Back to Journals » Cancer Management and Research » Volume 13
Factors Prognostic for Brain Metastases from Colorectal Cancer: A Single-Center Experience in China
Authors Wang D, Chen C , Ge X, Yang Q, Huang Y, Ling T, Jin T, Yu S, Wang J, Sun L
Received 14 May 2021
Accepted for publication 19 August 2021
Published 29 August 2021 Volume 2021:13 Pages 6767—6774
DOI https://doi.org/10.2147/CMAR.S320179
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Bilikere Dwarakanath
Da Wang,1,* Chao Chen,1,* Xiaoxu Ge,1,2 Qi Yang,2 Yuhuai Huang,1 Tianyi Ling,1 Tian Jin,1 Shaojun Yu,1 Jian Wang,1 Lifeng Sun1
1Department of Colorectal Surgery and Oncology, Key Laboratory of Cancer Prevention and Intervention, Ministry of Education, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang Province, People’s Republic of China; 2Department of Cancer Institute, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Da Wang; Lifeng Sun
Department of Colorectal Surgery and Oncology, Key Laboratory of Cancer Prevention and Intervention, Ministry of Education, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang Province, People’s Republic of China
Tel +86-571-87784720
; +86-571-87783586
Email [email protected]; [email protected]
Purpose: This study aimed to analyze clinicopathological, survival, prognostic factors, as well as the timing of brain metastases (BM) in colorectal cancer (CRC) using data from a Chinese center.
Patients and Methods: Data of 65 consecutive CRC patients with BM were collected from a single institution in China. The time from primary tumor surgery to the occurrence of BM was calculated. Kaplan-Meier analysis was used to evaluate cumulative survival of patients. Factors associated with prognosis of overall survival (OS) were explored using Cox’s proportional hazard regression models.
Results: The median time interval from CRC surgery to the diagnosis of BM was 24 months. After diagnosis of BM, median OS values for patients were 11 months. Extracranial metastases occurred in 45 cases (69.2%) when BM was diagnosed, and 58.5% of these patients with lung metastases Time of BMs (P=0.018), presence of extracranial metastases (P=0.033), treatment (P=0.003), CA199 (P=0.034), CA125 (P< 0.001), CA242 (P=0.018), and CA211 (P=0.012) were associated with OS of patients through univariate analysis. Multivariate analysis using a Cox regression model showed that only treatment was an independent predictor for OS (conservative treatment; HR=1.861, 95% CI=1.077– 3.441; P=0.048).
Conclusion: Surgical treatment of metastatic lesions may be an alternative choice for CRC patients with BM. Identifying the timing of brain metastases can help to detect this disease early, leading to a better survival outcome.
Keywords: colorectal cancer, brain metastases, prognosis factors, surgery
Introduction
Colorectal cancer (CRC) is the third most common cancer type in both males and females and the second leading cause of cancer-related death worldwide.1 According to the 2018 China Cancer Statistics Report, the mortality rate of CRC in China ranks the fifth among all malignant tumors, with 191,000 deaths.2 Previous studies report that the incidence of brain metastases (BM) in CRC is 1–3% and has been increasing in recent decades due to the progression of the diagnosis pathway.3 Although BM is much less frequent in CRC than other common metastatic targets such as the liver, lung, or peritoneum, BM is considered an end-stage disease with extremely low survival rates (median OS of 3–6 months).4
Cranial imaging is not a routine examination recommended by National Comprehensive Cancer Network (NCCN) guidelines for CRC to detect BM at early stage. Neurological examination is performed when patients show neurological symptoms which normally presented at a later stage.5 Therefore, BM detection before the onset of neurological symptoms could lead to a more effective treatment, higher quality-of-life, and better prognosis. However, little is known about the appropriate time to monitor brain lesions, so it is still a problem for a timely BM detection for CRC patients.
Whole brain radiotherapy (WBRT) and chemotherapy were standard therapeutic modalities administered to palliate neurologic symptoms. Unfortunately, they did not improve the prognosis of these patients (the survival rate ranges from 2.2–4 months).6,7 Patients achieved notable survival benefits (median OS of 6–10 months) after undergoing metastasectomy of BM compared with palliative treatment.7,8 However, to the best of our knowledge, few studies reported the survival predictors of metastasectomy of BM. Therefore, it is necessary to investigate parameters related to the presentation and outcome of patients with BM from CRC.
