakness, confusion and bed confinement). Extrinsic factors are related mainly to environmental situations (eg, inadequate lighting, presence of obstacles, slippery surfaces, absence of handrails, among others).8 In addition, Montero-Odasso (2018) highlights the importance of cognitive impairments such as lack of attention that compromises postural and gait stability and executive functions such as impairment of attention, inhibitory control, working memory and cognitive flexibility thus leading to falls.9 All these aspects must also take into consideration that older adults with falls exhibit reduced mobility and become more bed-confined due to further functional impairment associated with bedrest per se and fear of further falls. Specifically, fear of falling might lead to increased likelihood of falling as well as activity limitation or avoidance, reduced autonomy and self-esteem, social isolation and depression.10 This vicious cycle is associated with high risk of falls, increased morbidity and mortality.3–6
This narrative review assesses the relationships between fall risk and the potential role circadian rhythms and melatonin play in mitigating this risk. We aim to provide healthcare workers adequate information about fall risk in older persons, including the potential role of the circadian rhythms and/or melatonin, as well as to lay foundations for future fall prevention interventional studies.
Search Methodology
The narrative review on the subject of “falls, circadian rhythms and melatonin” examined both primary and secondary sources of literature on the topic. PubMed, Web of Science and Google Scholar served as search engines and were used to access relevant literature.
Articles available on human studies in English language, published within the last two decades, and articles focusing on falls, circadian rhythms and melatonin were included. Articles involving children or those with falls secondary to accidents were excluded. As the focus of this paper was on aging, and falls are usually associated with high morbidity (including longer periods of bedrest confinement) and mortality in older persons, special focus was placed on aging-related falls.
Aging, Circadian Rhythms and Falls
Aging is generally associated with weakening of the circadian system. The circadian amplitude of most physiological variables is reduced, while the circadian phase becomes more labile and tends to occur earlier with advancing age.11 Circadian disruption (as in shift work) is related to an increased risk of a host of different disease conditions. The elderly population faces additional environmental impact on their circadian rhythms; many drugs impact circadian rhythms and sleep synchronization (Figure 1). The chronically ill and institutionalized older persons as well as those in prolonged isolation, such as the lockdown conditions implemented during the COVID-19 pandemic are more vulnerable to circadian dysregulation, mainly due to reduced exposure to natural sunlight and excessive exposure to artificial light at night. Even in healthy older people living at home, lack of daily structure provided by having a regular job also heightens sleep pattern disruption.12 Such dysregulation could in turn lead to secondary disorders such as diabetes, immunosuppression, insomnia, depression as well as metabolic and cognitive disorders.13 Likewise, the presence of disease or a disease condition linked to the genetic makeup can also lead to a disturbed circadian system.
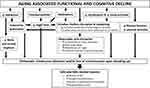 |
Figure 1 Aging-associated functional and cognitive decline and its relationship with autonomic function, circadian rhythms, melatonin and falls (orthostatic intolerance). |
One of the most relevant circadian rhythms is the sleep-wake cycle, since it encompasses and orchestrates many other bodily rhythms. In aging, circadian rhythms become more fragmented, slow wave sleep (SWS) is reduced (which plays a key role in memory consolidation) and there is an increase of wakeful periods during the night and of inactivity intervals during the day (naps), which represents an age-related vulnerability to sleep disorders. In turn, sleep disorders exacerbate cognitive symptoms in subjects with increased risk of developing neurodegenerative diseases, accelerating the rate of cognitive decline in such patients. In addition, sleep disorders have been reported to exacerbate behavioral, pathophysiological manifestations of dementia12 and changes in the circadian rhythm of activity are associated to higher risk of mild cognitive impairment and dementia.14
Sleep-wake cycle disruption is associated to alterations in the circadian rhythm of the autonomic nervous system (ANS) as the parasympathetic function is prevalent during sleep and sympathetic activity is prevalent during wake state. The link between aging processes and factors associated to an increased risk of developing autonomic dysfunction are well established and there is a strong association between heart rate variability indexes and aging.15 As there appears to be a relationship between cognitive status and cardiac sympatho-vagal balance, these factors may influence each other in older patients thus increasing their risk of developing dementia. Therefore, autonomic changes characteristic of normal aging could represent a vulnerability in front of the effects of underlying pathophysiological neurodegenerative processes.16
It has been suggested that circadian rhythms of ANS activity may play important role for maintenance of orthostatic tolerance (OT). One way to assess OT is through the study of the vascular (endothelial) function as it is involved in vascular tone regulation and may contribute to the development of orthostatic intolerance. Many studies have reported a link between orthostatic hypotension (OH) and cognitive impairment, and suggest OH as a possible early marker of dementia.
