Back to Journals » Cancer Management and Research » Volume 12
Factors Prognostic for Peritoneal Metastases from Colorectal Cancer Treated with Surgery
Authors Chen C , Wang J, Zhao Y , Ge X, Wang Z, Yu S, Song Y, Ding K, Zhang S, Zheng S , Sun L
Received 5 July 2020
Accepted for publication 14 September 2020
Published 27 October 2020 Volume 2020:12 Pages 10587—10602
DOI https://doi.org/10.2147/CMAR.S270830
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Chao Chen,1,* Jian Wang,1,* Yamei Zhao,1 Xiaoxu Ge,1,2 Zhanhuai Wang,1 Shaojun Yu,1 Yongmao Song,1 Kefeng Ding,1 Suzhan Zhang,1,2 Shu Zheng,1,2 Lifeng Sun1
1Department of Colorectal Surgery and Oncology, Key Laboratory of Cancer Prevention and Intervention, Ministry of Education, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang Province, People’s Republic of China; 2Department of Cancer Institute, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lifeng Sun
The Second Affiliated Hospital, Zhejiang University School of Medicine, 88 Jiefang Road, Hangzhou, Zhejiang 310009, People’s Republic of China
Tel +86-571-87783586
Email [email protected]
Purpose: To analysis factors prognostic for peritoneal metastases (PM) from colorectal cancer (CRC) treated with surgery using data from two sources and investigate the origin and effective treatment of ovarian metastases (OM).
Patients and Methods: Data from CRC patients with PM who had undergone surgery were collected from the Surveillance, Epidemiology, and End Results (SEER) database (n = 639) and a single Chinese institution (n = 60). Cumulative survival was evaluated by Kaplan–Meier analysis. Factors associated with overall survival (OS) and progression-free survival (PFS) prognosis were assessed using Cox’s proportional hazard regression models.
Results: Median OS values for patients who underwent surgery were 19 and 32 months in the SEER database and Chinese center, respectively. Age was an independent predictor of OS in both datasets. Signet-ring cell cancer and perineural invasion were independent predictors of inferior OS only in the SEER dataset, while completeness of cytoreduction (CC) and peritoneal carcinomatosis index were independent predictors for OS and PFS only in the Chinese center. Median OS was 24 months in CRC patients with PM alone and 36 months in those with both PM and OM (p = 0.181). Further, median PSF in patients with PM alone was 10 months, while that in individuals with both PM and OM was 20 months (p = 0.181).
Conclusion: Surgical treatment of the primary and metastatic sites is effective and safe for CRC patients with PM. CC-0 is recommended for improved prognosis. Moreover, OM should be recognized as a feature of PM, and cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy is beneficial for CRC patients with OM.
Keywords: ovarian metastases, prognosis factors, cytoreductive surgery, hyperthermic intraperitoneal chemotherapy
Introduction
Subdermal Colorectal cancer (CRC) is the third most common cancer both in males and females and the second most frequent cause of cancer death worldwide.1 Approximately 5% of patients with CRC develop synchronous peritoneal metastases (PM), while a further 5% develop metachronous PM during disease progression. Patients with PM are considered to have end-stage disease and have extremely low survival rates. Due to advances in surgical techniques, the safety and benefits of surgery for PM from CRC have improved;2–8 however, surgery is generally only performed when there is obstruction, perforation, or bleeding, and is not the standard treatment for PM from CRC.9 Few studies have analyzed prognostic factors in CRC patients with PM who have undergone surgery in single center. Here, we investigated predictors of prognosis in CRC patients with PM treated by surgery, using not only data from a single cancer center, but also from the Surveillance, Epidemiology, and End Results (SEER) database, which contains information from approximately 28% of the US population.10
OM is reported to always be coincident with PM in patients undergoing cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy (CRS/HIPEC),11 and patients with OM have dismal prognosis, due to rapid progression and relative chemoresistance.12 Transcoelomic spread was postulated by some scholars, because of the similar behavior of OM and PM and the frequent co-existence of OM and PM.13 In some institutions, CRS/HIPEC has been suggested for patients with OM;14 however, there is no clear understanding of the spread route or standard treatment for OM.
In this study, we reviewed data from the SEER database and a single Chinese center to evaluate prognostic factors for CRC patients with PM who underwent surgery. We also investigated the efficacy of surgical treatment for OM. Our results will provide information and treatment strategies for clinicians, as well as promoting related research.
