Back to Journals » Therapeutics and Clinical Risk Management » Volume 16
Failure to Reach a Consensus in Polypharmacy Definition: An Obstacle to Measuring Risks and Impacts—Results of a Literature Review
Authors Taghy N, Cambon L, Cohen JM, Dussart C
Received 2 May 2019
Accepted for publication 5 November 2019
Published 11 February 2020 Volume 2020:16 Pages 57—73
DOI https://doi.org/10.2147/TCRM.S214187
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Najwa Taghy, 1 Linda Cambon, 2 Jean-Marie Cohen, 3 Claude Dussart 4
1Laboratory P2S (Health Systemic Process), University of Lyon, University Claude Bernard of Lyon 1, Lyon, EA4129, France; 2Research Chair in Prevention, University of Bordeaux, ISPED, Inserm, Bordeaux Population Health Research Center, Team Methods for Population Health Intervention Research, Bordeaux, France; 3Open Rome, Paris, France, Laboratory P2S (Health Systemic Process), University of Lyon, University Claude Bernard Lyon 1, EA4129, France; 4Lyon Public Hospices, Central Pharmacy, Laboratory P2S (Health Systemic Process), University of Lyon, University Claude Bernard Lyon 1, EA4129, France
Correspondence: Najwa Taghy
University of Lyon, University Claude Bernard Lyon 1, Laboratory P2S (Health Systemic Process), Lyon EA4129, France
Tel +33 633740378
Email [email protected]
Introduction: The risk of polypharmacy is on the rise in most industrialized countries, threatening to burden their health systems. Although many definitions exist and numerous concepts are found in literature as synonyms, the phenomenon of polypharmacy remains poorly defined. The aim of this literature review is to provide an overview of available definitions of polypharmacy, to analyse their convergences and divergences and to discuss the consequences on the assessment of the problem.
Methods: A literature review was conducted to identify all published systematic reviews on definitions of polypharmacy available via Scopus and Pubmed databases. The Assessment of Multiple Systematic Reviews (AMSTAR) tool was used to appraise the methodological quality of the selected reviews. Available definitions and other characteristics were extracted; summarised in a table and analysed.
Results: Six systematic reviews were identified. They were published between 2000 and 2018. Three focussed on definitions of polypharmacy in the elderly; two in the general population and one in children. The strategy adopted in reviews is more rigorous in the most recent ones. However, they remain, at best, partially exhaustive. The definitions found in the literature used two main approaches, either (i) quantitative, applying varying thresholds and types of polypharmacy based on the number of medications being taken by the patient (ii) qualitative, based on the clinical indications and effects of a given drug regimen, with a growing number of characteristics to describe polypharmacy. The term “inappropriate” is increasingly associated with polypharmacy especially in studies that aimed to use this definition to identify possible solutions for healthcare providers in the field related to aging.
Conclusion: This review confirms a high variability and an evolution in the approaches defining “polypharmacy” in the absence of a consensus following standardized criteria. That makes it very difficult to estimate and measure the outcomes associated with this phenomenon.
Keywords: polypharmacy, definition, literature review
Introduction
The increasing life expectancy, the rising prevalence of chronic diseases and multi-morbidity and the growing range of therapies are currently challenging public health in most industrialized countries and leading to increased risk of polypharmacy. This phenomenon, which is on the rise today, affects the elderly and could seriously threaten health systems. Despite this, there is no consensus definition for polypharmacy. The World Health Organization (WHO) defines polypharmacy as “the administration of many drugs at the same time or the administration of an excessive number of drugs”.1 Whether in practice or in research, this term has numerous meanings and many terms and concepts are used in the literature interchangeably as synonyms for “polypharmacy”. This vagueness in polypharmacy definition creates confusion and makes it difficult to assess the extent of the problem, to measure its consequences and to search for solutions. In order to identify and apply relevant knowledge and effective interventions on polypharmacy, it is necessary to assess existing discrepancies in the literature. Given that systematic reviews are recognized as very useful in identifying evidence and basing health care on it,2 we conducted an extensive search of systematic reviews to answer the following question: how is the term “polypharmacy” defined and how it is used in the literature?
The results of a very recent review were published at the end of 2017 and provide a very precise quantitative summary of the existing definitions for polypharmacy.3 By using the results of this work and all other systematic reviews found, our literature review aims to provide an overview of the different approaches adopted, their evolution over time, the convergences and divergences of their results and to discuss their consequences on the evaluation of the problem.
