Back to Journals » Cancer Management and Research » Volume 12
Factors Predicting Severe Myelosuppression and Its Influence on Fertility in Patients with Low-Risk Gestational Trophoblastic Neoplasia Receiving Single-Agent Methotrexate Chemotherapy
Authors Tu X, Chen R , Huang G , Lu N, Chen Q , Bai X, Li B
Received 5 March 2020
Accepted for publication 6 May 2020
Published 2 June 2020 Volume 2020:12 Pages 4107—4116
DOI https://doi.org/10.2147/CMAR.S252664
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Xiaoyu Tu,1,* Ruizhe Chen,1,* Genping Huang,1 Nanjia Lu,1 Qin Chen,2 Xiaoxia Bai,1 Baohua Li1
1Department of Gynecologic Oncology, Women’s Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, People’s Republic of China; 2Department of Pathology, Women’s Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Baohua Li Email [email protected]
Purpose: To investigate the potential factors to predict severe myelosuppression among low-risk gestational trophoblastic neoplasia (GTN) patients with single-agent methotrexate (MTX) chemotherapy. To analyze reproductive outcomes of patients with or without severe myelosuppression after achieving complete remission (CR).
Patients and Methods: The retrospective study included 319 low-risk GTN patients registered from January 2008 to December 2018 in our hospital. Patients were divided into two groups according to myelosuppression grading. Their clinical data and reproductive outcomes were compared and analyzed.
Results: A higher proportion of patients in group A received second-line chemotherapy than group B (P< 0.001). The number of total chemotherapy courses was more in group A than group B (P=0.001), while the number of MTX chemotherapy courses was more in group B than group A (P=0.001). When the joint predictor of pretreatment albumin (ALB) was not more than 44.5 g/L, pretreatment serum creatinine (Scr) was not less than 75.6 μmol/L, and the number of MTX chemotherapy courses was not less than four, there was a moderate predictive value. There was no significant difference of reproductive outcomes between the two groups after achieving CR.
Conclusion: Although some patients developed severe myelosuppression, MTX was still the effective first-line treatment for low-risk GTN patients. Patient’s pretreatment ALB was not more than 44.5 g/L, pretreatment Scr was not less than 75.6 μmol/L, and the number of MTX chemotherapy courses not less than four could be used as combined predictors to recognize the risk of severe myelosuppression. Severe myelosuppression had no significant adverse influence on fertility after achieving CR.
Keywords: gestational trophoblastic neoplasia, methotrexate, myelosuppression, fertility
Introduction
Gestational trophoblastic neoplasia (GTN) is a group of pregnancy-related conditions arising from the abnormal development and proliferation of trophoblastic cells, including choriocarcinoma, invasive mole, placental-site trophoblastic tumor, and epithelioid trophoblastic tumor.1 Based on the modified International Federation of Gynecology and Obstetrics (FIGO)/World Health Organization (WHO) scoring system, low-risk GTN is defined as a FIGO stage of I–III with a WHO score of 0–6, while high-risk GTN includes those of stage IV or with WHO score ≥ 7. Each patient must be stratified for risk prior to treatment, because low-risk GTN patients are more likely to respond to single-agent therapy, whereas high-risk GTN patients have a higher risk of resistance to single-agent chemotherapy and require the use of combination chemotherapy agents.2 Fortunately, GTN is highly chemo-sensitive and has excellent prognosis, particularly for low-risk disease where the cure rate is almost 100%.3 Various single agent regimens for low-risk GTN have been used worldwide, such as methotrexate (MTX) and dactinomycin (ActD).4 However, there is no consensus on the single best regimen and there is no fair comparison on the benefits and risks of each regimen due to the differences in inclusion criteria among trials. Currently, the choice of the regimen usually depends on the clinicians’ personal experience or preference.5 Even so, MTX is widely recognized as a key first-line, single-agent therapy for low-risk GTN since its first reported use to treat choriocarcinoma successfully in 1956.6
As one of the antimetabolic chemotherapeutic agents, MTX chemotherapy may lead to adverse events: nausea and vomiting, myelosuppression, hepatic and renal dysfunction alopecia, diarrhea, stomatitis, mucositis, and so on.7 Although low-dose MTX therapy is usually well tolerated in clinical practice, and most adverse events are mild-to-moderate in most patients, some patients develop severe myelosuppression during single agent MTX therapy.8,9 However, there has not yet been a study exploring possible factors associated with the development of MTX-related myelosuppression among low-risk GTN patients. Only one study investigated factors associated with the MTX-related myelosuppression in inflammatory rheumatic diseases.10 Identifying risk factors for severe myelosuppression among low-risk GTN patients may allow better planning and choice of treatment in advance.