Patients and Methods
Ethics and Patients
CRC patients (n = 60) presenting with PM from January 2014 to December 2017 were included in this study, which was conducted with the approval of the Institutional Review Board of the Second Affiliated Hospital of Zhejiang University School of Medicine, and according to the Declaration of Helsinki. All patients in our center provided signed informed consent.
Inclusion and Exclusion Criteria
There is no single code for PM in the SEER database. According to Abdel-Rahman et al, we identified PM patients as those with code 36 for the variable “CS Mets at Dx,” and without metastases in liver, lungs, bone, or brain.15 All patients in the SEER database with the following criteria were enrolled: 1. Colorectal cancer diagnosed by positive histology between 2010 and 2015; 2. Exact stage, according to the TNM 7th staging system, was available. 3. CRC patents with code 36 for the variable “CS Mets at Dx” and without metastases in the liver, lungs, bone, or brain.
In our center, patients were enrolled according to the following criteria: 1. Pathological diagnosis of CRC with synchronous or metachronous PM; 2. Eastern Cooperative Group (ECOG) performance status 0 or 1, and no extra-abdominal disease on radiological investigation; 3. Extent of PM evaluated either via contrast-enhanced computed tomography (CT) or magnetic resonance imaging of the abdomen and pelvis, and the feasibility of CRS/HIPEC was discussed by the multidisciplinary cancer treatment team (MDT). Exclusion criteria were as follows: 1. Follow-up time <12 months from the date of performing CRS/HIPEC; 2. Extra-abdominal metastasis.
CRS/HIPEC
According to the size of residual tumors after CRS, the completeness of cytoreduction (CC) was classified as one of four grades (CC-0, −1, −2, and −3). CRS was performed to remove all macroscopic PM, or leave lesions <2.5 mm (CC-0/1), which was considered optimal cytoreduction. The extent of disease was assessed using the peritoneal cancer index (PCI) score, as described by Sugarbaker.16 HIPEC was performed immediately after the abdomen was closed in the operating room. Mitomycin C (30 mg) or oxaliplatin (400 mg) was administered for 60 min at 43°C in all cases. After postoperative recovery, patients received systemic chemotherapy for a maximum of 24 weeks.
Clinical Follow-Up
All patients were followed up in the outpatient unit at approximately two weeks after CRS/HIPEC, and at least every 3 months for 2 years, then every six months. Tumor markers (carcinoembryonic antigen, carbohydrate antigen 199, carbohydrate antigen 125), together with CT scans of the abdomen, pelvis, and thorax, were assessed at each follow-up visit. Recurrent lesions were recorded.
Statistical Analysis
Overall survival (OS) was defined as the date of performing HIPEC to the last known date of follow-up or date of death. Progression-free survival (PFS) was the period from HIPEC to observation of disease progression or the occurrence of death for any reason.
Cumulative survival was evaluated by Kaplan–Meier analysis using both data from our center and from the SEER database. Differences in survival curves between groups of patients were assessed using the Log rank test. Multivariate analyses were implemented using Cox’s proportional hazard regression models to identify factors associated with OS in the SEER dataset, as well as with OS and PFS in our center. A two-sided P value <0.05 was considered significant. All analyses were performed in SPSS for windows (version 25.0).
Results
Statistical Analysis of the SEER Dataset
Clinicopathologic Features
Data from CRC patients with PM between 2010 and 2015 (n = 3155) were extracted from the SEER database. Baseline characteristics of CRC patients with PM in the SEER database are summarized in Supplemental Table 1. Of these 3155 patients, 693 underwent surgery for primary cancer and metastatic lesions, and their baseline characteristics are presented in Table 1. Mean patient age was 63.5 years and 63.6% were female. Of primary tumor sites the largest proportion were cases of right colon cancer (n = 380, 54.8%), followed by left colon cancer (n = 263, 38%), and rectal cancer (n = 27, 3.9%). Among pathological subtypes, adenocarcinoma accounted for 62.5% of patients and signet-ring cell cancer was the least common (9.5%). The most common T stage among CRC patients with PM was T4 and 50.4% of primary tumors were >5 cm. Perineural invasion (PNI) occurred in 242 patients (34.9%) and 630 patients (93.7%) had >4 invaded lymph nodes.
