Back to Journals » Infection and Drug Resistance » Volume 14
Factors Influencing the Prognosis of Patients with Intra-Abdominal Infection and Its Value in Assessing Prognosis
Authors Pan J, Zhu Q, Zhang X, Xu J, Pan L, Mao X, Wu X
Received 18 June 2021
Accepted for publication 17 August 2021
Published 24 August 2021 Volume 2021:14 Pages 3425—3432
DOI https://doi.org/10.2147/IDR.S325386
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Jianfei Pan,1,* Quanwei Zhu,1,* Xiaoqian Zhang,2 Jun Xu,1 Linlin Pan,1 Xiang Mao,3 Xiao Wu1,4,*
1Department of Emergency, The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, 230022, People’s Republic of China; 2Department of Endocrinology, The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, 230022, People’s Republic of China; 3Department of Neurosurgery, The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, 230022, People’s Republic of China; 4Jiangsu Provincial Key Laboratory of Critical Care Medicine, Department of Critical Care Medicine, Zhongda Hospital, School of Medicine, Southeast University, Nanjing, Jiangsu, 210009, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiang Mao
Department of Neurosurgery, The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, 230022, People’s Republic of China
Tel +86 65908477
Email [email protected]
Purpose: To explore the distribution of pathogenic bacteria in patients with intra-abdominal infection, to clarify the independent factors that affect the prognosis of patients with intra-abdominal infection and its evaluation value for prognosis.
Patients and Methods: The pathogens, underlying diseases, and related clinical data of patients with intra-abdominal infection from January 2012 to December 2019 in our hospital were retrospectively collected and the APACHE II score was calculated. The patients were divided into survival group and death group according to the prognosis, and the index between the two groups was compared. Spearman correlation analysis was used to evaluate the correlation between each index and prognosis, multivariate logistic regression analysis was used to screen the independent prognostic factors.
Results: Spearman correlation analysis showed that ALB level was negatively correlated with prognosis, age and APACHE II score were positively correlated with prognosis. Logistic regression analysis showed that age, ALB level, and APACHE II score were independent prognostic factors. The formula of age combined ALB level and APACHE II score was Y = X1-3.6X2 + 6.5X3 (X1 was the age, X2 was the ALB level and X3 was the APACHE II score), Y was positively correlated with poor prognosis, and the optimal cutoff value was Y = 40.96.
Conclusion: Age, ALB level, and APACHE II score are independent factors that influencing the prognosis of patients with intra-abdominal infection, and the combination of age, ALB level, and APACHE II score can better assess the prognosis of patients with intra-abdominal infection.
Keywords: intra-abdominal infection, formula, prognostic factors, the value of independent factors, single center
Introduction
Intra-abdominal infection refers to infectious diseases caused by pathogenic bacteria invading the abdominal cavity, including infections of the abdominal cavity, retroperitoneum, and abdominal organs,1,2 involving many diseases (such as acute cholecystitis and biliary system infection, liver abscess, peritonitis, Acute pancreatitis complicated by pathogen infection, appendicitis, post-abdominal infection, etc.), the severity of the disease varies, and the severity of the disease often depends on the spread of inflammation.3 Some studies have pointed out that the mortality rate of abdominal infection is 10.5%,4 and some reports have exceeded this value,5 even more than 50% for elderly and critical cases.6 Timely and effective control and treatment of the source of infection, and rational use of antimicrobial drugs are beneficial to the prognosis. In the past, it was believed that antibacterial drugs should be treated in sufficient quantity and for a long period. However, more and more scientific researches now point out that short-term antibiotic treatment is equally effective and can significantly reduce antibiotic exposure, prolonged use of antibacterial drugs has not brought clinical benefit.7–9
The distribution of pathogenic bacteria in patients with intra-abdominal infection is not the same in different countries and regions,10–12 and the initial selection of anti-infection programs by clinicians in each region is different, and the distribution spectrum of pathogens is constantly being induced and changed, which makes the differences between different regions increase. As a clinician, it is very necessary to understand the distribution of pathogens in their area, because only by accurately knowing the distribution of pathogens can they make a correct judgment on the selection of initial anti-infection schemes. In the area where our hospital is located, we do not know the distribution of pathogenic bacteria causing intra-abdominal infection. We believe that it is necessary to analyze and report the distribution of pathogenic bacteria in patients with intra-abdominal infections in our hospital in recent years.