Many women with GTN are of reproductive age and may still have the desire to be pregnant. Previous studies show that the reproductive function was preserved after successful treatment of GTN with chemotherapy.11 However, whether development of severe myelosuppression during single-agent MTX chemotherapy would influence fertility has not been reported. Thus, the aim of this study was to investigate the potential factors predicting severe myelosuppression in low-risk GTN patients receiving single-agent MTX chemotherapy, and to compare reproductive outcomes between women with and without severe myelosuppression during chemotherapy.
Materials and Methods
Patients registered in the electronic database of Women’s Hospital, Zhejiang University School of Medicine, China with a diagnosis of low-risk GTN defined according to the FIGO/WHO scoring system between January 2008 to December 2018 were identified. GTN was diagnosed according to the following criteria: (1) β-human chorionic gonadotropin (hCG) plateau lasting for four measurements over a period of at least 3 weeks; (2) β-hCG increase of 10% or more in three measurements over a period of at least 2 weeks; (3) β-hCG level higher than 20,000 IU/L 4 weeks after uterine evacuation; (4) persistently elevated β-hCG levels 6 months after uterine evacuation; and (5) evidence of metastatic disease.1,12 The FIGO score was determined by age, antecedent pregnancy, duration of disease, pretreatment hCG level, site and number of metastases, size of largest tumor, and previously failed chemotherapy.13
Patients included in the present study all had initially received first-line chemotherapy with intravenous MTX 0.4 mg/kg daily for 5 days and repeated at fixed intervals of 2 weeks until hCG normalization. At least two courses of consolidation chemotherapy were given after the first negative hCG level. Patients developing resistance or severe toxicity to MTX were switched to second-line chemotherapy protocols such as intravenous ActD10-12 µg/kg daily for 5 days and repeated at fixed two-weekly intervals or the EMA-CO combination chemotherapy (etoposide, MTX, and ActD followed by cyclophosphamide and vincristine).14,15 Primary remission was defined as three consecutive weekly hCG levels that were within the normal range using the same chemotherapy regimen. After that, the patients were followed up with monthly hCG monitoring for at least 1 year and radiological examinations if clinically indicated. Sustained complete remission (CR) was defined as maintenance of normal serum hCG levels beyond 1 year. Resistance to first-line MTX was defined as a hCG plateau over 3 consecutive weeks or rising hCG of 10% or more over 2 consecutive weeks. Relapse was defined as a rise in hCG levels after the remission in the absence of new pregnancy.12,16,17
The patients’ medical records were collected and reviewed retrospectively for information on myelosuppression, which was graded according to the WHO Classification criteria for acute and subacute toxic side-effects of anticancer drugs18 (Supplementary Material Table S1). Our study was approved by the Institutional Review Board, Women’s Hospital, Zhejiang University School of Medicine. Patient consent statements were explaineded and signed by all individual participants included in the study. A total of 319 low-risk GTN patients were included in the study and divided into two groups according to myelosuppression grading. Group A included 248 patients with no or mild myelosuppression (grades 0, I, II) during MTX chemotherapy, while group B included 71 patients with severe myelosuppression (grades III, IV) during MTX chemotherapy.