Sleep disorders are among the earliest manifestations of many neurodegenerative diseases, even prior to cognitive decline due to the early affectation of brain regions responsible of circadian regulation, which impact clock genes, body temperature, nighttime melatonin secretion and daytime orexin secretion, as well sleep architecture (sleep fragmentation, sleep efficiency, rapid eye movement (REM), no-REM and SWS duration).12 Specifically, it has been shown that in Alzheimer’s disease (AD) - the main cause of dementia worldwide -, beta-amyloid production (AD’s pathophysiological hallmark) responds to a circadian rhythm. Thus, disrupted sleep affects both amyloid production and clearance. Amyloid accumulation due to disrupted sleep might generate a positive feedback loop, where amyloid accumulation would also affect sleep due to its impact on sleep-promoting regions.17
Sleep-wake disturbances and frailty present overlapping features (systemic inflammation, cognitive impairment, low physical activity, drowsiness, overactivation of the hypothalamic-pituitary axis (HPA), incident cardio- and cerebro-vascular disease, vascular and neurodegenerative disorders) and predict similar outcomes. Available evidence suggests they most likely have a bidirectional relationship. Frailty reduces physical function and socialization, which affect relevant external synchronizers (social activities, physical exercise, outdoor sunlight exposure, meal scheduling) that synchronize circadian rhythms and promote a healthy sleep-wake cycle (Figure 1). This association has not been thoroughly studied yet it represents and interesting approach since sleep-wake disturbances are potentially treatable and might reverse or prevent frailty progression.18
In this line, the concept of “circadian reserve” is of increasing relevance. Similar to that of “cognitive reserve”, it postulates that persons with healthier circadian rhythmicity cope with pathological insults better than those with low circadian reserve. This concept posits that individual differences in reserves may be responsible for the decline, which may begin at different times in relation to the development of pathology. Many of the aspects involved in this “circadian reserve” can be promoted in the aging population to foster a healthier lifestyle and quality of life.
Falls perhaps contribute most to the decline in the quality of life of older persons. It has been reported that being prone to falling is one of the strongest predictors of nursing home placement.19 Falls and their resulting injuries are responsible for major health, social, and economic problems. A systematic review of the literature concluded that the incidence of falls in older persons follows a circadian variation.20 A better understanding of conditions in which falls occur can lead to the implementation of countermeasures (such as adjusting the scheduling of hospital staff, or changing the timing of anti-hypertensive medication if falls are related to undesirable circadian patterns of blood pressure and/or heart rate). This includes knowing the times of the day, days of the week, and times of the year when falls are more likely to occur at home or in the hospital. The systematic recording of such events, and circumstances in which they occur, will be amenable to chronobiological analyses. A suite of computer programs for the assessment and comparison of rhythmic patterns has been developed at the Halberg Chronobiology Center, and has been made freely available online.21 Whether for prevention or as an adjuvant modality of care, strengthening the circadian system thus presents itself as a novel and promising strategy to examine falls in older persons.
Chronobiological Interventions
Falls were found to be more frequent on Fridays, Sundays, and Mondays compared with Tuesdays, and were more frequent in winter and spring.20 Circadian rhythms are genetically anchored. Their endogenous period differs only slightly, but statistically significantly, from exactly 24 hours. External synchronizers are important to keep circadian rhythms in sync. Strengthening the circadian system can thus be accomplished by enhancing external synchronizers.
The alternation between light and darkness, and between activity and rest/sleep, is of primary importance since the lighting regimen acts directly on the suprachiasmatic nuclei, thought to orchestrate the entire circadian system. Because light is sensed via the eyes and the optic nerve, preventing conditions such as glaucoma is important.22 Exposure to sun light during the day, notably in the morning, and turning lights off during the night is highly recommended.
The feeding schedule is also important since it synchronizes circadian clock genes in peripheral organs such as the liver. Circadian clocks are also tightly coupled to cellular metabolism. As a consequence, time-restricted feeding and other meal scheduling plans are currently been tested for their effect on geroprotection. In the experimental laboratory, time-restricted feeding has been shown to increase the circadian amplitude of important physiological variables, an indication of an enhanced circadian system. Caloric restriction, often associated with time-restricted feeding, has long been known to be associated with prolonged longevity.23 Regular exercise is another non-pharmacologic intervention known to be beneficial to health and to support a strong circadian system. The beneficial effects of exercise are well known in relation to rehabilitation, for instance.
The development of geroprotectors is an active field of research. For instance, the use of metformin has attracted much interest, also beyond its administration to patients with non-insulin-dependent diabetes mellitus. Molecular pathways underlying effects of metformin show cross-talk with circadian clock genes. Melatonin (see below) is another molecule, which has shown beneficial effects in relation to a number of disease conditions. One of its beneficial effects may be its action in increasing sleep quality, again related to the strengthening of the circadian system.
The longitudinal monitoring of physiological variables has been advocated as a means of health surveillance, notably when the data are interpreted chronobiologically. Indeed, the quantitative assessment of rhythmic behavior amounts to entering inside the physiological range, where deviations from norms can prompt the timely institution of prophylactic intervention aiming at primary prevention.11 An example of this approach relates to the ambulatory monitoring of blood pressure (ABPM). In the BIOCOS project, ABPM records were collected in clinically healthy individuals from the first few days of life to centenarians, and also during pregnancy. On their basis, time-specified reference values and reference standards for the circadian parameters were built for males and females in different age groups.24 These chronobiologic norms are now used to assess the health status of volunteers and patients who typically undergo a 7-day/24-hour ABPM session. The analysis of their data can determine whether their circadian variation in blood pressure and heart rate is within acceptable limits or whether it deviates from norms. In such a case, the diagnosis goes beyond the detection of a mere elevated blood pressure, since the circadian amplitude and/or phase can also be abnormal. Several outcome studies have shown that such abnormal behavior of the circadian amplitude and/or phase is also associated with an increased cardiovascular disease risk, and that this risk can be higher than the risk associated with an elevated blood pressure alone.25 Some indirect evidence also exists showing that the restoration of a healthy circadian profile of blood pressure can reduce the incidence of strokes and that of overall cardiovascular mortality.26
A chronobiologic approach can thus save lives. It can also save costs, as suggested by a few case studies. One notorious case is that of a pregnant woman who had an excessive circadian amplitude of her blood pressure profile. Since this observation was made at the beginning of a new research study, the obstetrician took no special action since the average blood pressure was well within acceptable limits. Eight weeks later, however, this pregnant woman delivered prematurely, and her infant boy had to be hospitalized for most of the first two years of his life. Cost-accounted medical costs related to the first year of care amounted to over 1 million dollars.27
Evaluation of the autonomic nervous system as a tool for detecting the risk of falling is becoming increasingly relevant and is being actively researched.28–32 Different studies have used ANS evaluation and, in particular, heart rate variability (HRV) to detect and predict the risk of falling in various populations.19,33 The identification of autonomic parameters as markers of risk of falling will help detect and monitor subjects prone to falling early on as well as allow for the implementation of preventive strategies.34,35 There is no literature on the study of the circadian rhythm of the ANS, yet given the existing research on the subject and the fact that HRV is a fast, non-invasive, affordable and easily implemented technique, a 24hr HRV assessment presents and attractive and promising approach to the study of frailty and falls in older persons.