Intra-abdominal infections vary in severity and prognosis. Preoperative weight loss, obesity, liver cirrhosis, long operation time, low serum albumin (ALB) level, and long drainage tube indwelling time are all independent risk factors for intra-abdominal infection,13–16 and in patients with abdominal infection However, there are few reports on the factors affecting the prognosis of intra-abdominal infection and the evaluation value of these factors on the prognosis. This article aims to explore the distribution of pathogenic bacteria in patients with intra-abdominal infection, clarify the independent factors affecting the prognosis of patients with intra-abdominal infection and their prognostic evaluation value, clinically screen patients with poor prognosis and high-risk patients, to implement timely and effective targeted treatment and improve patient prognosis.
Patients and Methods
General Materials
The age, gender, underlying disease, pathogenic microorganisms, and relevant clinical data on the day of infection of patients with intra-abdominal infection in our hospital from January 2012 to December 2019 were collected, and the APACHE II score was calculated. Taking the patient’s ICU discharge or death as the time node, the patients were divided into survival group and death group according to the different prognosis of patients. All procedures performed in studies involving human participants were in accordance with the ethical standards of the the First Affiliated Hospital of Anhui Medical University research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Data Collection
Clinically confirmed intra-abdominal infection, and age ≥18 years old. Diagnostic criteria for intra-abdominal infection: fever (high fever >39°C), abdominal pain, abdominal distension or ascites; abdominal tenderness with or without rebound pain; ascites or other abdominal cavity specimens microbiological culture examination was positive. Exclusion criteria: 1) Under 18 years of age; 2) The latter cannot be predicted due to the patient’s factors (such as withdrawal in the middle of the treatment, etc.); 3) without surgical interventions; 4) with microbiological culture negative. For the part of the treatment, empirical medication was used before the microbiological culture results. Medication was adjusted according to the drug sensitivity results.
Statistical Analysis
SPSS19.0 software was used for data statistics. The quantitative variables of normal distribution were expressed as mean ± standard deviation (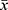 ±s). t-test was used for homogeneity of variance and t ‘test for variance heterogeneity; Quantitative variables of skew distribution were expressed as median (quartile) [M(Q25, Q75)] and rank sum test was used; categorical variables were expressed as a rate (number), using
±s). t-test was used for homogeneity of variance and t ‘test for variance heterogeneity; Quantitative variables of skew distribution were expressed as median (quartile) [M(Q25, Q75)] and rank sum test was used; categorical variables were expressed as a rate (number), using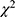 test; multivariate analysis using Spearman correlation Analysis and Logistic regression analysis; logistic regression formula was used as a model to establish a new formula for independent prognostic factors; ROC curve was drawn for independent prognostic factors, The AUC of each independent prognostic factor was compared to determine its prognostic value. The difference was statistically significant (P < 0.05).
test; multivariate analysis using Spearman correlation Analysis and Logistic regression analysis; logistic regression formula was used as a model to establish a new formula for independent prognostic factors; ROC curve was drawn for independent prognostic factors, The AUC of each independent prognostic factor was compared to determine its prognostic value. The difference was statistically significant (P < 0.05).
Results
General Situation and Pathogenic Microorganism Results
282 patients were included in the statistics, including 183 males and 99 females, aged 56.99±15.34 years old. A total of 343 strains of non-repetitive pathogenic bacteria were collected, of which 62.10% (213 strains) of gram-negative bacteria, 34.40% (118 strains) of gram-positive bacteria, and 3.50% (12 strains) of fungi. The top five pathogens are Escherichia coli (22.45%), Klebsiella pneumonia (11.08%), Acinetobacter baumannii (8.16%), Enterococcus faecium (8.16%), and Enterococcus faecalis (5.25%, Table 1).
 |
Table 1 Distribution and Composition of 343 Strains of Pathogens Causing Intra-Abdominal Infection |
Comparison of the Basic Situation
According to the different prognoses, 218 cases were divided into the survival group and 64 cases in the death group. The total mortality of abdominal infection was 22.70%. There were differences between the survival group and the death group in age, serum ALB level, APACHE II score, underlying cardiovascular disease or diabetes (P<0.05); There was no statistical difference between the two groups in white blood cell (WBC) count, procalcitonin (PCT), C-reactive protein (CRP), and cancer (P>0.05). Different types of pathogen infections did not affect the prognosis of patients (P>0.05, Table 2).
 |
Table 2 Comparison of General Data Between Survival Group and Death Group |
Research on the Correlation of Each Indicator
In order to clarify the correlation between various indicators and prognosis, age, ALB level, APACHE II score, underlying cardiovascular disease, and diabetes were included in the Spearman correlation analysis. The results showed that ALB level was negatively correlated with poor prognosis, while age, APACHE II score, Basic cardiovascular disease, diabetes, etc. are positively correlated with poor prognosis.