Clinical data collected included age, body mass index (BMI), gravidity and parity, nature of the antecedent pregnancy (molar pregnancy, live birth, stillbirth, or abortion), time between the end of antecedent pregnancy and the beginning of chemotherapy with MTX, GTN stage, FIGO/WHO prognostic score, number of MTX courses, number of courses of other chemotherapy, pretreatment serum hCG levels, as well as the pretreatment indices of the full blood count, liver, and renal functions. The post-treatment full blood count indices were also taken at the time of the most severe myelosuppression if it happened, or the last measurement was taken if no myelosuppression occurred.
Considering the GTN therapeutic variables, we evaluated the chemotherapy regimens of all patients, the number of MTX cycles and total chemotherapy courses in both groups, the number of patients to switch to second-line chemotherapy due to MTX resistance or MTX myelosuppression toxicity, the number of patients receiving CR after chemotherapy, the number of patients suffering relapse, and the number of patients who received different support treatment in both groups.
Regarding the reproductive outcomes of the patients after achieving CR, we contacted them all by phone call or by interview at the clinic. The following variables in both groups were recorded: their desire for pregnancy, outcomes of any pregnancy (live birth, abortion, ectopic pregnancy, or stillbirth), cumulative pregnancy rate and cumulative live birth rate from CR to the final follow-up date in August 2019.
Continuous data were presented as mean±standard deviation if normally distributed, or as median (25th–75th percentile) if not normally distributed. Categorical data were presented as frequencies and percentages. Patients’ characteristics between the two groups were compared using the Student’s t-test, Mann–Whitney U-test, Chi-square test, or Fisher’s exact test where appropriate. Logistic regression analysis was used to evaluate predictors of binary outcome measures, with multivariate analysis being subsequently performed on factors determined to be statistically significant in univariate analysis. Features that have been found to be significant in multivariate analysis were studied further for their prediction capability by the receiver-operator characteristic (ROC) curve, which evaluated the sensitivity, specificity, and area under the curve (AUC). The cumulative pregnancy rates and cumulative live birth rate of the two groups were compared by the Log rank test of the Kaplan–Meyer survival curve. All statistical tests were two-sided, and a P-value<0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS Statistics software, version 22 for Mac (IBM, USA) and MedCalc (Version 8.0.0.1MedCalc Software, Mariakerke, Germany).
Results
Of the 319 patients included in this study, 165 (51.7%) patients received MTX alone, while 154 (48.3%) patients developed resistance or toxicity to MTX and therefore switched to a second-line treatment. A higher proportion of patients in group A received second-line chemotherapy than group B (P<0.001). The number of total chemotherapy courses was more in group A than group B (P=0.001), while the number of MTX chemotherapy courses was more in group B than group A (P=0.001). The primary remission rate with a single-agent MTX was higher in group B than group A (P<0.001), but the sustained CR rate was similar in both groups (P=0.383). Although there were five (2.0%) patients in group A and three (4.2%) patients in group B who relapsed, they received remission again after treatment (Supplementary Material Table S2).
The number of patients with different grades of myelosuppression in both groups are shown in Supplementary Material Table S3. All patients in group B suffered bicytopenia or pancytopenia. The occurring percentages of leukopenia, neutropenia, anemia, as well as thrombocytopenia were all significantly higher in group B than in group A. In order to prevent or relieve severe myelosuppression, all patients in group B were given oral leukocyte-raising drugs or granulocyte colony-stimulating factor (G-CSF) during the chemotherapy. Besides, 18 (25.4%) patients were given iron therapy or erythropoietin (EPO), 10 (14.1%) patients were given thrombopoietin (TPO), one (1.4%) patient even had the blood transfusion treatment. Among them, six patients discontinued the round of chemotherapy during which occurred severe myelosuppression with MTX 0.4 mg/kg daily only for 3 days. However, their blood routine examination recovered before the next round of chemotherapy. In group A, a significantly smallerproportion of patients received the above-mentioned support treatment. None of the patients had blood transfusion treatment or discontinued treatment because of toxicity.