Longitudinal monitoring of physical and cognitive functions can be helpful in detecting early signs of frailty. A battery of tests can assess physical and cognitive function in older persons. In a Japanese community, central blood pressure was found to be a long-term predictor of cognitive decline in older adults, whereas preserved physical activity was found to be a short-term predictor of cognitive improvement.36 Any changes in cognitive function may be accompanied by changes in sleep characteristics. Intervening early may hence mitigate the development of insomnia-related diseases.
Whether one is concerned with disease prediction and prevention or maintenance of healthy aging, the study of circadian rhythms and the broader time structure underlying physiopathology is helpful in terms of screening, early diagnosis and prognosis, as well as the timely institution of prophylactic and/or palliative/curative treatment. Timing the administration of such treatment as a function of circadian (and other) rhythms also brings considerable benefits. Indeed, chronotherapy (timed treatment) can make the difference between life and death in the experimental laboratory and between health and disease in humans.37
Melatonin and Falls: A Possible Relationship?
Melatonin can be assessed in blood, saliva and urine. A prominent circadian rhythm characterizes melatonin, which peaks during the night in both diurnal and nocturnal mammals. Interestingly, melatonin also undergoes about-weekly (circaseptan) and about-yearly (circannual) variations.24,38,39 While most investigators focus on nocturnal melatonin, the circadian characteristics of melatonin can also be informative. The circadian amplitude of melatonin differs both in terms of breast cancer risk40 and the presence of overt cancer.41 Some genetic mutations are also associated with a shift in the circadian phase of melatonin.27
The circadian amplitude of melatonin decreases as a function of age,11 raising the questions whether such a decrease in the circadian amplitude of melatonin relates to a higher risk of falls (Figure 1) and, if so, whether melatonin supplementation may be an effective countermeasure. Indeed, a direct link between sleep quality, falls risk and melatonin has been shown. For instance, sleep disturbances – independent of confounders – have been shown to be associated with increased risk of falls in older men.42 By improving sleep in older persons, melatonin could play an important role in falls prevention in older persons.
As a chronobiotic/cytoprotective agent, melatonin occupies a special place in the prevention and treatment of age-related disorders.43 Melatonin improves sleep efficiency and has antioxidant and anti-inflammatory properties, in part because of its function as a metabolic regulator and mitochondrial protector.44–46 Melatonin, an unusually phylogenetic conserved molecule present in all known aerobic organisms, is effective both as a chronobiotic and as a cytoprotective agent.
The light-dark variation in the synthesis of melatonin by pinealocytes is the essential fact that explains the role of melatonin as a chronobiotic that coordinates the physiology of biological rhythms.47 The action of melatonin as a chronobiotic is twofold: on the one hand, it “opens the doors of sleep” by inhibiting the promoting activity of late awakening driven by suprachiasmatic nuclei (SCN).48,49 On the other hand, melatonin is the “hormone of darkness”, a chemical code of the duration of the night, and has established itself as crucial in the transmission of information from light to the neuroendocrine system. Melatonin represents a “hand” of the biological clock in the sense that it responds to the signals of the SCN, the temporal variation of the melatonin rhythm indicating the state of the clock, both in terms of phase (time in the internal clock in relation to external time) and amplitude.50
In mammals, circulating melatonin is derived almost exclusively from the pineal gland. In addition, melatonin is synthesized locally in many cells, tissues and organs. Indeed, there is now strong evidence that melatonin is produced in every animal cell that has mitochondria.51 In both animals and humans, melatonin participates in diverse physiological functions that indicate not only the duration of the night, but also improve the elimination of free radicals and the immune response, showing relevant neuroprotective properties.52
The chronobiotic action of melatonin is mediated via the melatonin (MT) receptors, which have been identified both in the central nervous system (CNS) and in the periphery.53 MT1 and MT2 receptors all belonging to the superfamily of membrane receptors associated with G proteins (G-protein coupled receptors, GPCR) have been cloned. More recently, another member, GPR50, was included in the melatonin receptor subfamily. GPR50 shows high sequence homology to MT1 and MT2 but does not bind to melatonin or any other known ligand. An interesting feature of these receptors is their capacity to form homo- and heteromers between each other and also with other GPCRs.54
The cytoprotective activity of melatonin exceeds that mediated via receptors. Almost every cell in the human body contains melatonin, in quantities much higher than those circulating in blood derived from the pineal gland.55 The mitochondrial capacity to synthesize melatonin is now confirmed, but for reasons that remain unexplained, intracellular melatonin does not get the extracellular space. To modify intracellular melatonin levels, doses much higher than those employed as a chronobiotic are needed.56 Most studies on neuroprotective and anti-inflammatory effects in animals employ pharmacological doses, which clearly exceed the saturation of the receptor.