Methods
A literature review was conducted to find all published systematic reviews on polypharmacy definitions. Scopus and Pubmed databases were searched from inception to December 2018 using keywords: “polypharmacy” and “definition”.
Selection of Articles
Inclusion criteria were as followed: (a) systematic reviews focusing on polypharmacy definition; (b) systematic reviews addressing polypharmacy issues and including also polypharmacy definitions allowing for relevant data extraction; (c) publications in English or French.
A first selection was conducted based on titles and abstracts followed by a second selection on full text. The Assessment of Multiple Systematic Reviews (AMSTAR) tool was used to appraise the methodological quality of the selected reviews.4 The 16 elements leading to its assessment criteria provide a reliable basis for evaluating systematic reviews of randomized and non-randomized controlled studies.2 Some criteria were not relevant for our study, particularly those related to meta-analyses and risks of bias (on the table = NA for Not Applicable). Possible answers were “Yes” “partial Yes” “No” or “NP” when the information was not provided.
Data Analysis
The selected articles were analysed through a full-text reading and the following characteristics were extracted: author, author’s country, year of publication, title, aim and purpose, review method (consulted databases, keywords, period), number of studies analysed, concerned population. Finally, all elements related to polypharmacy definition were also extracted in order to analyse convergences and divergences.
Results
Analyses and results are presented below in four main sections:
Selection of Articles
Ninety-nine articles were initially identified; among which 79 were considered irrelevant after titles and abstract reading (not systematic review, lack of a focus on polypharmacy definition; no other relevant definition elements; research protocol). Twenty were selected for full-text reading. Finally, only five reviews were selected after full-text reading and one paper mentioned in the selected reviews was manually retrieved. Figure 1 illustrates the review flow chart (see Figure 1)
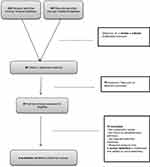 |
Figure 1 Flow chart for review of systematic reviews of polypharmacy definition. |
Description of Included Reviews
Five reviews were in English and one in French. They were published between June 2000 and November 2018. Of the six reviews selected, three focussed on the elderly (one on elderly,5 one on people aged 65 and over6 and one people aged 60 and over7). Two others defined polypharmacy in the general population3,8 and 1 review, the most recent, focused on paediatric polypharmacy.9 The studies included in these included reviews were published between 1985 and July 2017.
Quality Assessment
The methodology used in reviews was analysed using the AMSTAR grid. However, the score provided by the AMSTAR tool was not calculated. Instead, available information was summarised in a table providing some indications on the quality of the methodology used in each review (Table 1). The strategy adopted in these reviews has evolved and is more rigorous in the most recent reviews. However, they remain, at best, partially exhaustive.
 |
Table 1 Quality Assessment Using the AMSTAR Tool |
Definition of Polypharmacy
The analysis of the selected reviews provided data on different ways in which polypharmacy was defined in the literature. As the content varied from one review to another (quantitative and/or descriptive synthesis, table or narrative text), th e extracted definition elements have been summarised in a table (See Table 2).
 |  |  |  |  |  |  |
Table 2 Definitions of Polypharmacy |
Towards Two Approaches to Define Polypharmacy
Among the reviews that we excluded because they were not systematic, some presented nevertheless interesting summaries of existing polypharmacy definitions.10–13 The first of these, which is important to mention because it is cited as a reference in most of the works studied, was published back in 1998 by a general practitioner (R. David Lee) in the Journal of the American Board of Family Practice.10 This article already identified polypharmacy as a serious problem because of the lack of relevant research data on its prevalence, complications and management solutions. According to the author, the definition of polypharmacy varies from one study to another, making it difficult to translate research results into useful information for primary care. Lee defines polypharmacy in its strictest sense as the concomitant use of many drugs and describes it as a practice that implies the prescribing of excessive medication. He also introduces two main approaches that we found in all selected reviews. The first approach focuses on the number of medications the patient is taking. The authors disagree, however, on the number of medications and on whether or not to consider medications over-the-counter drugs or herbal and alternative medications. The second approach which allows for an individualized approach to a patient’s drug regimen focus on the clinical indications and effects of a given drug regimen, regardless of the number of medications used. Polypharmacy would therefore imply that more medications are used or prescribed than those that are clinically indicated.