The clinical characteristics of patients in both groups are summarized in Table 1. Regarding the nature of the antecedent pregnancy, there was a higher proportion of mole and live births in group B than group A (P=0.002). The pretreatment ALB and GFR were higher in group A than in group B (P=0.001 and <0.001, respectively), while patients’ BMI and pretreatment Scr were higher in group B than in group A (P=0.015 and 0.001, respectively). As patients in group B had severe myelosuppression, the posttreatment WBC, neutrophil, RBC, and Hb were significantly lower than patients in group A (data not shown). There was no statistical differences in other parameters.
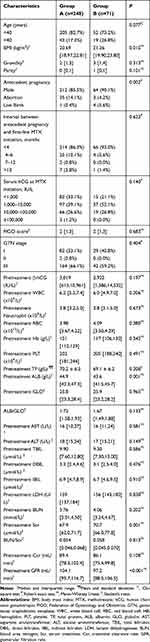 |
Table 1 The Clinical Characteristics of Patients in Both Groups |
Univariate analysis showed that BMI, pretreatment ALB, pretreatment Scr, pretreatment GFR, number of MTX chemotherapy courses were significantly correlated with occurrence of severe myelosuppression (Table 2). No significant association was found between severe myelosuppression and other blood parameters. Multivariate analysis by backward elimination of nonsignificant associated factors in the univariate analysis showed that pretreatment ALB, pretreatment Scr and number of MTX chemotherapy courses were independent risk factors for development of severe myelosuppression with MTX chemotherapy.
 |
Table 2 Correlation Analysis of Clinical Characteristics and Patients With Severe Myelosuppression |
The predictive performance of these factors as well as their joint predictor (pretreatment ALB+pretreatment Scr+number of MTX chemotherapy courses, P joint predictor=1/(1+e‒Z), Z=−0.834−0.135xPretreatment ALB+0.065xPretreatment Scr+0.232xNumber of MTX chemotherapy courses) on severe myelosuppression was evaluated by ROC curves (Figure 1). The diagnostic values are shown in Table 3 including their sensitivity, specificity, as well as cut-off values. The area under the ROC curve (AUC) was 0.628 (95% CI=0.572–0.681) for pretreatment ALB, 0.630 (95% CI=0.574–0.683) for pretreatment Scr, 0.624 (95% CI=0.568–0.677) for number of MTX chemotherapy courses, each of which were significantly lower than that of joint predictor (AUC of 0.735, 95% CI=0.683–0.783) (Supplementary Material Table S4).
 |
Table 3 The Diagnostic Values of Pretreatment ALB, Pretreatment Scr, Number of MTX Chemotherapy Courses and Their Joint Predictor to Predict Severe Myelosuppression after MTX Chemotherapy |
Concerning the influence of severe myelosuppression on fertility, we followed up all the patients after their CR. The median follow-up was 61 [17–92] months. In group A, 187 (75.4%) patients were successfully followed-up and 61 (24.6%) were lost to follow-up, while in group B, 54 (76.1%) patients were successfully followed-up and 17 (23.9%) were lost to follow-up; the percentage of drop-outs was comparable in both groups (P=0.910). Among those who were followed-up successfully, 103 (55.1%) patients in group A and 33 (61.1%) patients in group B had no fertility desire (P=0.431). Among those who had a pregnancy desire (84 in group A and 21 in group B), 73 (86.9%) patients in group A and 15 (71.4%) patients in group B conceived (P=0.102). 60 (82.2%) patients in group A and 10 (66.7%) patients in group B had a live birth (P=0.179). Eleven (15.1%) patients of group A and five (33.3%) patients of group B had an abortion (P=0.137). Two (2.7%) patients of group A and 0 (0.0%) patients of group B had an ectopic pregnancy (P=1.000) (Table 4). Kaplan–Meier analysis was used to compare the cumulative pregnancy rate and cumulative live birth rate of patients with and without severe myelosuppression after chemotherapy (Figure 2). The 10-year cumulative pregnancy rates of group A and group B were 86.9% and 71.4%, respectively (P=0.819). The 10-year cumulative live birth rates of group A and group B were 71.4% and 47.6%, respectively (P=0.466). Therefore, there was no significant difference in reproductive outcomes between the two groups.