Concerning falls and frailty, several aspects of melatonin action must be considered. In a study designed to identify the role of melatonin in cerebellum balance disturbances and cognitive disorders, spatial memory was highly altered in subjects with inversion of circadian melatonin rhythm, an indication of circadian disruption.57 This possible negative correlation among melatonin, the cerebellum and human balance was suggested by a double-blind pilot study in which 10 mg of melatonin was administered to 14 healthy volunteers at 10.00 a.m. to reproduce the abnormally high diurnal values of the pineal methoxyindole.58 Since melatonin is often used as a sleeping aid in older adults, it was important to determine whether treatment does not cause any hangover effect that could potentially lead to falls and fractures. In a double-blind placebo-controlled study, 81 postmenopausal women with osteopenia received 1 or 3 mg melatonin, or placebo nightly for 12 months.59 Postural balance as well as muscle function was measured. Compared to placebo, one-year treatment with melatonin did not affect postural balance or risk of falls nor modified muscle strength.
A number of studies point out to a preventive role of melatonin in sarcopenia.60,61 For example, in postmenopausal women low levels of 6-sulfatoxymelatonin excretion correlated with sarcopenia.62 Moreover, enhanced urinary melatonin metabolite was associated with more grip and quadriceps muscle strength in aged persons.63 However, in previously sedentary men and women aged 40–75 years after one year of moderate exercise, the urinary melatonin metabolite levels were unchanged.64
Sarcopenia is characterized by the occurrence of insulin-resistance65 and exogenous melatonin decreases insulin resistance, ameliorates mitochondrial function and prevented chemically induced apoptosis and endoplasmic reticulum stress in skeletal muscle cells in vivo and in vitro.61 Therefore, exogenous melatonin can be useful as an antioxidant and an anti-inflammatory agent the prolong muscle strength and adaptation during exercise in adulthood and aging. For example, in a study examining a possibly beneficial effect of a 1year-long administration of melatonin (3 mg/day) on body composition and lipid metabolism increases in lean mass and decreases in fat mass were observed.66
Accumulating evidence has also indicated that melatonin may be involved in the homeostasis of bone metabolism. Age‐related reductions in melatonin are considered to be critical factors in bone loss and osteoporosis with aging, and serum melatonin levels may serve as a biomarker for the early detection and prevention of osteoporosis.67 In comparison to conventional antiosteoporotic agents, which primarily inhibit bone loss, melatonin both suppresses bone loss and promotes new bone formation. By activating melatonin receptor 2, melatonin upregulates the gene expression of alkaline phosphatase, bone morphogenetic protein 2 (BMP2), BMP6, osteocalcin, and osteoprotegerin to promote osteogenesis while inhibiting the receptor activator of NF‐kB ligand pathway to suppress osteolysis.67 In humans, bone mineral density in the femoral neck of postmenopausal osteopenic women increased in response to melatonin in a dose‐dependent manner when compared with a placebo.68 Likewise, in a one-year double blind randomized control trial assessing the effects of nightly melatonin, strontium (citrate), vitamin D3 and vitamin K2 on bone mineral density in postmenopausal osteopenic women treatment increased it in lumbar spine and left femoral neck.69
Melatonin exhibits both hypnotic and chronobiotic properties and has been used therapeutically for the treatment of insomnia related to age, as well as other primary and secondary insomnia. Several meta-analyses support this role.70–72 A consensus of the British Association of Psychopharmacology on the evidence-based treatment of insomnia, parasomnia and sleep disorders in the circadian rhythm concluded that melatonin is the first-choice treatment when a hypnotic is indicated in patients older than 55 years.73 A similar conclusion was reached by a recent consensus of five Italian Medical Societies74 as well as by the expert recommendations from the French institute of medical research on sleep concerning melatonin use in adult psychiatric disorders.75
As discussed recently,56 studies using 2–5 mg of melatonin/day are not adequate to provide an adequate comparison with data on the protection in metabolic syndrome or neurodegenerative disorders derived from animal studies. Hence, clinical trials with doses in the 40–100 mg/day range are urgently needed. It should be noted, however, that purpose for melatonin administration must be considered for its optimal dosing and timing: while such high doses for receptor-independent antioxidant effects are warranted,76 high doses may desensitize specific melatonin receptors77 thereby preventing its pro-circadian78 and other receptor-mediated effects. For example, lack of hypotensive effect could occur due to too high doses (24mg) of administered melatonin.79
It should be noted that melatonin is remarkably non-toxic, and its safety is very high. In humans, melatonin has a high safety profile and, in general, is very well tolerated.
Vagal Tone and Fall Risk
An additional factor influencing the fall risk in older persons might be the decrease of vagal activity with age (Figure 1). Although vagal tone over-all diminishes arterial blood pressure important for a good blood supply to the brain, the decrease of vagal activity also reduces the ability to regulate blood pressure fast, which might be more important for position control than a high blood pressure.
Vagal neurons, utilizing acetylcholine in their final synapses, allow a faster control of heart rate than their sympathetic sisters.80 Vagal acetylcholine is cleared by a fast chemical decay mediated by cholinesterase, whereas the sympathetic norepinephrine depend on a clearing of the synapses by pre-synaptic re-uptake of the transmitter molecules after excitation. It is known that respiratory oscillations of heart rate counter-regulate the oscillations of carotid blood pressure due to respiratory movements and intrathoracic pressure changes.81 Up to the intrinsic heart rate of about 110 b/min, most of the blood regulation is carried out via the vagus nerve.