From the first review we selected in 2000 to the last one published in 2018, we find this notion of “qualitative approach vs. quantitative approach” in the definition of polypharmacy. What changes are the nuances found in the results of each review; nuances that are a consequence of approaches and methods that have evolved and that are different from on review to another according to the purpose of each (for example: specific population, more or less in-depth analysis of the original articles). The progressive gain of ground in the definition by increasingly precise concepts (qualitative approach) as well as the progressive emergence of notion of inappropriate polypharmacy are also reflected in the comparison of the results of the different reviews.
An interesting approach to definition by attempting to measure polypharmacy is found in the French Institute for Research and Information in Health Economics (IRDES) systematic review from 2014.8 Their results include all the aforementioned varieties of definitions (based on thresholds, on the number of medications or on other related characteristics) and focus specifically on time slots to measure polypharmacy. This approach distinguishes several types of polypharmacy: simultaneous, cumulative (or multiple medication) and continuous (see Table 2).
As mentioned above, in 2017, an Australian team published in the BMC Geriatric the results of a systematic literature review including 110 articles defining polypharmacy.3 This review describes and quantifies available definitions by categories (numerical only; numerical with an associated duration of therapy or healthcare setting; descriptive). The vast majority of existing definitions (80.4%) are only quantitative; the most commonly used threshold is five or more daily medications (51 studies). Only one study, published in 2011 in the British Medical Journal defined polypharmacy as the number of drug classes used by patient.14 Nearly eleven percent of studies added the criterion of duration of therapy or healthcare setting to the numerical definition. In addition, 8.9% of revised studies had a descriptive approach to define polypharmacy while some of them used different terms when referring to the same definition. Finally, this review identifies studies that define appropriate or rational polypharmacy as opposed to potentially inappropriate medication. Of the 110 studies included, it highlights a recent article (2015) that highlights the inconsistency in the definitions of polypharmacy and refers to situations where “patients visiting multiple pharmacies which may be associated with safety concerns relating to potential outcomes such as medication duplication, drug–drug interactions and adverse effects”.15
The last selected review is a scoping review published by an American team in November 2018, including 363 articles.9 It describes definitions and terminology of paediatric polypharmacy and provides an overview of the wide range of definitions associated to the term “polypharmacy” in paediatric studies. This review’s results are similar to those found in other reviews concerning the general population: the vast majority of definitions are quantitative; the difference is the number of medications. In over 80% of the reviewed studies, polypharmacy among children was defined on the basis of two or more medications or two or more therapeutic classes. Commonly used terms included “polypharmacy”, “polytherapy”, “combination pharmacotherapy”, “average number”, and “concomitant medications”. The term “polypharmacy” was more common in the psychiatric literature, while the term “polytherapy“ was more frequently found in epilepsy literature.
A Need for a Consensus Definition to Enable Action: A Field of Application Related to Ageing
Between 2000 and 2008, we found three reviews with the same objective: to identify a consensus definition for polypharmacy in the literature.5–7 All of them focused on the elderly. This is quite logical as the elderly are affected by polypharmacy. The definitions found in the literature concern the two approaches described above: (i) quantitative, with varying thresholds and types of polypharmacy determined by the number of medications (e.g., minor, major, excessive); (ii) qualitative, with a growing number of concepts and characteristics to describe polypharmacy, including treatment duration and many other contextual elements and concepts (example: Bushardt in 2008 identified 24 different definitions,5 the most cited being “Medication does not match the diagnosis”).