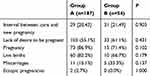 |
Table 4 The Reproductive Outcomes of Follow-Up Patients in Two Groups |
 |
Figure 2 10-year follow-up cumulative pregnancy rates (A) and cumulative live birth rates (B) of group A and group B. |
Discussion
The present study evaluated factors predicting severe myelosuppression with MTX chemotherapy among 319 low-risk GTN patients. Although 71 (22.6%) patients developed grade III or IV myelosuppression, nearly all patients achieved sustained CR in both groups. The main finding was that patients developing severe myelosuppression (group B) received more courses of first-line MTX chemotherapy, achieved a higher remission rate with single agent MTX therapy, but required a lower total number of chemotherapy courses than those with no or mild myelosuppression (group A), and fewer of those in group B required second-line chemotherapy. What’s more, we found that pretreatment ALB, pretreatment Scr, and the number of MTX chemotherapy courses were independent predictors of severe myelosuppression with MTX treatment, and the composite index of these factors had a moderate predictive value. Therefore, patient’s pretreatment ALB was not more than 44.5 g/L, pretreatment Scr was not less than 75.6 μmol/L, and the number of MTX chemotherapy courses was not less than four could be used as the combined predictors by clinicians to recognize a high risk of occurring severe myelosuppression. To our knowledge, this is the first retrospective cohort study to explore the factors predicting the risk of severe myelosuppression with MTX chemotherapy for low risk GTN patients.
It is generally believed that MTX-associated toxicity and its severity are related to several factors including drug dose, the duration and frequency of MTX administration, and genetic factors.19,20 In our study, patients with severe myelosuppression had a higher number of MTX courses than those without myelosuppression. Further analysis showed that receiving more than four courses of MTX was one of the high risk factors. Meanwhile, serum ALB levels not more than 44.5 g/L was identified as one of the predisposing factors for severe myelosuppression as well in our study. MTX is moderately bound to plasma proteins, mainly albumin, with the fraction bound ranging from 46.5–54%.21 The binding of a drug to plasma or serum proteins may exert profound effects on drug distribution and elimination, thus influencing its therapeutic, pharmacodynamic, and toxicological actions.22 The reduction of serum albumin levels meant an increase in unbonded MTX entering cells via the reduced folate carrier, which increased intracellular drug concentration and exposure duration, contributing to the development of severe myelosuppression. Although no previous studies have reported a correlation between albumin levels and MTX side-effects in GTN patients yet, many studies found that hypoalbuminemia contributed to MTX-induced myelosuppression in regard to treatment for inflammatory rheumatic diseases.10,23 As for the association between pretreatment Scr and myelosuppression, we found that pretreatment Scr not less than 75.6 μmol/L would predispose GTN patients to MTX toxicity. This is because the MTX toxicity not only depends on its concentration, but also its duration of exposure to the cells.24 The main excretion route of MTX is through the kidney.25 A rise in serum creatinine concentration above normal values indicates potential renal dysfunction and delayed MTX elimination.26,27 It’s worth mentioning that we also found that pretreatment GFR was significantly lower in patients with severe myelosuppression, though it was only a significant correlation in univariate analysis but not in multivariate analysis. Data from earlier studies supported the findings and indicate that any renal clearance impairment would lead to sustained higher serum levels of the drug that might induce toxicities and extend the therapy period.24,28