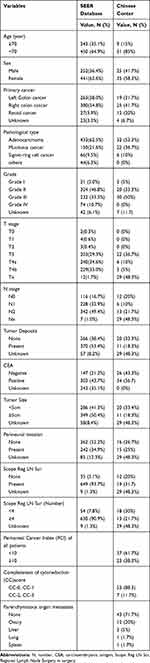 |
Table 1 Demographic Characteristics of the Patients in SEER Database and Chinese Center |
Survival Outcomes
The 5-year OS rates of patients with or without surgery for both primary and metastatic cancer were 14% and 1.4% (Figure 1). When predictors of prognosis for CRC patients with PM treated with surgery were analyzed using the Kaplan–Meier method, factors significantly associated with increased risk of all-cause mortality were age (P < 0.001), site of primary cancer (p < 0.001), pathological subtype (p < 0.001), grade (p < 0.001), N stage (p < 0.001), tumor size (p = 0.045), and PNI (p = 0.037).
 |
Figure 1 Overall survival Kaplan–Meier curves for Surgery of primary cancer or metastatic cancer in SEER database. |
Analysis using Cox’s proportional hazard regression models revealed factors influencing prognosis in CRC patients with PM (Table 2). The following factors were associated with significantly increased risk of mortality: age (<70 years; hazard ratio (HR), 0.414, 95% confidence interval (CI), 0.342 to 0.500; p < 0.001), pathological subtype (signet-ring cell cancer (SRC); HR, 1.827, 95% CI, 1.356 to 2.462; p < 0.001); and PNI (present; HR, 1.304, 95% CI, 1.073 to 1.586; p = 0.008) (Figure 2).
 |
Table 2 Univariate Analysis and Multivariate Analysis of Affecting OS with a Cox Regression Model in SEER Center |
 |
Figure 2 Overall survival Kaplan–Meier curves for significant prognostic variables in surgery for CRC patients with PM in SEER database. (A) Age. (B) pathological subtypes. (C) Perineural Invasion. |
Statistical Analysis of Data from Our Cancer Center
Clinicopathologic Features
A total of 60 consecutive patients with PM underwent CRS/HIPEC from January 2014 to December 2017 in our cancer center. Mean patient age was 55.1 years and 41.7% were male. Simultaneous PM was present in 51.7% of patients and the majority of primary cancers with PM were in the right colon cancer (n = 25, 41.7%). Synchronous extra-peritoneal metastasis occurred in 17 patients and in 12 cases with OM. Among pathological subtypes, mucinous cancer accounted for 36.7% and initial surgery for primary tumor was not performed in our center for 29 patients; therefore, precise details of surgery were unavailable for these cases. Patients who underwent surgery in our center most commonly had T3 and N3 stage tumors (36.7% and 21.7%, respectively). Vascular invasion was detected in 20 patients (33.3%) and with perineural invasion in 15 (25%). Lymph node invasion was present in a large proportion of cases (31.7%), and the number of lymph nodes invaded was >4 in 13 patients (21.7%). Demographic and histologic data are summarized in Table 1.
Survival Outcomes
Median follow-up time was 39 (range, 1 to 58) months from the date of CRS/HIPEC. Median OS for all patients was 32 months, while median PFS was 12 months, with overall 1- and 3-year survival rates of 83.1% and 44.5%, respectively.
Analysis of predictors using the Kaplan–Meier method identified the following factors that significantly influenced OS: age at diagnosis (P = 0.006), CEA level (P = 0.021), PCI (P = 0.001), CC score (P < 0.001), and parenchymatous organ metastasis (P = 0.005). There were no significant associations with T stage, N stage, grade, pathological subtype, primary cancer site, vascular invasion, perineural invasion, or lymph node invasion. On multivariate analysis using a Cox regression model, only incomplete cytoreduction was an independent predictor for poor OS (CC >1; HR, 4.522, 95% CI, 1.082 to 11.345; P = 0.001) (Table 3, Figure 3).
 |
Table 3 Univariate Analysis and Multivariate Analysis of Affecting OS with a Cox Regression Model in Chinese Center |
 |
Figure 3 Overall survival Kaplan–Meier curves for different prognostic variables in surgery for CRC patients with PM in Chinese center. Completeness of cytoreduction (CC). |
Age at diagnosis (P = 0.02), CC score (P = 0.064), and PCI (P = 0.011) were significantly associated with PFS (Log rank test). Age at diagnosis (≥70 years; HR, 2.757, 95% CI, 1.272 to 5.975; P = 0.010) and higher PCI (PCI ≥ 10; HR, 2.329, 95% CI, 1.268 to 4.278; P = 0.006) were independently associated with unfavorable PFS on multivariate analysis (Table 4, Figure 4).