The term “inappropriate” is increasingly associated with polypharmacy, particularly in reviews that aimed to use this definition to identify possible solutions for healthcare providers. Several evaluation tools and various methods have been developed for this purpose: The most well known are the Beers’ criteria developed in 1997.16 Beers’ criteria help clinicians identify adverse reactions and medications to be avoided or used with caution among the elderly. These criteria were included in several research studies on inappropriate prescriptions in the late 1990s.17–20 Other interventions for assess and control polypharmacy include “brown bag” approach21, using mnemonics such as SAIL or TIDE,22 or the “10-step approach”.23
The three reviews conducted between 2000 and 2008 identify many variations in definitions from one school to another. Some authors note that European studies often defined polypharmacy according to the number of medications taken, while studies conducted in the United States tend to define polypharmacy according to the clinical indication of medication.7 This qualitative approach is essential to studies focused on onco-geriatrics for example. In this multi-morbidity context, beyond the number of medications prescribed and used, polypharmacy is defined more broadly than potentially inappropriate medication (PIM) use “Medications of a specific drug type or class that may not be appropriate for a given patient because of age or a concurrent illness/condition”, Medication underuse “Medications with a clear benefit for a given illness/condition that a patient is not taking”, and Medication duplication “Medications of the same or a similar drug class or therapeutic effect concurrently being used that may not be beneficial”.11
Discussion
A Lack of Consensus on Polypharmacy Definition
This literature review further confirms the lack of standardization in the use of the term “polypharmacy” both, in research and practice. The WHO definition (“the administration of many drugs at the same time or the administration of an excessive number of drugs“) is broad enough so as to allow for the emergence of different definitions1. The first part suggests an administration of “many” drugs without specifying to which number this excessive nature corresponds. Moreover, the notion of “same time” raises the issue of the temporal dimension according to which polypharmacy is considered and measured. The other part of the definition refers to an “excessive number of drugs” introducing, thus, another notion; that of drug misuse.8 A literature review conducted by a Canadian team, published in 1981, seeking to define “polypsychopharmacy” (polypharmacy in the field of psychiatry), had already revealed a large variability in definitions in the medical literature.24 While being used for more than a century and a half,25 this term has not reached a consensus yet in its definition. Instead, the most recent publications reveal a great heterogeneity in approaches and the impossibility of establishing a standard definition for polypharmacy.
Moreover, although not standardised, these definitions have been enriched and refined over time to include new characteristics and concepts linked to the quality of prescription (appropriate, rational vs. inappropriate), duration or context of therapy …, etc. This qualitative approach moves away from stricter definitions that are limited to the number of drugs consumed. Nevertheless, the most recent review shows that more than 80% of the definitions used in the literature are quantitative.3
Definition by Measurement: Different Approaches
Several research studies have focused on the study and development of indicators and potential tools to reliably measure polypharmacy. We mentioned the work of IRDES researchers, which defines indicators and assesses the technical feasibility of their calculation.8 They compare five of the most frequently used measurement tools and test them on the basis of IMS-Health data to assess the ability of these indicators to identify polypharmacy. From this review of the literature, they retained four polypharmacy indicators. Three indicators represent simultaneous polypharmacy and one, cumulative polypharmacy. To these, they added a continuous polypharmacy indicator, also found in the literature and retained within the PAERPA program framework.26 A table summarizes the name, calculation and sources of each indicator (8, table T).
Two additional publications deal with the same issue. The first one27 refers to explicit and implicit instruments, such as the Beers Criteria, STOPP/START criteria, and Medication Appropriateness Index, which are common criteria that can be used to identify high-risk medications that suggest reconsideration.28 It also discusses the relevance of other ways to assess the medication burden in older adults using tools that consider pharmacological principles (i.e. dose–response and cumulative effects) and target-specific medications such as those with clinically significant anticholinergic effects and sedative effects (i.e. Anticholinergic Drug Score, Anticholinergic Risk Scale, Anticholinergic Cognitive Burden Scale, Sedative Load, and Drug Burden Index). The authors emphasize the importance of establishing clinically relevant cutoffs for polypharmacy, meaning that they must be measured and interpreted according to the clinical context, multimorbidity, patient preferences and goals of care.
The most recent paper in this field is a systematic review and expert consensus study that identifies what they consider to be the key elements of a measure of prescribing appropriateness in the context of polypharmacy.29 Panel members particularly valued indicators concerned with adverse drug reactions, contraindications, drug–drug interactions, and the conduct of medication reviews. A set of 12 indicators of clinical importance considered relevant to polypharmacy appropriateness has been identified (29, Table 2). This review concludes by recalling that the use of these indicators in clinical practice and informatics systems is dependent on their operationalization and their utility (e.g. risk stratification, targeting and monitoring polypharmacy interventions) requires subsequent evaluation.
No Standard Definition: An Obstacle to Measuring Outcomes
This lack of consensus makes it very difficult to estimate and measure the outcomes associated with polypharmacy. Several of these reviews aimed at defining polypharmacy in order to study its prevalence in a given population (geriatric, onco-geriatric, paediatric or psychiatric) or to prove and quantify the association between polypharmacy and its associated consequences (falls, hospitalization, non-compliance with treatment, increased expenses …, etc.). The definition of these parameters is a fundamental step and a real challenge for researchers interested in developing adequate solutions based on evidence and proven methods including recommendations to guide practice and interventions targeting patients or healthcare providers. All reviews that attempted to conduct meta-analyses or to compare the results of previous work share a common limitation related to the use of different methodologies from one study/school to another. Several elements may vary: population profile, threshold definition (duration, number), healthcare environment (home, hospital, institution … ), and specificities of contexts and health systems from one country to another.