Apart from exploring the predictive factors of severe myelosuppression during MTX chemotherapy for low risk GTN patients, we also compared their reproductive outcomes via follow-up and found there was no significant difference between two groups. To our knowledge, this is the first retrospective cohort study to analyze the impact of occurrence of severe myelosuppression on fertility in low risk GTN patients. It suggested that fertility is preserved regardless of the occurrence of severe myelosuppression, totally consistent with previous studies on long-term fertility effects after chemotherapy for low-risk GTN patients in general, and up to around 60% of such patients were able to conceive subsequently.9,29 Another study even revealed that multiagent EMA-CO chemotherapy did not significantly alter menstrual or reproductive outcomes in high-risk GTN patients compared to single-agent methotrexate chemotherapy for low-risk GTN.30 However, there was a parallel study reporting that single-agent chemotherapy advanced the age at menopause by 1 year compared with 3 years for those treated with combination chemotherapy.31 However, another larger study with 1,903 patients and a mean follow-up of 16.9 years showed that MTX-FA therapy had no significant impact on the risk of early menopause, while EMA-CO therapy increased the risk of early menopause, particularly for older patients.32 Overall, low-risk GTN patients with single-agent MTX chemotherapy, even developing severe myelosuppression during treatment, can generally anticipate a normal future reproductive outcome. Patients with difficulty conceiving may even consider assisted reproductive technology for fertility treatment.11
One main limitation of our study is the small sample size of patients having severe myelosuppression, which prevented us from drawing a more definitive conclusion. Nevertheless, this study represents the largest series to date reporting on the predictor of severe myelosuppression among low-risk GTN patients with first-line MTX chemotherapy. Meanwhile, the data all are collected from a single-center, so a multicenter and large sample setting is further needed to exclude possible single-center biases. Second, many patients were lost to follow-up, which limited the accuracy of analysis on reproductive outcomes in our study. Third, due to the retrospective nature of this study, adverse events during the chemotherapy were identified through medical record review, rather than in real time. This may confer certain inherent limitations, such as ascertainment bias and confounding, or even be incomplete.
Conclusion
Our study showed that some low-risk GTN patients would develop severe myelosuppression during MTX treatment, but these patients had better MTX responses and efficacy after symptomatic and supportive treatment. Therefore, MTX as first-line chemotherapy is indeed suitable for low-risk GTN patients even in those with myelosuppression. Meanwhile, patient’s pretreatment ALB was not more than 44.5 g/L, pretreatment Scr was not less than 75.6 μmol/L, and the number of MTX chemotherapy courses was not less than four could be used as the combined predictor to recognize a high risk of developing severe myelosuppression with MTX chemotherapy among low-risk GTN patients, which helps clinicians identify it and take timely measures, such as folic acid rescue, supporting therapy, even stopping or changing chemotherapy regimens, to deal with or prevent serious adverse events. What’s more, those with severe myelosuppression during MTX treatment had comparable reproductive outcomes than those without, which helps clinicians provide more consulting information on fertility for low-risk GTN patients.
Acknowledgments
The authors are very grateful to Hang Wun Raymond Li (Department of Obstetrics and Gynaecology, The University of Hong Kong, Queen Mary Hospital, Hong Kong) for his advice to explain the results. This study was supported by Zhejiang Provincial Natural Science Foundation of China (Grant No. LY18H160004).
Disclosure
The authors declare no conflict of interest in this work.