Predictors of Patient Prognosis
Select statistically significant age, ALB level, underlying cardiovascular disease, diabetes, and APACHE II score in the survival group and death group for multivariate logistic regression analysis. The results showed that age, ALB level, and APACHE II score were independent factors influencing the prognosis of patients (P < 0.05). In order to combine age, ALB level, and APACHE II score to evaluate the prognosis of patients, the logistic regression equation formula ln(p/(1-p)=aX1+bX2+cX3+d was used as the model, and this formula was transformed into (ln(p/ (1-p)-d)/a=X1+b/a*X2+c/a*X3 (X1 was the age, X2 was the ALB level and X3 was the APACHE II score), let Y=(ln(p/(1-p)-d)/a, the new formula is Y= X1+b/a*X2+c/a*X3. Use Logistic regression analysis to obtain regression coefficients for independent prognostic factors (Table 3), and substitute them into the formula Then Y = X1-3.7X2+7.1X3 (X1 was the age, X2 was the ALB level and X3 was the APACHE II score), Spearman correlation analysis shows that Y (ie, age combined with ALB level and APACHE II score) was positively correlated with poor prognosis.
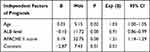 |
Table 3 Logistic Regression Analysis of Independent Prognostic Factors in Patients with Intra-Abdominal Infection |
ROC Curve of Age, ALB Level, APACHE II Score, and the Combination of the Three Factors for Prognosis Evaluation
The optimal cut-off values of age, ALB level, APACHE II score, and the combination of the three factors Y were 64.5 years, 26g/L, 12.5 points, and 40.96, respectively. The AUC was 0.624, 0.698, 0.807 and 0.842, respectively. The sensitivity was 56.30%, 57.80%, 79.70% and 89.10%, respectively. The specificity was 69.30%, 75.20%, 68.80% and 65.10%, respectively. (Table 4 and Figure 1).
 |
Table 4 The Evaluation Value of Age, ALB Level, APACHE II Score, and the Combination of the Three on the Prognosis of Patients with Intra-Abdominal Infection |
 |
Figure 1 The ROC curve of age, ALB level, APACHE II score, and Y for prognostic evaluation of patients with intra-abdominal infection. |
Test the AUC of Each Independent Prognostic Factor
The AUC of Y was compared with that of age, ALB level, and APACHE II score by z-test. The results showed that Y’s AUC for the poor prognosis of patients with intra-abdominal infection was greater than those of age, ALB, and APACHE II for the prognosis of patients with abdominal infection, and the differences were statistically significant (P<0.05), (Table 5).
 |
Table 5 The Comparison of Area Under ROC Curve |
Discussion
Intra-abdominal infections include infections of the abdominal cavity, retroperitoneum, and abdominal organs.1,2 The distribution of pathogenic bacteria in intra-abdominal infection is different in different countries or regions, and the overall prognosis of patients is also different,17,18 the prognosis of patients infected with different pathogens is also different.19,20 Timely control of the source of infection and appropriate antibacterial treatment is beneficial to the prognosis.21,22 The top five pathogens in our hospital are Escherichia coli (22.45%), Klebsiella pneumoniae (11.08%), Acinetobacter baumannii (8.16%), Enterococcus faecium (8.16%), and Enterococcus faecalis (5.25%). At the same time, our study found that which pathogenic bacteria are infected by patients with abdominal cavity infection does not affect the prognosis of patients.