 |
Table 4 Univariate Analysis and Multivariate Analysis of Affecting PFS with a Cox Regression Model in Chinese Center |
 |
Figure 4 Progression-free survival Kaplan–Meier curves for different prognostic variables in univariate analysis. (A) Age at diagnosis. (B) Peritoneal Carcinomatosis Index (PCI). |
Analysis of Patients with PM Alone Compared with Both PM and OM
Thirty-five female CRC patients with PM underwent CRS-HIPEC in our center and OM was detected in 12 patients (34.3%). Median OS and PSF were 24 and 10 months, respectively, in patients with PM alone, with corresponding values of 36 and 20 months in patients with both PM and OM. Estimated 1- and 3-year OS rates were 82.6% and 40.2%, respectively, in patients with PM alone and 83.3% and 53.6% in those with PM and OM (p = 0.181) (Figure 5A). The PFS rates at 1- and 3-years were 34.8% and 0% in patients with PM alone, compared with 58.3% and 25.0% in patients with both PM and OM, respectively (p = 0.181) (Figure 5B).
Discussion
This is the first study to analyze factors predicting prognosis for CRC patients with PM who underwent surgery using data from the SEER database and to compare the findings with results from a Chinese center. Developments in surgery have improved outcomes for patients with early-stage CRC and metastasis to the liver or lung; however, the outcomes of CRC patients with PM are inferior to those for patients with only liver or lung metastasis.17 Although several studies have suggested a potential role for CRS/HIPEC in the management of selected CRC patients with PM,18–21 is not the standard treatment for these individuals.9 Median OS was 19 months in the SEER dataset, while in the Chinese cohort median OS and PFS were 32 and 12 months, respectively, which were longer than those of patients who did not undergo surgical treatment. In a phase-III randomized trial, the OS and PFS of CRC patients with PM who received palliative care were 12.6 and 7.7 months, respectively.22 Our results are consistent with these published findings and indicate that surgery can improve the outcomes of selected CRC patients with PM.
SRC is a specific mucinous carcinoma subtype with the worst prognosis in patients with PM after CRS/HIPEC.23–25 Our data also confirmed this phenomenon, since the 2-year OS rate for patients with SRC carcinoma in our cohort was 1.8%. This may be attributable to the aggressive biological behavior of SRC, which is characterized by deeper infiltration, lymph node invasion, and distant metastasis. Further, patients with SRC features are more likely to have high PCI scores.26–28 Only 1% of CRC tumors are SRC pathological subtype,29 while 9.5% of patients with PM from CRC have SRC, and reported 5-year survival rates from CRC with SRC range from 9.1% to 25.3%.30–32 While treatment with CRS/HIPEC clearly improves long-term survival, patient prognosis remains worse than for those with other histological subtype tumors.33,34 Hence, PM with SRC remains a challenging condition clinically.
Recently, the newly-discovered biological feature, PNI, has attracted increasing attention in CRC research. Of CRC patients with PM in this study, 34.9% were diagnosed PNI, which is more than previous reports of 9% and 30%.35 Infiltrating nerve fibers can stimulate tumor growth and spread, and tumor cells can drive nerve growth via signaling cascades that promote tumor progression.36 PNI has been reported to contribute to inferior OS in patients with stage II or III CRC, and chemotherapy is useful for these individuals;37 however, Cao et al found that PNI is not an independent prognostic factor for CRC patients.37 In our study, the OS of CRC patients with PM presenting with PNI was shorter than that of patients without PNI. PNI influences the outcome of surgery, but not CRS/HIPEC, for CRC patients with PM; hence, such patients maybe benefit from HIPEC. Sato et al found that harvesting more than 5 lymph nodes during surgery was associated with a better prognosis,38 while our data did not support the number of lymph nodes collected during surgery as an independent poor prognostic factor in CRC patients with PM.