Data used in this study were retrieved from a single center in China. Data were used to evaluate clinicopathological, survival, and prognostic factors for CRC patients with BM. In addition, the time interval for BM occurrence was analyzed, which would help the follow-up monitoring and early diagnosis of BM from CRC in the future. This study’s findings will provide information and treatment strategies for clinicians, and serve as a basis for further research.
Materials and Methods
Patients
This study included 65 consecutive CRC patients presenting with BM from January 2009 to December 2019 in the Second Affiliated Hospital of Zhejiang University School of Medicine, which is a large center with over 1,000 CRC cases per year in China. All patients were diagnosed with CRC pathologically and BM was confirmed by radiologic imaging. Follow-ups were carried out for all patients in the outpatient unit approximately 2 weeks after treatment, and at least every 3 months for 2 years, then every 6 months after the first 2 years. CT or MRI scans of the brain, abdomen, pelvis, and thorax were assessed at each follow-up visit. The study was approved by the Institutional Review Board of the Second Affiliated Hospital of Zhejiang University School of Medicine and conducted according to the Declaration of Helsinki. All patients included in the study provided signed informed consent.
Study Design
BM patients were divided into two groups to compare surgery vs non-surgery treatment options. Demographic data, Characteristics of BM, treatment strategy, and survival were collected and analyzed.
Statistical Analysis
Overall survival (OS) was defined as the period between the date patients were diagnosed with BM to the last known date of follow-up or date of death. Cumulative survival was evaluated by Kaplan-Meier analysis. Differences in survival curves between groups of patients were assessed using the Log rank test. Significant factors associated with prognosis of overall survival (OS) in univariate analysis were included in multivariate analyses. Multivariate analyses were performed using Cox’s proportional hazard regression models to identify factors associated with OS. A two-sided P-value <0.05 was considered statistically significant. All analyses were performed in IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.
Results
Clinicopathologic Features
A total of 65 CRC patients diagnosed with BM from January 2009 to December 2019 in our cancer center were included in this study. The flowchart of patients included in this study is shown in Figure 1. The median age of patients was 63 (37–72) years old and 55.4% of the patients were more than 60 years old; 6.2% of patients presented with simultaneous BM and most primary cancers with BM were in rectal cancer (n=41, 63.1%). Extra-brain metastases occurred in 45 patients, among who 38 were lung metastases (58.5%) and 11 liver metastases (16.9%). BM with maximal dimension less than 3 cm accounted for 50.8% and patients diagnosed with BM in our center mainly presented with Supratentorial BM and Combinational BM (43.1% and 43.1%, respectively). The number of brain lesions was single in 37 patients (56.9%). CEA was positive in 53.8% of patients, on the contrary, more than half of the patients have a negative tumor marker including CA199, CA125, CA242, or CA211 (63.1%, 73.8%, 66.2%, or 64.6%, respectively). A total of 28 patients underwent surgery for BM in our center. Nine patients who underwent surgery have a recurrence of BM. Demographic and histologic data of patients were summarized in Table 1.
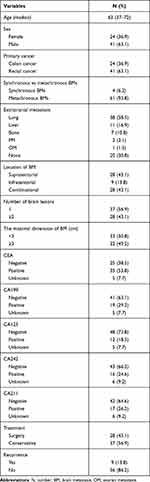 |
Table 1 Demographic Characteristics of Patients |
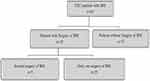 |
Figure 1 A flowchart of patients included in this study. |
Timing of BM Occurrence
The median time interval from diagnosing CRC to BM was 24 months (Figure 2). Among them, 32.3% of the BM occurred within the first year after surgery of primary tumor, 49.2% in the first 2 years, 70.8% in the first 3 years.