For patients with underlying cardiovascular diseases and diabetes, the risk of infection is high, and it is difficult to control after infection,23–25 and the length of hospital stay is a factor that affects the prognosis of patients, but it is not an independent factor that affects the final prognosis of patients. In all abdominal cavities In infected patients, indicators of infection, such as white blood cell count, CRP, and PCT, were all higher than normal. CRP is an acute time item protein synthesized during infection or tissue injury,26 which can be increased by 1000 times in infection and inflammation.27 Our study found that the level of CRP is not related to the prognosis of patients with intra-abdominal infection, and Miguel-Bayarri et al28 is consistent with our study. As a biomarker for the diagnosis of infection and sepsis, PCT has been widely used in clinical practice.29,30 We found that there was no statistical difference between the death group and the survival group in PCT, which was different from that of some researchers,31,32 which may be due to the different times of clinical specimen collection. The clinical indicators measured in this study were all measured on admission or the day of definite infection. The time of obtaining specimens was earlier, and the difference between the two groups may not be obvious. According to our observation, both increased in the early stage of intra-abdominal infection, but there was no difference between the death group and the survival group. Dynamic monitoring of changes in these indicators may be a more reasonable method. Some studies have pointed out that dynamic monitoring of PCT changes can help assess the recovery of patients,33 and calculating clearance rates such as PCT is also a good method.34
This study found that age is an independent risk factor for the poor prognosis of patients with intra-abdominal infection. With the increase of age, the metabolism gradually slows down, the function of various organs gradually declines, and the compensatory ability is weak after the infection and the death rate increases. However, the AUC of the ROC curve for assessing the prognosis of patients based on age alone is only 0.626, and the sensitivity is only 56.45%, so the prognosis is very limited, some studies have even shown that age does not affect the prognosis of patients with intra-abdominal infection.35 The APACHE II score is a scoring system used to assess the severity of a patient’s condition and estimate the risk of death,36,37 This scoring system is widely used in clinical practice. The higher the score, the more severe the patient’s condition and the higher the risk of death during hospitalization.38 Our study further confirmed this conclusion. APACHE II score was an independent factor affecting the prognosis. ROC curve showed that the optimal cut-off value was 12.5, AUC was 0.807, the sensitivity was 79.70%, and the specificity was 68.80%. Because Spearman correlation analysis shows that APACHE II score was positively correlated with poor prognosis, that is, APACHE II scores of 13 points and above indicate a poor prognosis for patients. This score is relatively good for evaluating the prognosis of patients with intra-abdominal infection.
ALB is a protein synthesized by the liver with a half-life of about 18 to 19 days.39 It is an independent factor that affects the prognosis of patients with intra-abdominal infections. It plays an important role in maintaining colloidal osmotic pressure, combining and transporting internal and external substances, scavenging free radicals, and maintaining acid-base balance.40 Excessive consumption of protein during infection, the body preferentially synthesizes acute-time proteins (such as C-reactive protein), inflammatory mediators affect ALB synthesis, and increased capillary permeability lead to ALB leakage,41 all make ALB levels progressively decrease. For every 10g/L decrease in ALB level in patients with hypoproteinemia, the risk of death increases by 137%.39 Spearman correlation analysis showed that ALB level was negatively correlated with poor prognosis of patients, that is, the lower the ALB level, the higher the risk of death in patients. As a carrier for the transportation of internal and external substances, many drugs require ALB to be transported in the body, which is one of the important factors affecting the prognosis of the disease.42 Our study showed that the optimal cut-off value of ALB level for prognosis assessment was 26g/L, that is, ALB level below 26g/L indicates a poor prognosis for patients. Since ALB can maintain colloidal osmotic pressure, it can increase hypertension and improve organ perfusion in severe sepsis,43 but a large amount of supplementation will increase the cardiac load, especially for patients with underlying heart diseases, which will increase the risk of a cardiovascular accident. To control the ALB level in what range can benefit patients outweigh the risks still needs follow-up research. As a protein synthesized by the liver, ALB reflects the synthetic function of the liver. The patients in the death group are older, more patients with cardiovascular disease and diabetes, and the ability of organic synthesis of ALB is reduced. Whether exogenous ALB can improve the prognosis of patients is still a question worthy of discussion. Some studies believe that the use of ALB for sepsis resuscitation can reduce patient mortality,44 and some studies have pointed out that supplementing ALB above 30g/L does not improve patient prognosis.45 While actively giving resuscitation, how to improve the patient’s liver synthesis function is a problem that needs to be considered by clinicians.
To combine age, ALB level, and APACHE II score to evaluate the prognosis of patients with intra-abdominal infection, we established a new formula based on the Logistic regression formula, namely Y = X1-3.7X2+7.1X3 (X1 was the age, X2 was the ALB level and X3 was the APACHE II score), the optimal cut-off value of Y was 40.96 and Y was positively correlated with poor prognosis, that is, Y greater than 40.96 indicates a poor prognosis. The ROC curve showed that the AUC of Y for poor prognosis assessment of patients with intra-abdominal infection was 0.842, which was higher than age, ALB level, and APACHE II score to assess the prognosis separately, and the difference was statistically significant, therefore, Y had the highest value in evaluating the prognosis of patients with intra-abdominal infection.