Results show that the prevalence of polypharmacy in the elderly can vary from 13% to 92% depending on the definition used and the characteristics of the population studied.11 A recent study exploring the correlation between polypharmacy and falls in a cohort of people over 60 years old in the United Kingdom, illustrated this variation: using the threshold of 4 or more drugs (adjusting for socio-demographic, medical and other lifestyle factors), the rate of falls is 18% higher among polymedicated people than among others (IRR 1.18, 95% CI 1.08 to 1.28), while the use of the threshold of 10 or more drugs was 50% higher (IRR 1.50, 95% CI 1.34 to 1.67).30 The results of another systematic review that assessed prevalence and associated clinical signs through variations in polypharmacy definition and mood variation in adults with bipolar disorder showed a prevalence that varied between 85% and 36% depending on whether the study used a “permissive” (2 drugs simultaneously) or more “conservative” (4 and more) definition.31
It is also difficult to estimate the cost of polypharmacy and its burden on health systems. For instance, in Great Britain, we can have the total number of drugs dispensed and its evolution (1.08 billion ≈ 19.9 drugs/person in 2015 Vs. 962 million ≈183 drugs/per cent in 201132); in USA the cost associated with the management of falls among people over 65 years old (£6million/day or £2.3 billion/year for the NHS in 201033 has been estimated in $20 billion in 200634). However, even if we accept the proven link between polypharmacy and falls,35–41 again, the absence of a standard definition makes it difficult to precisely quantify this cost.
Limitation
The fact that we have limited ourselves to systematic reviews without studying all the original articles could be considered as a limitation to this work. But this option was chosen in order to get an overview of the existing literature and to be able to assess whether there was a consensus definition of polypharmacy.
Conclusion
Published literature reports a high variability in the use of the term “polypharmacy” in the absence of a consensus following standardized criteria. In all reviews, we find this notion of “qualitative approach vs quantitative approach” in the definition of polypharmacy. The results (existing definitions of polypharmacy) were different from one review to another depending on the purpose and method of each and have, a fortiori, evolved over time.
Some researchers make trade-offs by choosing a definition for the purposes of their research. Others are trying to define relevant indicators to move towards a precise measurement of polypharmacy whether comparing the ability of indicators to identify polypharmacy and evaluating the technical feasibility of their calculations,8 proposing alternative terminologies15 or, much more recently, establishing a standard definition of the term “polypharmacy” based on an index including the many parameters associated with comorbidity and multi-morbidity.42
Research on polypharmacy is moving towards an increasingly holistic approach. Polypharmacy could be defined, therefore, within the intersection between its many etiological or risk factors (health status, general frailty, comorbid conditions, certain diagnoses, prescribing cascades, self-medication, inappropriate prescription, etc.); significant demographic factors (age, sex, level of education); healthcare environment; and the other factors influencing the number of doctor visits prescribers and hospital admissions. Paradoxically, this approach brings us further away from a standard definition because it defines several situations or types of polypharmacy. By combining all these considerations, some definitions qualify polypharmacy as “appropriate” when “the prescription of numerous medications is justified”, and as “inappropriate” when “wrongly or indiscriminately prescribed”.25,43 In the same vein, we can define “inappropriate polypharmacy” as opposed to situations where the use of several medications would be desirable, justified and even necessary. This practice corresponds to the simultaneous administration of several medications, at least one of which would be inappropriate regarding its indications and/or the iatrogenic risks potentially implied by its administration.
Abbreviations
AMSTAR, Assessment of Multiple Systematic Reviews; IRDES, Institute for Research and Information in Health Economics; IRR, Incident Rate Ratio; MAI, Medication Appropriateness Index; NA, Not applicable; NHS, National Health Service; NP, Not provided; OTC, Over the counter; PIM, Potentially inappropriate medication; WHO, World Health Organization.
Disclosure
The authors report no conflicts of interest in this work.