In our center, the principal factors influencing the outcomes of patients with PM included age at diagnosis, CEA level, PCI score, CC score, and parenchymatous organ metastasis. We also analyzed other clinicopathologic characteristics in the SEER dataset; however, they were not identified as prognostic factors for CRC patients with PM who underwent CRS/HIPEC; this may be due to the relatively small number of patients from our center included in the study.
Almost all studies have found that complete cytoreductive surgery is a crucial factor affecting long-term prognosis and recurrence of patients with CRC;5,26,39–42 however, the degree of cytoreduction is not only related to tumor burden but also to the technique of the surgeon. In this study, incomplete cytoreduction was an independent risk factor for OS on multivariate analysis. Further, studies in different countries have found that CRS/HIPEC is a safe, feasible, and effective treatment for diverse advanced tumors, and that completeness of cytoreduction and PCI score are critical factors for survival.5,7,43,44 As our study demonstrates, higher PCI independently predicts unfavorable prognosis, especially in terms of PFS. Increased PCI score was associated with a significant decrease in the proportion of CC-0 patients, while the proportion of CC-3 or CC-4 patients was significantly increased; however, in some cases, although some patients had relatively small tumors and low PCI scores, tumors invaded the mesenteric root or penetrated the vital organs, and were therefore not conducive to the implementation of cytoreductive surgery. In contrast, optimal cytoreduction can be achieved of some tumors with superficial spreading on the serosal surface, even where the PCI score was high. Hence, biological behavior related to tumor malignancy should also be considered, including the growth pattern, location, and extent of tumor invasion.
OM affects young women, develops rapidly, and is relatively chemoresistant.12 Understanding the spread pathway of OM from CRC could facilitate the development of suitable treatments for patients with OM. There is no specific code for OM in the SEER database; therefore, we reviewed the patients in our center, and found that 34.3% of female CRC patients with PM who underwent CRS-HIPEC presented with OM, which is lower than a previous report (52%),11 but still higher than the OM incidence of 2%–8% in female patients with gastrointestinal cancer.45 Some hypotheses on how cancers metastasize to the ovary have been proposed by researchers, including hematogenous spread, lymphogenous spread, and transcoelomic spread; however, the exact pathway of spread remains unclear, and there are no effective treatments for patients with OM. As the omentum and ovarian epithelium belong to the same lineage,46 and biological behavior and response to treatment in patients with OM or PM are similar, we suggest that OM should be recognized as a feature of PM spread. We also found there was no significant difference between patients with isolated PM and those with both PM and OM, in terms of OS and PFS, and that OM is not an independent factor influencing survival after CRS-HIPEC. Our results are similar to those reported by Kuijpers et al,14 who suggested that there were no differences in the outcomes of patients with PM who underwent CRS-HIPEC, according to the presence or absence of OM. There are different ideas about the management of CRC patients with OM, with some surgeons recommending CRC/HIPEC, because it can improve patient survival duration;47 however, others hold the view that this approach is ineffective.48 In this study, median OS was 36 months in CRC patients with OM group who underwent surgery, compared with the 7–17 months for patients receiving palliative treatment, reported by Gilliland et al.49 Hence, CRS-HIPEC should be advised for these patients.
We analyzed factors prognostic for CRC patients with PM in data from our center and the SEER dataset, which represents a large population; however, our study has some limitations. First, this was a retrospective study, leading to bias. Further, all patients in our center underwent surgery indicating that they were healthier than others who could not receive surgery. Second, there is no information about OM and CRS-HIPEC in the SEER database, and only 12 patients with OM were included in our center. Third, the sample size in our center was small, comprising 60 CRC patients with PM. These limitations could be ameliorated by including a code for OM and more information about CRS-HIPEC in the SEER database, as well as by the recruitment of more patients for inclusion in a future prospective study. Studies including more samples are needed to assess the effectiveness of treatments and explore pathways to clinical transfer.
Conclusion
We conclude that surgery is an effective and safe treatment modality for CRC patients with PM. Age >70 years, signet-ring cell cancer, and PIN were associated with poorer outcomes of patients with CRC and PM who underwent surgery. PCI and CC score are clear prognostic factors for CRS/HIPEC. In contrast, our data do not support the influence of OM on the outcome of CRS/HIPEC for CRC patients with PM. In conclusion, we suggest that surgery of the primary or metastatic site to achieve CC-0 should be recommended for CRC patients with PM. Our study also indicates that OM should be considered as a feature of PM; therefore, we suggest patients with OM should also be advised to accept CRS/HIPEC, which may improve their prognosis.