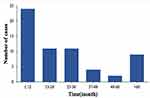 |
Figure 2 Time interval from surgery of CRC to diagnosis of BM. |
Survival Outcomes
Median OS for all patients was 7 months, with overall 1- and 3-year survival rates of 27.5% and 7.8%, respectively. A total of 37 patients rejected surgery after BM diagnosis. Median OS for the 37 patients was 4 months compared with 11 months of patients who underwent surgery (Figure 3).
 |
Figure 3 Overall survival Kaplan-Meier curves for significant prognostic variables for CRC patients with OM who underwent surgery. |
Analysis of predictors using Kaplan-Meier method showed that time of BMs (P=0.018), presence of extracranial metastases (P=0.033), treatment (P=0.003), CA199 (P=0.034), CA125 (P<0.001), CA242 (P=0.018), and CA211 (P=0.012) were significantly associated with OS (Table 2). However, sex, primary cancer, location of BM, or the number of brain lesions were not correlated with survival time. Factors with a P-value less than 0.05 were used for multivariable analysis, and only conservative management was identified as an independent predictor for poorer OS (Conservative treatment; HR=1.884, 95% CI=1.077–3.296; P<0.001) (Table 2, Figure 3).
 |
Table 2 Univariate Analysis and Multivariate Analysis of Factors Associated with OS Using a Cox Regression Model for Patients Who Underwent CRS in Our Center |
Discussion
BM affects young patients, develops rapidly, and is considered as the worst prognostic. Therefore, it is necessary to develop effective treatment for BM patients. Quan et al9 investigated the influence of different treatments on CRC patients with BM, concluding that patients who underwent surgery with or without radiotherapy have more prolonged survival (OS=17 months) than radiotherapy only (OS=7 months). Other researchers also concluded that aggressive intervention was associated with a better prognosis of CRC patients with BM.10 In this study, median OS for CRC patients in the BM group who underwent surgery was 11 months, compared with a median OS of 4 months for patients receiving palliative treatment. However, we did not analyze the difference between surgery with or without radiotherapy due to the fact that only seven patients received surgery combined with radiotherapy. Previous studies have reported controversial results on surgery combined with radiotherapy for CRC patients with BM.11 Some surgeons recommend surgery combined with radiotherapy as it improves CRC patient survival better than surgery only, and others found no differences between the two treatments.12–14 Prospective studies including more samples should be carried out to assess treatments’ effectiveness and explore effective prognostic factors for CRC patients with BM.
Previous studies have explored factors associated with prognosis of CRC patients with BM, including extracranial metastases, number of BMs, and treatment of BM;6,7,15,16 However, conclusions were varied among different studies.17 The results of the current study showed that time of BMs, presence of extracranial metastases and treatment were significantly associated with OS in univariate analyses, however, only treatment was the independent factor in multivariate analyses for prognosis of CRC patients with BM.
In this study, we analyzed the timing of BM occurrence, and found that the median time interval from CRC surgery to BM was 24 months, similar to other studies.5,18 The late manifestations of BM may be influenced by the development of surgery and anticancer therapy and the blood–brain barrier (BBB).19,20 The mechanism of brain metastases is multifactorial and extremely complex, BBB may be only one point in the mechanism of BM. BBB stop cancer cells invading because of the continuous tight junctions.21 On the contrary, BBB lost the solid blocking effect on cancer cells once BM occurs and anti-cancer drugs are too large to pass the BBB. As a result, the brain becomes a kind of store-house for metastatic cancer cells.
According to Kim et al,22,23 patients with rectal cancer have a higher tendency for BM. This is in accordance with our study. The likely explanation may be the vascular anatomy of the rectum and lung. BM from CRC occurs at the late stage of the disease, and there is often a high proportion of concurrent extracranial metastases.20,24 Previous researchers investigated that the most concomitant extracranial metastases at diagnosis of BM is lung metastases (42.1–65%), followed by liver metastases (30–44.7%), and bone metastases (12–21.2%).25 The results of the current study show that 69.2% of all patients had combined extracranial metastases. Among these extracranial metastases, the lung was the most common location (58.5%), consistent with previous studies.26 A few authors also showed that patients with lung metastases had a statistically significantly increased risk of BM.27 According to previous investigations, the metastatic pathway of BM from CRC may be cancer cells transfer to the lung and thereafter the brain via a circulatory venous system.18,24 Other scholars also investigated the direct metastases from the primary to the brain via vertebral plexus.14,28
Limitations
We analyzed the prognosis factors of CRC patients with BM in our center, which can help provide information and treatment strategies for clinicians, as well as promoting related research. But limitations still exist. First, there is selection bias in this retrospective study, for example, all patients in our center received brain surgery, which means they are potentially healthier than others who received nonsurgical regimens. Second, it is a small sample size (only 65 CRC patients with BM), which may restrict the reliability of the conclusion. Third, we only focus on the effect of surgery of BM. Limited details about chemotherapy and radiotherapy are collected because of the unavailability of detailed medical records from other institutions. Thus, we do not investigate if radiation therapy would have affected survival outcome similar to surgery. These limitations can be against if employ more patients in prospective study in the future. Studies including more samples are urgently needed to assess the effectiveness of these therapeutics and to reach a solid conclusion for the management of CRC patients with BM.