References
1. Seckl MJ, Sebire NJ, Berkowitz RS. Gestational trophoblastic disease. Lancet. 2010;376(9742):717–729. doi:10.1016/S0140-6736(10)60280-2
2. Lurain JR. Gestational trophoblastic disease II: classification and management of gestational trophoblastic neoplasia. Am J Obstet Gynecol. 2011;204(1):11–18. doi:10.1016/j.ajog.2010.06.072
3. Bolze PA, Riedl C, Massardier J, et al. Mortality rate of gestational trophoblastic neoplasia with a FIGO score of >/=13. Am J Obstet Gynecol. 2016;214(3):390e391–398. doi:10.1016/j.ajog.2015.09.083
4. Li J, Li S, Yu H, Wang J, Xu C, Lu X. The efficacy and safety of first-line single-agent chemotherapy regimens in low-risk gestational trophoblastic neoplasia: a network meta-analysis. Gynecol Oncol. 2018;148(2):247–253. doi:10.1016/j.ygyno.2017.11.031
5. Foulmann K, Guastalla JP, Caminet N, et al. What is the best protocol of single-agent methotrexate chemotherapy in nonmetastatic or low-risk metastatic gestational trophoblastic tumors? A review of the evidence. Gynecol Oncol. 2006;102(1):103–110. doi:10.1016/j.ygyno.2006.02.038
6. Ngan HYS, Seckl MJ, Berkowitz RS, et al. Update on the diagnosis and management of gestational trophoblastic disease. Int J Gynaecol Obstet. 2018;143(Suppl 2):79–85. doi:10.1002/ijgo.12615
7. Livshits Z, Rao RB, Smith SW. An approach to chemotherapy-associated toxicity. Emerg Med Clin North Am. 2014;32(1):167–203. doi:10.1016/j.emc.2013.09.002
8. Chalouhi GE, Golfier F, Soignon P, et al. Methotrexate for 2000 FIGO low-risk gestational trophoblastic neoplasia patients: efficacy and toxicity. Am J Obstet Gynecol. 2009;200(6):643e641–646. doi:10.1016/j.ajog.2009.03.011
9. Khan F, Everard J, Ahmed S, Coleman RE, Aitken M, Hancock BW. Low-risk persistent gestational trophoblastic disease treated with low-dose methotrexate: efficacy, acute and long-term effects. Br J Cancer. 2003;89(12):2197–2201. doi:10.1038/sj.bjc.6601422
10. Mori S, Hidaka M, Kawakita T, et al. Factors associated with myelosuppression related to low-dose methotrexate therapy for inflammatory rheumatic diseases. PLoS One. 2016;11(4):e0154744. doi:10.1371/journal.pone.0154744
11. Brown J, Naumann RW, Seckl MJ, Schink J. 15years of progress in gestational trophoblastic disease: scoring, standardization, and salvage. Gynecol Oncol. 2017;144(1):200–207. doi:10.1016/j.ygyno.2016.08.330
12. Mangili G, Cioffi R, Danese S, et al. Does methotrexate (MTX) dosing in a 8-day MTX/FA regimen for the treatment of low-risk gestational trophoblastic neoplasia affect outcomes? The MITO-9 study. Gynecol Oncol. 2018;151(3):449–452. doi:10.1016/j.ygyno.2018.09.025
13. Ngan HY, Bender H, Benedet JL, et al. Gestational trophoblastic neoplasia, FIGO 2000 staging and classification. Int J Gynaecol Obstet. 2003;83(Suppl 1):175–177. doi:10.1016/S0020-7292(03)90120-2
14. McNeish IA, Strickland S, Holden L, et al. Low-risk persistent gestational trophoblastic disease: outcome after initial treatment with low-dose methotrexate and folinic acid from 1992 to 2000. J Clin Oncol. 2002;20(7):1838–1844. doi:10.1200/JCO.2002.07.166
15. Abu-Rustum NR, Yashar CM, Bean S, et al. Gestational trophoblastic neoplasia, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(11):1374–1391. doi:10.6004/jnccn.2019.0053
16. Prouvot C, Golfier F, Massardier J, et al. Efficacy and safety of second-line 5-day dactinomycin in case of methotrexate failure for gestational trophoblastic neoplasia. Int J Gynecol Cancer. 2018;28(5):1038–1044. doi:10.1097/IGC.0000000000001248
17. Maesta I, Nitecki R, Horowitz NS, et al. Effectiveness and toxicity of first-line methotrexate chemotherapy in low-risk postmolar gestational trophoblastic neoplasia: the New England trophoblastic disease center experience. Gynecol Oncol. 2018;148(1):161–167. doi:10.1016/j.ygyno.2017.10.028