During aging, vagal activity and vagal oscillations of heart beat dynamic reduce,82 only in some individuals, usually the almost centenarians, this is not the case.83 Until the early 70th there is a continuous decline, whereas elder people display no more reduction of vagal tone.83 Acutely administered melatonin stimulates vagal activity and reduces the heart rate.84 It may therefore be used to support the reduction of blood pressure in hypertensives, likely by this activation of vagal tone, and might be helpful to support fast blood pressure regulation due to vagal enhancement. To our knowledge this aspect has not yet been fully investigated. Only one study has investigated the effectiveness of melatonin in regulation of BP specifically in the older population. In this randomized trial carried out in adults between 63 −91 years, the hypotensive effect of melatonin was most pronounced between 3:00 and 8:00 in the morning, pointing at timing of its pronounced effect during hours corresponding to greatest cardiovascular risks.85
Conclusions and Future Directions
Whether one is concerned with disease prediction and prevention or maintenance of healthy aging, the study of circadian rhythms and the broader time structure underlying physiopathology is helpful in terms of screening, early diagnosis and prognosis, as well as the timely institution of prophylactic and/or palliative/curative treatment. Timing the administration of such treatment as a function of circadian (and other) rhythms also could lead to reduction of falls in older persons.
There is no literature on the study of the circadian rhythm of the ANS, yet given the existing research on the subject and the fact that HRV is a fast, non-invasive, affordable and easily implemented technique, a 24hr HRV assessment presents an attractive and promising approach to the study of frailty and falls in older persons.
Finally, a prominent circadian rhythm characterizes melatonin, which peaks during the night. The circadian amplitude of melatonin decreases as a function of age, raising the questions whether such a decrease in the circadian amplitude of melatonin relates to a higher risk of falls and, if so, whether melatonin supplementation may be an effective countermeasure.
Disclosure
The authors report no conflicts of interest for this work.
References
1. European Stakeholders Alliance for Active Ageing through Falls Prevention. Active ageing through preventing falls: “Falls prevention is everyone’s business”. Published 2015. Available from: http://www.eunaapa.org/wp-content/uploads/2015/09/Joint-Declaration_Sept-2015.pdf.
2. World Health Organisation. WHO global report on falls prevention in older age. Published 2007.
3. Bousquet J, Bewick M, Cano A, et al. Building bridges for innovation in ageing: synergies between action groups of the EIP on AHA. J Nutr Health Aging. 2017;21(1):92–104. doi:10.1007/s12603-016-0803-1
4. Blain H, Masud T, Dargent-Molina P, et al. A comprehensive fracture prevention strategy in older adults: the European Union geriatric medicine society (EUGMS) statement. J Nutr Health Aging. 2016;20(6):647–652. doi:10.1007/s12603-016-0741-y
5. Goswami N. Falls and fall-prevention in older persons: geriatrics meets spaceflight! Front Physiol. 2017;8:1–9. doi:10.3389/fphys.2017.00603
6. Goswami N, Blaber AP, Hinghofer-Szalkay H, Montani JP. Orthostatic intolerance in older persons: etiology and countermeasures. Front Physiol. 2017;8. doi:10.3389/fphys.2017.00803
7. World Health Organisation. Falls. Published 2018. Available from: https://www.who.int/news-room/fact-sheets/detail/falls.
8. Saraiva D. Quedas-indicador de qualidade assistencial. Nursing. 2008;19(235):28–35.
9. Montero-Odasso M, Speechley M. Falls in cognitively impaired older adults: implications for risk assessment and prevention. J Am Geriatr Soc. 2018;66(2):367–375. doi:10.1111/jgs.15219
10. Terroba‐Chambi C, Bruno V, Millar Vernetti P, Bruce D, Brockman S, Merello M. Design and validation of a new instrument to assess fear of falling in Parkinson’s disease. Mov Disorders. 2019;34:1496–1504. doi:10.1002/mds.27820
11. Cornelissen G, Otsuka K. Chronobiology of aging: a mini-review. Gerontology. 2017;63(2):118–128. doi:10.1159/000450945
12. Ju YE, McLeland JS, Toedebusch CD, et al. Sleep quality and preclinical Alzheimer disease. JAMA Neurol. 2013;70(5):587–593. doi:10.1001/jamaneurol.2013.2334
13. Baron KG, Reid KJ. Circadian misalignment and health. Int Rev Psychiatry. 2014;26(2):139–154. doi:10.3109/09540261.2014.911149
14. Tranah GJ, Blackwell T, Stone KL, et al. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann Neurol. 2011;70(5):722–732. doi:10.1002/ana.22468
15. da Silva VP, Ramalho Oliveira BR, Tavares Mello RG, Moraes H, Deslandes AC, Laks J. Heart rate variability indexes in dementia: a systematic review with a quantitative analysis. Curr Alzheimer Res. 2018;15(1):80–88.