Abbreviations
SEER, Surveillance, Epidemiology, and End Results; CRS/HIPEC, cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy; CRC, colorectal cancer; PCI, Peritoneal Cancer Index; CEA, carcinoembryonic antigen; Reg LN Sur, Regional Lymph Node Surgery in surgery; PM, Peritoneal Metastasis; OM, Ovarian Metastasis; PNI, perineural invasion.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Ethical Approval
This study was reviewed and approved by Institutional Review Board of the Second Affiliated Hospital of Zhejiang University School of Medicine.
Informed Consent
Informed consents were obtained from all participants in the study.
Acknowledgments
Our special acknowledgments to Mr. Xiang Zhou and Mr. Liu Bo for helping us with editing.
Funding
The study was funded by National Natural Science Foundation of China (No. 81472819, No. 81672342), the Zhejiang Provincial Key R&D Program of China (No. 2019C03018), the Zhejiang Provincial Natural Science Foundation of China (No. LY20H160038), the Fundamental Research Funds for the Central Universities (No. 2019QNA7028, No. 2019FZJD009).
Disclosure
The authors declare that they have no conflict of interests.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30.
2. Alzahrani NA, Valle SJ, Fisher OM, et al. Iterative cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy for colorectal peritoneal metastases: a multi-institutional experience. J Surg Oncol. 2018. doi:10.1002/jso.25277
3. Caro CR, Manzanedo I, Pereira F, Carrion-Alvarez L, Serrano A, Perez-Viejo E. Cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with gastric cancer and peritoneal carcinomatosis. Ejso. 2018;44(11):1805–1810. doi:10.1016/j.ejso.2018.06.036
4. Chua TC, Moran BJ, Sugarbaker PH, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012;30(20):2449–2456. doi:10.1200/JCO.2011.39.7166
5. Di Giorgio A, De Iaco P, De Simone M, et al. Cytoreduction (peritonectomy procedures) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in advanced ovarian cancer: retrospective italian multicenter observational study of 511 cases. Ann Surg Oncol. 2017;24(4):914–922. doi:10.1245/s10434-016-5686-1
6. Fujimoto S, Shrestha RD, Kokubun M, et al. Intraperitoneal hyperthermic perfusion combined with surgery effective for gastric cancer patients with peritoneal seeding. Ann Surg. 1988;208(1):36–41. doi:10.1097/00000658-198807000-00005
7. Park EJ, Baik SH, Hur H, et al. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for appendiceal and colorectal cancer with peritoneal carcinomatosis: clinical outcomes at 2 tertiary referral centers in Korea. Medicine. 2017;96(21):e6632. doi:10.1097/MD.0000000000006632
8. van Driel WJ, Koole SN, Sikorska K, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med. 2018;378(3):230–240. doi:10.1056/NEJMoa1708618
9. van de Velde CJ, Aristei C, Boelens PG, et al. EURECCA colorectal: multidisciplinary mission statement on better care for patients with colon and rectal cancer in Europe. Eur J Cancer. 2013;49(13):2784–2790. doi:10.1016/j.ejca.2013.04.032
10. Surveillance E, and End Results (SEER) Program. SEER*Stat Database: incidence-SEER 9 Regs research data with delay-adjustment, malignant only, Nov. 2018 Sub (1975–2016) <Katrina/Rita population adjustment>-linked to county attributes-total US, 1969–2017 counties. National Cancer Institute, Division of Cancer Control and Population Sciences, Surveillance Research Program, Surveillance Systems Branch; 2019.
11. Evers DJ, Verwaal VJ. Indication for oophorectomy during cytoreduction for intraperitoneal metastatic spread of colorectal or appendiceal origin. Br J Surg. 2011;98(2):287–292. doi:10.1002/bjs.7303
12. Sekine K, Hamaguchi T, Shoji H, et al. Retrospective analyses of systemic chemotherapy and cytoreductive surgery for patients with ovarian metastases from colorectal cancer: a single-center experience. Oncol Rep. 2018;95(4):220–228.