References
1. WHO Centre for Health Development (Kobe J. A glossary of terms for community health care and services for older persons. 2004
2. Pieper D, Mathes T, Eikermann M Can AMSTAR also be applied to systematic reviews of non-randomized studies? BMC Res Notes [Internet]. September 6, 2014
3. Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE What is polypharmacy? A systematic review of definitions. BMC Geriatr [Internet]. October 10, 2017
4. Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10. doi:10.1186/1471-2288-7-10
5. Bushardt RL, Massey EB, Simpson TW, Ariail JC, Simpson KN. Polypharmacy: misleading, but manageable. Clin Interv Aging. 2008;3(2):383–389. doi:10.2147/CIA.S2468
6. Veehof LJG, Meyboom-De Jong B, Haaijer-Ruskamp FM. Polypharmacy in the elderly – a literature review. Eur J Gen Pract. 2000;6(3):98–106. doi:10.3109/13814780009069956
7. Fulton MM, Allen ER. Polypharmacy in the elderly: a literature review. J Am Acad Nurse Pract. 2005;17(4):123–132. doi:10.1111/jaan.2005.17.issue-4
8. Monégat M, Sermet C. (IRDES), In collaboration with Perronnin M. (IRDES) and Rococo E. (Institut Gustave Roussy – IGR). Polypharmacy: definitions, measurement and stakes involved. Review of the literature and measurement tests. Questions d’économie de la santé: 2014 (état de santé, protection sociale, enquête, analyses économiques, comparaisons internationales) - IRDES [Internet].
9. Bakaki PM, Horace A, Dawson N, et al. Defining pediatric polypharmacy: a scoping review. PLoS One. 2018;13(11):e0208047. doi:10.1371/journal.pone.0208047
10. Lee RD. Polypharmacy: a case report and new protocol for management. J Am Board Fam Pract. 1998;11(2):140–144. doi:10.3122/15572625-11-2-140
11. Maggiore RJ, Gross CP, Hurria A. Polypharmacy in older adults with cancer. Oncologist. 2010;15(5):507–522. doi:10.1634/theoncologist.2009-0290
12. Pire V, Fournier A, Schoevaerdts D, Spinewine A, Swine C. Polypharmacy among the elderly. Louvain Med. 2009;128(7):235–240.
13. Hersh LR, Beldowski K, Hajjar ER. Polypharmacy in the geriatric oncology population. Curr Oncol Rep. 2017;19(11):73. doi:10.1007/s11912-017-0632-3
14. Barnett K, McCowan C, Evans JMM, Gillespie ND, Davey PG, Fahey T. Prevalence and outcomes of use of potentially inappropriate medicines in older people: cohort study stratified by residence in nursing home or in the community. BMJ Qual Saf. 2011;20(3):275–281. doi:10.1136/bmjqs.2009.039818
15. Gillette C, Prunty L, Wolcott J, Broedel-Zaugg K. A new lexicon for polypharmacy: implications for research, practice, and education. Res Soc Adm Pharm. 2015;11(3):468–471. doi:10.1016/j.sapharm.2014.08.010
16. Beers MH. Explicit criteria for determining potentially inappropriate medication use by the elderly. An update. Arch Intern Med. 1997;157(14):1531–1536. doi:10.1001/archinte.1997.00440350031003
17. Aparasu RR, Sitzman SJ. Inappropriate prescribing for elderly outpatients. Am J Health Syst Pharm. 1999;56(5):433–439. doi:10.1093/ajhp/56.5.433
18. Gallagher LP. The potential for adverse drug reactions in elderly patients. Appl Nurs Res. 2001;14(4):220–224. doi:10.1053/apnr.2001.26788
19. Golden AG, Preston RA, Barnett SD, Llorente M, Hamdan K, Silverman MA. Inappropriate medication prescribing in homebound older adults. J Am Geriatr Soc. 1999;47(8):948–953. doi:10.1111/jgs.1999.47.issue-8
20. Hanlon JT, Fillenbaum GG, Kuchibhatla M, et al. Impact of inappropriate drug use on mortality and functional status in representative community dwelling elders. Med Care. 2002;40(2):166–176. doi:10.1097/00005650-200202000-00011
21. Polypharmacy in the elderly: clinical challenges in emergency practice | 2002- 06-03| AHC media: continuing medical education publishing [Internet].