Conclusion
In summary, surgery for BM may be an effective and safe treatment approach for CRC patients with BM. It’s helpful to detect BM before the onset of neurological symptoms through confirming the timing from primary tumor surgery to BM. In addition, BM is coincident with lung metastases, the mechanism of BM from CRC merits comprehensive investigation and these potential targets might be a novel approach in curing BM from CRC.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Ethical Approval
This study was reviewed and approved by the Institutional Review Board of the Second Affiliated Hospital of Zhejiang University School of Medicine.
Consent to Participate
Informed consent was obtained from all participants in the study.
Consent to Publication
Informed consents was obtained from all authors in the study.
Acknowledgments
Our special thanks to Mr. Bo Liu for helping us with editing.
Funding
The study was funded by the National Natural Science Foundation of China (No. 81472819, No. 81672342), the Zhejiang Provincial Key R&D Program of China (No. 2019C03018), the Zhejiang Provincial Natural Science Foundation of China (No. LY20H160038, LQ17H160008), and the Fundamental Research Funds for the Central Universities (No. 2019QNA7028, No. 2019FZJD009).
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30.
2. [Chinese Protocol of Diagnosis and Treatment of Colorectal Cancer (2020 edition)]. Zhonghua Wai Ke Za Zhi. 2020;58(8):561–585. Chinese.
3. Go PH, Klaassen Z, Meadows MC, Chamberlain RS. Gastrointestinal cancer and brain metastasis: a rare and ominous sign. Cancer. 2011;117(16):3630–3640. doi:10.1002/cncr.25940
4. Sun J, Wang C, Zhang Y, et al. Genomic signatures reveal DNA damage response deficiency in colorectal cancer brain metastases. Nat Commun. 2019;10(1):3190. doi:10.1038/s41467-019-10987-3
5. Noura S, Ohue M, Shingai T, et al. Brain metastasis from colorectal cancer: prognostic factors and survival. J Surg Oncol. 2012;106(2):144–148. doi:10.1002/jso.23055
6. Suzuki Y, Yamaguchi T, Matsumoto H, et al. Prognostic factors and treatment effects in patients with curatively resected brain metastasis from colorectal cancer. Dis Colon Rectum. 2014;57(1):56–63. doi:10.1097/01.dcr.0000436998.30504.98
7. Gu XD, Cai YT, Zhou YM, Li ZY, Xiang JB, Chen ZY. Prognostic factors and multidisciplinary treatment modalities for brain metastases from colorectal cancer: analysis of 93 patients. BMC Cancer. 2015;15:902. doi:10.1186/s12885-015-1933-2
8. Nieder C, Pawinski A, Balteskard L. Colorectal cancer metastatic to the brain: time trends in presentation and outcome. Oncology. 2009;76(5):369–374. doi:10.1159/000210026
9. Quan J, Ma C, Sun P, et al. Brain metastasis from colorectal cancer: clinical characteristics, timing, survival and prognostic factors. Scand J Gastroenterol. 2019;54(11):1370–1375. doi:10.1080/00365521.2019.1686056
10. Tapia Rico G, Price TJ, Karapetis C, et al. Brain metastasis in advanced colorectal cancer: results from the South Australian metastatic colorectal cancer (SAmCRC) registry. Cancer Biol Med. 2017;14(4):371–376. doi:10.20892/j.issn.2095-3941.2017.0068
11. Del Carpio Huerta L, Virgili Manrique AC, Szafranska J, et al. Brain metastases in colorectal cancer: prognostic factors and survival analysis. Int J Colorectal Dis. 2018;33(11):1517–1523. doi:10.1007/s00384-018-3107-6
12. Mege D, Sans A, Ouaissi M, Iannelli A, Sielezneff I. Brain metastases from colorectal cancer: characteristics and management. ANZ J Surg. 2018;88(3):140–145. doi:10.1111/ans.14107