13. Riihimäki M, Hemminki A, Sundquist J, Hemminki K. Patterns of metastasis in colon and rectal cancer. Sci Rep. 2016;6(1):1–9. doi:10.1038/srep29765
14. Kuijpers A, Mehta A, Aalbers A, van Driel W, Boot H, Verwaal V. Treatment of ovarian metastases of colorectal and appendiceal carcinoma in the era of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur J Surg Oncol. 2014;40(8):937–942. doi:10.1016/j.ejso.2014.02.238
15. Abdel-Rahman O. A real-world, population-based study of the outcomes of patients with metastatic colorectal cancer to the peritoneum treated with or without cytoreductive surgery. Int J Colorectal Dis. 2020;35(4):719–725. doi:10.1007/s00384-020-03525-x
16. Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359–374.
17. Abdel-Rahman O. Effect of body mass index on 5-FU-based chemotherapy toxicity and efficacy among patients with metastatic colorectal cancer; a pooled analysis of 5 randomized trials. Clin Colorectal Cancer. 2019;18(4):e385–e393. doi:10.1016/j.clcc.2018.12.006
18. Leimkühler M, Hemmer PH, Reyners AKet al. Neoadjuvant chemotherapy followed by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal cancer: a feasibility and safety study. World J Surg Oncol. 2019;17(1):14.
19. Fuzun M. Treatment of intraoperatively detected peritoneal carcinomatosis of colorectal origin with cytoreductive surgery and intraperitoneal chemotherapy. World J Surg Oncol. 2018;16(1):70. doi:10.1186/s12957-018-1369-7
20. Ahmed S, Stewart JH, Shen P, Votanopoulos KI, Levine EA. Outcomes with cytoreductive surgery and HIPEC for peritoneal metastasis. J Surg Oncol. 2014;110(5):575–584. doi:10.1002/jso.23749
21. Riss S, Mohamed F, Dayal S, et al. Peritoneal metastases from colorectal cancer: patient selection for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur J Surg Oncol. 2013;39(9):931–937. doi:10.1016/j.ejso.2013.06.001
22. Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15(9):2426–2432. doi:10.1245/s10434-008-9966-2
23. Cashin PH, Graf W, Nygren P, Mahteme H. Patient selection for cytoreductive surgery in colorectal peritoneal carcinomatosis using serum tumor markers: an observational cohort study. Ann Surg. 2012;256(6):1078–1083. doi:10.1097/SLA.0b013e318254f281
24. Kwakman R, Schrama AM, van Olmen JP, et al. Clinicopathological parameters in patient selection for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal cancer metastases: a meta-analysis. Ann Surg. 2016;263(6):1102–1111. doi:10.1097/SLA.0000000000001593
25. van Oudheusden TR, Braam HJ, Nienhuijs SW, et al. Poor outcome after cytoreductive surgery and HIPEC for colorectal peritoneal carcinomatosis with signet ring cell histology. J Surg Oncol. 2015;111(2):237–242. doi:10.1002/jso.23784
26. Solomon D, DeNicola N, Feingold D, et al. Signet ring cell features with peritoneal carcinomatosis in patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy are associated with poor overall survival. J Surg Oncol. 2019;119(6):758–765. doi:10.1002/jso.25379
27. Piessen G, Messager M, Leteurtre E, Jean-Pierre T, Mariette C. Signet ring cell histology is an independent predictor of poor prognosis in gastric adenocarcinoma regardless of tumoral clinical presentation. Ann Surg. 2009;250(6):878–887. doi:10.1097/SLA.0b013e3181b21c7b
28. Taghavi S, Jayarajan SN, Davey A, Willis AI. Prognostic significance of signet ring gastric cancer. J Clin Oncol. 2012;30(28):3493–3498. doi:10.1200/JCO.2012.42.6635
29. Hyngstrom JR, Hu CY, Xing Y, et al. Clinicopathology and outcomes for mucinous and signet ring colorectal adenocarcinoma: analysis from the National Cancer Data Base. Ann Surg Oncol. 2012;19(9):2814–2821. doi:10.1245/s10434-012-2321-7
30. Chen JS, Hsieh PS, Hung SY, et al. Clinical significance of signet ring cell rectal carcinoma. Int J Colorectal Dis. 2004;19(2):102–107. doi:10.1007/s00384-003-0515-y