18. WHO. WHO Handbook for Reporting Results of Cancer Treatment.pdf. Geneva: WHO; 1979.
19. Ozdemir ZC, Bozkurt Turhan A, Duzenli Kar Y, Bor O. The frequency of hepatotoxicity and myelotoxicity in leukemic children with different high doses of methotrexate. Int J Pediatr Adolesc Med. 2016;3(4):162–168. doi:10.1016/j.ijpam.2016.08.008
20. Shimasaki N, Mori T, Samejima H, et al. Effects of methylenetetrahydrofolate reductase and reduced folate carrier 1 polymorphisms on high-dose methotrexate-induced toxicities in children with acute lymphoblastic leukemia or lymphoma. J Pediatr Hematol Oncol. 2006;28(2):64–68. doi:10.1097/01.mph.0000198269.61948.90
21. Inoue K, Yuasa H. Molecular basis for pharmacokinetics and pharmacodynamics of methotrexate in rheumatoid arthritis therapy. Drug Metab Pharmacokinet. 2014;29(1):12–19. doi:10.2133/dmpk.DMPK-13-RV-119
22. Paxton JW. Protein binding of methotrexate in sera from normal human beings: effect of drug concentration, pH, temperature, and storage. J Pharmacol Methods. 1981;5(3):203–213. doi:10.1016/0160-5402(81)90088-7
23. Kivity S, Zafrir Y, Loebstein R, Pauzner R, Mouallem M, Mayan H. Clinical characteristics and risk factors for low dose methotrexate toxicity: a cohort of 28 patients. Autoimmun Rev. 2014;13(11):1109–1113. doi:10.1016/j.autrev.2014.08.027
24. Wang W, Zhou H, Liu L. Side effects of methotrexate therapy for rheumatoid arthritis: a systematic review. Eur J Med Chem. 2018;158:502–516. doi:10.1016/j.ejmech.2018.09.027
25. Grim J, Chladek J, Martinkova J. Pharmacokinetics and pharmacodynamics of methotrexate in non-neoplastic diseases. Clin Pharmacokinet. 2003;42(2):139–151. doi:10.2165/00003088-200342020-00003
26. Stoller RG, Hande KR, Jacobs SA, Rosenberg SA, Chabner BA. Use of plasma pharmacokinetics to predict and prevent methotrexate toxicity. N Engl J Med. 1977;297(12):630–634. doi:10.1056/NEJM197709222971203
27. Howard SC, McCormick J, Pui CH, Buddington RK, Harvey RD. Preventing and managing toxicities of high-dose methotrexate. Oncologist. 2016;21(12):1471–1482. doi:10.1634/theoncologist.2015-0164
28. Seideman P, Beck O, Eksborg S, Wennberg M. The pharmacokinetics of methotrexate and its 7-hydroxy metabolite in patients with rheumatoid arthritis. Br J Clin Pharmacol. 1993;35(4):409–412. doi:10.1111/j.1365-2125.1993.tb04158.x
29. Berkowitz RS, Tuncer ZS, Bernstein MR, Goldstein DP. Management of gestational trophoblastic diseases: subsequent pregnancy experience. Semin Oncol. 2000;27(6):678–685.
30. Wong JM, Liu D, Lurain JR. Reproductive outcomes after multiagent chemotherapy for high-risk gestational trophoblastic neoplasia. J Reprod Med. 2014;59(5–6):204–208.
31. Bower M, Rustin GJ, Newlands ES, et al. Chemotherapy for gestational trophoblastic tumours hastens menopause by 3 years. Eur J Cancer. 1998;34(8):1204–1207. doi:10.1016/S0959-8049(98)00059-8
32. Savage P, Cooke R, O’Nions J, et al. Effects of single-agent and combination chemotherapy for gestational trophoblastic tumors on risks of second malignancy and early menopause. J Clin Oncol. 2015;33(5):472–478. doi:10.1200/JCO.2014.57.5332
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