16. Toledo MA, Junqueira LF. Cardiac autonomic modulation and cognitive status in Alzheimer’s disease. Clin Auton Res. 2010;20(1):11–17. doi:10.1007/s10286-009-0035-0
17. Holth J, Patel T, Holtzman DM. Sleep in Alzheimer’s disease – beyond amyloid. Neurobiol Sleep Circadian Rhythms. 2017;2:4–14. doi:10.1016/j.nbscr.2016.08.002
18. Vaz Fragoso CA, Gahbauer EA, Van Ness PH, Gill TM. Sleep-wake disturbances and frailty in community-living older persons. J Am Geriatr Soc. 2009;57(11):2094–2100. doi:10.1111/j.1532-5415.2009.02522.x
19. Terroba-Chambi C, Bruno V, Vigo D, Merello M. Heart rate variability and falls in Huntington’s disease. Clin Auton Res. 2020. doi:10.1007/s10286-020-00669-2
20. López-Soto PJ, Manfredini R, Smolensky MH, Rodríguez-Borrego MA. 24-hour pattern of falls in hospitalized and long-term care institutionalized elderly persons: a systematic review of the published literature. Chronobiol Int. 2015;32(4):548–556. doi:10.3109/07420528.2014.987295
21. Lee Gierke C, Cornelissen G. Chronomics analysis toolkit (CATkit). Biol Rhythm Res. 2016;47(2):163–181. doi:10.1080/09291016.2015.1094965
22. Gubin DG, Malishevskaya ТN, Astakhov YS, et al. Progressive retinal ganglion cell loss in primary open-angle glaucoma is associated with temperature circadian rhythm phase delay and compromised sleep. Chronobiol Int. 2019;36(4):564–577. doi:10.1080/07420528.2019.1566741
23. Cornélissen G. Altered circadian energy metabolism and chronobiological risk factors of chronic diseases. In: The Role of Functional Food Security in Global Health. Academic Press; 2019:513–524.
24. Cornélissen G, Halberg F, Bakken EE, et al. Chronobiology of high blood pressure. Scr Med (Brno). 2007;80(4):157–166.
25. Halberg F, Powell D, Otsuka K, et al. Diagnosing vascular variability anomalies (VVAs), not only MESOR-hypertension (MH). Am J Physiol Heart Circ Physiol. 2013;305:H279–H294. doi:10.1152/ajpheart.00212.2013
26. Shinagawa M, Kubo Y, Otsuka K, Ohkawa S, Cornélissen G, Halberg F. Impact of circadian amplitude and chronotherapy: relevance to prevention and treatment of stroke. Biomed Pharmacother. 2001;55(Suppl 1):125s–132s. doi:10.1016/S0753-3322(01)90017-4
27. Cornélissen G, Rigatuso J, Wang ZR, et al. Case report of an acceptable average but overswinging blood pressure in circadian hyper-amplitude-tension, CHAT. Neuro Endocrinol Lett. 2003;24:84–91.
28. Goswami N, Rössler A, Lackner HK, Schneditz D, Grasser E, Hinghofer-Szalkay H. Heart rate and stroke volume response patterns to augmented orthostatic stress. Clin Auton Res. 2009;19:157–165. doi:10.1007/s10286-009-0001-x
29. Lackner HK, Goswami N, Papousek I, Jezova D, Hinghofer-Szalkay H, Montani JP. Time course of cardiovascular responses induced by mental and orthostatic challenges. Int J Psychophysiol. 2010;75:48–53. doi:10.1016/j.ijpsycho.2009.11.003
30. Patel K, Rössler A, Lackner HK, et al. Effect of postural changes on cardiovascular parameters across gender. Medicine (Baltimore). 2016;95(28):e4149. doi:10.1097/MD.0000000000004149
31. Sachse C, Trozic I, Brix B, Roessler A, Goswami N. Sex differences in cardiovascular responses to orthostatic challenge in healthy older persons: a pilot study. Physiol Int. 2019;106(3):236–249. doi:10.1556/2060.106.2019.16
32. Trozic I, Platzer D, Fazekas F, et al. Postural hemodynamic parameters in older persons have a seasonal dependency: a pilot study. Z Gerontol Geriatr. 2020;53:145–55.
33. Rodriguez J, Blaber AP, Kneihsl M, et al. Poststroke alterations in heart rate variability during orthostatic challenge. Medicine. 2017;96(14). doi:10.1097/MD.0000000000005989
34. Xu D, Tremblay MF, Verma AK, Tavakolian K, Goswami N, Blaber AP. Cardio-postural interactions and muscle-pump baroreflex are severely impacted by 60-day bedrest immobilization. Sci Rep. 2020;10(1):12042. doi:10.1038/s41598-020-68962-8
35. Zorko A, Frühwirth M, Goswami N, Moser M, Levnajić Z. Heart rhythm analyzed via shapelets distinguishes sleep from awake. Front Physiol. 2019;10:1554. doi:10.3389/fphys.2019.01554
36. Otsuka K, Kubo Y, Yamanaka G, et al. Tokyo cognitive assessment for mild cognitive impairment, ToCA-MCI: validity and utility in elderly Japanese. Ther Res. 2017;38:579–621.
37. Halberg CG. A maverick ahead of his time. Herald of the International Academy of Sciences. Russian Section. 2018;1:78–84.
38. Herold M, Cornélissen G, Rawson M, et al. About-daily (Circadian) and about-weekly (Circaseptan) patterns of human salivary melatonin. J Anti Aging Med. 2000;3:263–267. doi:10.1089/rej.1.2000.3.263
39. Tarquini B, Cornélissen G, Perfetto F, Tarquini R, Halberg F. Chronome assessment of circulating melatonin in humans. In Vivo (Brooklyn). 1997;11(6):473–484.
40. Wetterberg L, Halberg F, Halberg E, et al. Circadian characteristics of urinary melatonin from clinically healthy young women at different civilization disease risks. Acta Med Scand. 1986;220(1):71–81. doi:10.1111/j.0954-6820.1986.tb02732.x
41. Tarquini B, Cornélissen G, Tarquini R, Perfetto F, Halberg F. General and unspecific damping by malignancy of the circadian amplitude of circulating human melatonin? Neuro Endocrinol Lett. 1999;20(1–2):25–28.