13. Silva IL, Iskandarani M, Hotouras A, et al. A systematic review to assess the management of patients with cerebral metastases secondary to colorectal cancer. Tech Coloproctol. 2017;21(11):847–852. doi:10.1007/s10151-017-1707-8
14. Magni E, Santoro L, Ravenda PS, et al. Brain metastases from colorectal cancer: main clinical factors conditioning outcome. Int J Colorectal Dis. 2014;29(2):201–208. doi:10.1007/s00384-013-1781-y
15. Imaizumi J, Shida D, Narita Y, et al. Prognostic factors of brain metastases from colorectal cancer. BMC Cancer. 2019;19(1):755. doi:10.1186/s12885-019-5973-x
16. Roussille P, Auvray M, Vansteene D, et al. Prognostic factors of colorectal cancer patients with brain metastases. Radiother Oncol. 2021;158:67–73. doi:10.1016/j.radonc.2021.02.006
17. Nieder C, Hintz M, Grosu AL. Predicted survival in patients with brain metastases from colorectal cancer: is a current nomogram helpful? Clin Neurol Neurosurg. 2016;143:107–110. doi:10.1016/j.clineuro.2016.02.022
18. Tanriverdi O, Kaytan-Saglam E, Ulger S, et al. The clinical and pathological features of 133 colorectal cancer patients with brain metastasis: a multicenter retrospective analysis of the Gastrointestinal Tumors Working Committee of the Turkish Oncology Group (TOG). Med Oncol. 2014;31(9):152. doi:10.1007/s12032-014-0152-z
19. Jiang XB, Yang QY, Sai K, Zhang XH, Chen ZP, Mou YG. Brain metastases from colorectal carcinoma: a description of 60 cases in a single Chinese cancer center. Tumour Biol. 2011;32(6):1249–1256. doi:10.1007/s13277-011-0229-7
20. Kruser TJ, Chao ST, Elson P, et al. Multidisciplinary management of colorectal brain metastases: a retrospective study. Cancer. 2008;113(1):158–165. doi:10.1002/cncr.23531
21. Zang YW, Gu XD, Xiang JB, Chen ZY. Brain metastases from colorectal cancer: microenvironment and molecular mechanisms. Int J Mol Sci. 2012;13(12):15784–15800. doi:10.3390/ijms131215784
22. Kim DY, Ryu CG, Jung EJ, Paik JH, Hwang DY. Brain metastasis from colorectal cancer: a single center experience. Ann Surg Treat Res. 2018;94(1):13–18. doi:10.4174/astr.2018.94.1.13
23. Lassman AB, DeAngelis LM. Brain metastases. Neurol Clin. 2003;21(1):1–23, vii. doi:10.1016/S0733-8619(02)00035-X
24. Damiens K, Ayoub JP, Lemieux B, et al. Clinical features and course of brain metastases in colorectal cancer: an experience from a single institution. Curr Oncol. 2012;19(5):254–258. doi:10.3747/co.19.1048
25. Thurmaier J, Heinemann V, Engel J, et al. Patients with colorectal cancer and brain metastasis: the relevance of extracranial metastatic patterns predicting time intervals to first occurrence of intracranial metastasis and survival. Int J Cancer. 2021;148(8):1919–1927. doi:10.1002/ijc.33364
26. Christensen TD, Spindler KL, Palshof JA, Nielsen DL. Systematic review: brain metastases from colorectal cancer–Incidence and patient characteristics. BMC Cancer. 2016;16:260. doi:10.1186/s12885-016-2290-5
27. Yaeger R, Cowell E, Chou JF, et al. RAS mutations affect pattern of metastatic spread and increase propensity for brain metastasis in colorectal cancer. Cancer. 2015;121(8):1195–1203. doi:10.1002/cncr.29196
28. Bartelt S, Momm F, Weissenberger C, Lutterbach J. Patients with brain metastases from gastrointestinal tract cancer treated with whole brain radiation therapy: prognostic factors and survival. World J Gastroenterol. 2004;10(22):3345–3348. doi:10.3748/wjg.v10.i22.3345
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