31. Lee WS, Chun HK, Lee WY, et al. Treatment outcomes in patients with signet ring cell carcinoma of the colorectum. Am J Surg. 2007;194(3):294–298. doi:10.1016/j.amjsurg.2006.12.041
32. Messerini L, Palomba A, Zampi G. Primary signet-ring cell carcinoma of the colon and rectum. Dis Colon Rectum. 1995;38(11):1189–1192. doi:10.1007/BF02048335
33. Grotz TE, Overman MJ, Eng C, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for moderately and poorly differentiated appendiceal adenocarcinoma: survival outcomes and patient selection. Ann Surg Oncol. 2017;24(9):2646–2654. doi:10.1245/s10434-017-5938-8
34. Massalou D, Benizri E, Chevallier A, et al. Peritoneal carcinomatosis of colorectal cancer: novel clinical and molecular outcomes. Am J Surg. 2017;213(2):377–387. doi:10.1016/j.amjsurg.2016.03.008
35. Knijn N, Mogk SC, Teerenstra S, Simmer F, Nagtegaal ID. Perineural invasion is a strong prognostic factor in colorectal cancer: a systematic review. Am J Surg Pathol. 2016;40(1):103–112. doi:10.1097/PAS.0000000000000518
36. Jobling P, Pundavela J, Oliveira SM, Roselli S, Walker MM, Hondermarck H. Nerve-cancer cell cross-talk: a novel promoter of tumor progression. Cancer Res. 2015;75(9):1777–1781. doi:10.1158/0008-5472.CAN-14-3180
37. Cao Y, Deng S, Yan L, et al. Perineural invasion is associated with poor prognosis of colorectal cancer: a retrospective cohort study. Int J Colorectal Dis. 2020;35(6):1067–1075. doi:10.1007/s00384-020-03566-2
38. Sato H, Kotake K, Sugihara K, Takahashi H, Maeda K, Uyama I. Clinicopathological factors associated with recurrence and prognosis after R0 resection for stage IV colorectal cancer with peritoneal metastasis. Dig Surg. 2016;33(5):382–391. doi:10.1159/000444097
39. Elias D, Honore C, Dumont F, et al. Results of systematic second-look surgery plus HIPEC in asymptomatic patients presenting a high risk of developing colorectal peritoneal carcinomatosis. Ann Surg. 2011;254(2):289–293. doi:10.1097/SLA.0b013e31822638f6
40. Jimenez W, Sardi A, Nieroda C, et al. Predictive and prognostic survival factors in peritoneal carcinomatosis from appendiceal cancer after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2014;21(13):4218–4225. doi:10.1245/s10434-014-3869-1
41. Kim DW, Park DG, Song S, Jee YS. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy as treatment options for peritoneal metastasis of advanced gastric cancer. J Gastric Cancer. 2018;18(3):296–304. doi:10.5230/jgc.2018.18.e32
42. Lieu CH, Lambert LA, Wolff RA, et al. Systemic chemotherapy and surgical cytoreduction for poorly differentiated and signet ring cell adenocarcinomas of the appendix. Ann Oncol. 2012;23(3):652–658. doi:10.1093/annonc/mdr279
43. Alzahrani N, Ferguson JS, Valle SJ, Liauw W, Chua T, Morris DL. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: long-term results at St George Hospital, Australia. ANZ J Surg. 2016;86(11):937–941. doi:10.1111/ans.13152
44. Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21(20):3737–3743. doi:10.1200/JCO.2003.04.187
45. Segelman J, Flöter‐Rådestad A, Hellborg H, Sjövall A, Martling A. Epidemiology and prognosis of ovarian metastases in colorectal cancer. Br J Surg. 2010;97(11):1704–1709. doi:10.1002/bjs.7196
46. Tan DS, Agarwal R, Kaye SB. Mechanisms of transcoelomic metastasis in ovarian cancer. Lancet Oncol. 2006;7(11):925–934. doi:10.1016/S1470-2045(06)70939-1
47. Al-Busaidi IS, Bailey T, Dobbs B, Eglinton TW, Wakeman CJ, Frizelle FA. Complete resection of colorectal cancer with ovarian metastases combined with chemotherapy is associated with improved survival. ANZ J Surg. 2019;89(9):1091–1096. doi:10.1111/ans.14930
48. Mrad K, Morice P, Fabre A, et al. Krukenberg tumor: a clinico-pathological study of 15 cases. Ann Pathol. 2000;20(3):202–206.
49. Gilliland R, Gill PJ. Incidence and prognosis of Krukenberg tumour in Northern Ireland. Br J Surg. 1992;79(12):1364–1366. doi:10.1002/bjs.1800791241
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