42. Stone KL, Blackwell TL, Ancoli-Israel S, et al. Sleep disturbances and risk of falls in older community-dwelling men: the outcomes of sleep disorders in older men (MrOS sleep) study. J Am Geriatr Soc. 2014;62(2):299–305. doi:10.1111/jgs.12649
43. Cardinali DP. Melatonin as a chronobiotic/cytoprotector: its role in healthy aging. Biol Rhythm Res. 2019;50(1):28–45. doi:10.1080/09291016.2018.1491200
44. Hardeland R, Cardinali DP, Brown GM, Pandi-Perumal SR. Melatonin and brain inflammaging. Prog Neurobiol. 2015;127–128:46–63. doi:10.1016/j.pneurobio.2015.02.001
45. Sharafati-Chaleshtori R, Shirzad H, Rafieian-Kopaei M, Soltani A. Melatonin and human mitochondrial diseases. J Res Med Sci. 2017;22:2. doi:10.4103/1735-1995.199092
46. Tan DX, Manchester LC, Esteban-Zubero E, Zhou Z, Reiter RJ. Melatonin as a potent and inducible endogenous antioxidant: synthesis and metabolism. Molecules. 2015;20(10):18886–18906. doi:10.3390/molecules201018886
47. Pandi-Perumal SR, Srinivasan V, Maestroni GJM, Cardinali D, Poeggeler B, Hardeland R. Melatonin: nature’s most versatile biological signal? FEBS J. 2006;273:2813–2838. doi:10.1111/j.1742-4658.2006.05322.x
48. Lavie P. Melatonin: role in gating nocturnal rise in sleep propensity. J Biol Rhythms. 1997;12(6):657–665. doi:10.1177/074873049701200622
49. Lewy AJ, Emens J, Jackman A, Yuhas K. Circadian uses of melatonin in humans. Chronobiol Int. 2006;23(1–2):403–412. doi:10.1080/07420520500545862
50. Pandi-Perumal SR, Trakht I, Srinivasan V, et al. Physiological effects of melatonin: role of melatonin receptors and signal transduction pathways. Prog Neurobiol. 2008;85(3):335–353. doi:10.1016/j.pneurobio.2008.04.001
51. Reiter RJ, Rosales-Corral S, Tan DX, Jou MJ, Galano A, Xu B. Melatonin as a mitochondria-targeted antioxidant: one of evolution’s best ideas. Cell Mol Life Sci. 2017;74(21):3863–3881. doi:10.1007/s00018-017-2609-7
52. Cardinali DP. Melatonin: clinical perspectives in neurodegeneration. Front Endocrinol (Lausanne). 2019;10:480.
53. Dubocovich ML, Delagrange P, Krause DN, Sugden D, Cardinali DP, Olcese J. International union of basic and clinical pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol Rev. 2010;62(3):343–380.
54. Cecon E, Oishi A, Jockers R. Melatonin receptors: molecular pharmacology and signalling in the context of system bias. Br J Pharmacol. 2018;175(16):3263–3280. doi:10.1111/bph.13950
55. Acuña-Castroviejo D, Escames G, Venegas C, et al. Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol Life Sci. 2014;71(16):2997–3025.
56. Cardinali DP. Are melatonin doses employed clinically adequate for melatonin-induced cytoprotection? 2019.
57. Alpini D, Cesarani A, Fraschini F, Kohen-Raz R, Capobianco S, Cornelio F. Aging and vestibular system: specific tests and role of melatonin in cognitive involvement. Arch Gerontol Geriatr Suppl. 2004;9:13–25. doi:10.1016/j.archger.2004.04.005
58. Fraschini F, Cesarani A, Alpini D, Esposti D, Stankov BM. Melatonin influences human balance. Biol Signals Recept. 1999;8(1–2):111–119. doi:10.1159/000014578
59. Amstrup AK, Sikjaer T, Mosekilde L, Rejnmark L. The effect of melatonin treatment on postural stability, muscle strength, and quality of life and sleep in postmenopausal women: a randomized controlled trial. Nutr J. 2015;14:102. doi:10.1186/s12937-015-0093-1
60. Coto-Montes A, Boga JA, Tan DX, Reiter RJ. Melatonin as a potential agent in the treatment of sarcopenia. Int J Mol Sci. 2016;17(10):1771. doi:10.3390/ijms17101771
61. Stacchiotti A, Favero G, Rodella LF. Impact of melatonin on skeletal muscle and exercise. Cells. 2020;9(2):288. doi:10.3390/cells9020288
62. Lee JY, Kim JH, Lee DC. Urine melatonin levels are inversely associated with sarcopenia in postmenopausal women. Menopause. 2014;21(1):39–44. doi:10.1097/GME.0b013e318291f6c8
63. Obayashi K, Saeki K, Maegawa T, et al. Melatonin secretion and muscle strength in elderly individuals: a cross-sectional study of the HEIJO-KYO cohort. J Gerontol a Biol Sci Med Sci. 2016;71(9):1235–1240. doi:10.1093/gerona/glw030
64. Thrift AP, Xiao L, Patel SR, Tworoger SS, McTiernan A, Duggan C. Effects of physical activity on melatonin levels in previously sedentary men and women. Cancer Epidemiol Biomarkers Prev. 2014;23(8):1696–1699. doi:10.1158/1055-9965.EPI-14-0299
65. Dardevet D, Rémond D, Peyron MA, Papet I, Savary-Auzeloux I, Mosoni L. Muscle wasting and resistance of muscle anabolism: the “anabolic threshold concept” for adapted nutritional strategies during sarcopenia. Sci World J. 2012;2012:269531. doi:10.1100/2012/269531
66. Amstrup AK, Sikjaer T, Pedersen SB, Heickendorff L, Mosekilde L, Rejnmark L. Reduced fat mass and increased lean mass in response to 1 year of melatonin treatment in postmenopausal women: a randomized placebo-controlled trial. Clin Endocrinol (Oxf). 2016;84(3):342–347. doi:10.1111/cen.12942
67. Li T, Jiang S, Lu C, et al. Melatonin: another avenue for treating osteoporosis? J Pineal Res. 2019;66(2):e12548. doi:10.1111/jpi.12548
68. Amstrup AK, Sikjaer T, Heickendorff L, Mosekilde L, Rejnmark L. Melatonin improves bone mineral density at the femoral neck in postmenopausal women with osteopenia: a randomized controlled trial. J Pineal Res. 2015;59(2):221–229. doi:10.1111/jpi.12252
69. Maria S, Swanson MH, Enderby LT, et al. Melatonin-micronutrients osteopenia treatment study (MOTS): a translational study assessing melatonin, strontium (citrate), vitamin D3 and vitamin K2 (MK7) on bone density, bone marker turnover and health related quality of life in postmenopausal osteopenic women following a one-year double-blind RCT and on osteoblast-osteoclast co-cultures. Aging (Albany NY). 2017;9(1):256–285.