22. Managing polypharmacy: walking the fine line between help and harm | mDedge Psychiatry [Internet].
23. Carlson JE. Perils of polypharmacy: 10 steps to prudent prescribing. Geriatrics. 1996;51(7):26–30, 35.
24. Rapp MS, Kaplan A. Polypsychopharmacy revisited. Can J Psychiatry. 1981;26(8):569–573. doi:10.1177/070674378102600811
25. Polypharmacy and medicines optimisation [Internet]. The King’s Fund. 2013 [cité 8 janv 2020]. Disponible sur: https://www.kingsfund.org.uk/publications/polypharmacy-and-medicines-optimisation.
26. Santé M des S et de la, Santé M des S et de la. Le dispositif Paerpa [Internet]. Ministère des Solidarités et de la Santé. 2020
27. Gnjidic D, Tinetti M, Allore HG. Assessing medication burden and polypharmacy: finding the perfect measure. Expert Rev Clin Pharmacol. 2017;10(4):345–347. doi:10.1080/17512433.2017.1301206
28. Hilmer SN, Gnjidic D. The effects of polypharmacy in older adults. Clin Pharmacol Ther. 2009;85(1):86–88. doi:10.1038/clpt.2008.224
29. Burt J, Elmore N, Campbell SM, Rodgers S, Avery AJ, Payne RA. Developing a measure of polypharmacy appropriateness in primary care: systematic review and expert consensus study. BMC Med. 2018;16(1):91. doi:10.1186/s12916-018-1078-7
30. Dhalwani NN, Fahami R, Sathanapally H, Seidu S, Davies MJ, Khunti K. Association between polypharmacy and falls in older adults: a longitudinal study from England. BMJ Open. 2017;7(10):e016358. doi:10.1136/bmjopen-2017-016358
31. Fornaro M, De Berardis D, Koshy AS, et al. Prevalence and clinical features associated with bipolar disorder polypharmacy: a systematic review. Neuropsychiatr Dis Treat. 2016;12:719–735. doi:10.2147/NDT.S100846
32. Prescription cost analysis - England, 2015 [Internet]. NHS Digital.
33. Falls Awareness. Programmes and innovation. Age UK [Internet].
34. Stevens JA, Corso PS, Finkelstein EA, Miller TR. The costs of fatal and non-fatal falls among older adults. Inj Prev J Int Soc Child Adolesc. 2006;12(5):290–295. doi:10.1136/ip.2005.011015
35. Kojima T, Akishita M, Nakamura T, et al. Association of polypharmacy with fall risk among geriatric outpatients. Geriatr Gerontol Int. 2011;11(4):438–444. doi:10.1111/j.1447-0594.2011.00703.x
36. Wong H, Heuberger R, Logomarsino J, Hewlings S. Associations between alcohol use, polypharmacy and falls in older adults. Nurs Older People. 2016;28(1):30–36. doi:10.7748/nop.28.1.30.s22
37. Baranzini F, Diurni M, Ceccon F, et al. Fall-related injuries in a nursing home setting: is polypharmacy a risk factor? BMC Health Serv Res. 2009;9:228. doi:10.1186/1472-6963-9-228
38. Helgadóttir B, Laflamme L, Monárrez-Espino J, Möller J. Medication and fall injury in the elderly population; do individual demographics, health status and lifestyle matter? BMC Geriatr. 2014;14:92. doi:10.1186/1471-2318-14-92
39. Zia A, Kamaruzzaman SB, Tan MP. Polypharmacy and falls in older people: balancing evidence-based medicine against falls risk. Postgrad Med. 2015;127(3):330–337. doi:10.1080/00325481.2014.996112
40. Ziere G, Dieleman JP, Hofman A, Pols HAP, van der Cammen TJM, Stricker BHC. Polypharmacy and falls in the middle age and elderly population. Br J Clin Pharmacol. 2006;61(2):218–223. doi:10.1111/bcp.2006.61.issue-2
41. Kojima T, Akishita M, Nakamura T, et al. Polypharmacy as a risk for fall occurrence in geriatric outpatients. Geriatr Gerontol Int. 2012;12(3):425–430. doi:10.1111/j.1447-0594.2011.00783.x
42. Kadam UT, Roberts I, White S, et al. Conceptualizing multiple drug use in patients with comorbidity and multimorbidity: proposal for standard definitions beyond the term polypharmacy. J Clin Epidemiol. 2019;106:98–107. doi:10.1016/j.jclinepi.2018.10.014
43. Aronson JK. In defence of polypharmacy. Br J Clin Pharmacol. 2004;57(2):119–120. doi:10.1111/bcp.2004.57.issue-2
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