70. Auld F, Maschauer EL, Morrison I, Skene DJ, Riha RL. Evidence for the efficacy of melatonin in the treatment of primary adult sleep disorders. Sleep Med Rev. 2017;34:10–22. doi:10.1016/j.smrv.2016.06.005
71. Ferracioli-Oda E, Qawasmi A, Bloch MH. Meta-analysis: melatonin for the treatment of primary sleep disorders. PLoS One. 2013;8(5):e63773. doi:10.1371/journal.pone.0063773
72. Li T, Jiang S, Han M, et al. Exogenous melatonin as a treatment for secondary sleep disorders: a systematic review and meta-analysis. Front Neuroendocrinol. 2019;52:22–28. doi:10.1016/j.yfrne.2018.06.004
73. Wilson S, Anderson K, Baldwin D, et al. British association for psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders: an update. J Psychopharmacol. 2019;33(8):923–947. doi:10.1177/0269881119855343
74. Palagini L, Manni R, Aguglia E, et al. Expert opinions and consensus recommendations for the evaluation and management of insomnia in clinical practice: joint statements of five Italian scientific societies. Front Psychiatry. 2020;11:558. doi:10.3389/fpsyt.2020.00558
75. Geoffroy PA, Micoulaud Franchi J-A, Lopez R, Schroder CM. The use of melatonin in adult psychiatric disorders: expert recommendations by the French institute of medical research on sleep (SFRMS). L’Encéphale. 2019;45(5):413–423. doi:10.1016/j.encep.2019.04.068
76. Reiter RJ, Tan DX, Galano A. Melatonin: exceeding expectations. Physiology (Bethesda). 2014;29(5):325–333.
77. Gerdin MJ, Masana MI, Rivera-Bermúdez MA, et al. Melatonin desensitizes endogenous MT2 melatonin receptors in the rat suprachiasmatic nucleus: relevance for defining the periods of sensitivity of the mammalian circadian clock to melatonin. FASEB J. 2004;18(14):1646–1656. doi:10.1096/fj.03-1339com
78. Lewy AJ, Emens JS, Sack RL, Hasler BP, Bernert RA. Low, but not high, doses of melatonin entrained a free-running blind person with a long circadian period. Chronobiol Int. 2002;19(3):649–658. doi:10.1081/CBI-120004546
79. Rahbari-Oskoui FF, Abramson JL, Bruckman AM, et al. Nighttime administration of high-dose, sustained-release melatonin does not decrease nocturnal blood pressure in African-American patients: results from a preliminary randomized, crossover trial. Complement Ther Med. 2019;43:157–164. doi:10.1016/j.ctim.2019.01.026
80. Moser M, Lehofer M, Sedminek A, et al. Heart rate variability as a prognostic tool in cardiology. A contribution to the problem from a theoretical point of view. Circulation. 1994;90:1078–1082. doi:10.1161/01.CIR.90.2.1078
81. Piepoli M, Sleight P, Leuzzi S, et al. Origin of respiratory sinus arrhythmia in conscious humans – an important role for arterial carotid baroreceptors. Circulation. 1997;95(7):1813–1821. doi:10.1161/01.CIR.95.7.1813
82. Moser M, Lehofer M, Hoehn-Saric R, et al. Increased heart rate in depressed subjects in spite of unchanged autonomic balance? J Affect Disord. 1998;48(2–3):115–124. doi:10.1016/S0165-0327(97)00164-X
83. Zulfiqar U, Jurivich DA, Gao W, Singer DH. Relation of high heart rate variability to healthy longevity. Am J Cardiol. 2010;105(8):1181–1185. doi:10.1016/j.amjcard.2009.12.022
84. Nishiyama K, Yasue H, Moriyama Y, et al. Acute effects of melatonin administration on cardiovascular autonomic regulation in healthy men. Am Heart J. 2001;141(5):E9. doi:10.1067/mhj.2001.114368
85. Gubin DG, Gubin GD, Gapon LI, Weinert D. Daily melatonin administration attenuates age-dependent disturbances of cardiovascular rhythms. Curr Aging Sci. 2016;9(1):5–13. doi:10.2174/1874609809666151130220011
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