The shortcomings of this study are as follows: 1). The samples collected in this study were data from the day the patients were clinically diagnosed as having an intra-abdominal infection, that is, if the patients were admitted to the hospital due to infection, the data on the day of admission were collected; if the infection occurred in the hospital, the data on the day of clinical diagnosis of the infection were collected. There were patients transferred to our hospital due to poor diagnosis and treatment results in lower-level hospitals in the samples, since the interval between transfer time and infection time varies in each sample, the results of the study may be biased. 2). The sample size of the data in this study was relatively small and was a single-center study. Whether the optimal cut-off value we obtained was also effective in other centers has not been verified. Large samples and multi-center studies are still needed.
Conclusions
In summary, age, ALB level, and APACHE II score are independent factors for evaluating the prognosis of patients with intra-abdominal infection. The combination of age, ALB level, and APACHE II score can better identify high-risk patients and evaluate the prognosis of patients, and then actively give targeted treatment may improve the prognosis of patients.
Ethics Approval and Consent to Participate
This observational study was conducted according to the terms and regulations of the local institutional review boards (Anhui Medical University, Hefei, Anhui, agreement; AHMU 202211). According to Chinese Law, no informed consent was required since this observational study did not modify the physician’s treatment decisions.
Acknowledgments
This project was supported by Beijing Postdoctoral Research Foundation (2020-ZZ-008), Returned overseas students from Anhui Province in 2020 Innovation and Entrepreneurship Support Plan Project (2020LCX016), University Natural Science Research Project of Anhui Province (KJ2020A0172), and Cultivate Funding of the National Natural Science Foundation of China (2016KJ12).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
1. Zhang S, Huang W. Epidemiological study of community- and hospital-acquired intraabdominal infections. Chin J Traumatol. 2015;18(2):84–89. doi:10.1016/j.cjtee.2015.07.003
2. Han G, Qiao X, Ma Z. Diagnosis and treatment of intra-abdominal infection complicated with hypothyroidism. Zhonghua Wei Chang Wai Ke Za Zhi. 2018;21(12):1356–1360.
3. Menichetti F, Sganga G. Definition and classification of intra-abdominal infections. J Chemother. 2009;21(Suppl 1):3–4. doi:10.1179/joc.2009.21.Supplement-1.3
4. Sartelli M, Catena F, Ansaloni L, et al. Complicated intra-abdominal infections worldwide: the definitive data of the CIAOW Study. World J Emerg Surg. 2014;9:37. doi:10.1186/1749-7922-9-37
5. Blot S, Antonelli M, Arvaniti K, et al. Epidemiology of intra-abdominal infection and sepsis in critically ill patients: “AbSeS”, a multinational observational cohort study and ESICM trials group project. Intensive Care Med. 2019;45(12):1703–1717. doi:10.1007/s00134-019-05819-3
6. Dupont H, Friggeri A, Touzeau J, et al. Enterococci increase the morbidity and mortality associated with severe intra-abdominal infections in elderly patients hospitalized in the intensive care unit. J Antimicrob Chemother. 2011;66(10):2379–2385. doi:10.1093/jac/dkr308
7. Montravers P, Tubach F, Lescot T, et al. Short-course antibiotic therapy for critically ill patients treated for postoperative intra-abdominal infection: the DURAPOP randomised clinical trial. Intensive Care Med. 2018;44(3):300–310. doi:10.1007/s00134-018-5088-x
8. Rattan R, Allen CJ, Sawyer RG, et al. Patients with complicated intra-abdominal infection presenting with sepsis do not require longer duration of antimicrobial therapy. J Am Coll Surg. 2016;222(4):440–446.
9. Mazuski JE, Tessier JM, May AK, et al. The surgical infection society revised guidelines on the management of intra-abdominal infection. Surg Infect (Larchmt). 2017;18(1):1–76. doi:10.1089/sur.2016.261
10. Shree N, Arora BS, Mohil RS, Kasana D, Biswal I. Bacterial profile and patterns of antimicrobial drug resistance in intra-abdominal infections: current experience in a teaching hospital. Indian J Pathol Microbiol. 2013;56(4):388–392. doi:10.4103/0377-4929.125321
11. Zhang S, Ren L, Li Y, et al. Bacteriology and drug susceptibility analysis of pus from patients with severe intra-abdominal infection induced by abdominal trauma. Exp Ther Med. 2014;7(5):1427–1431. doi:10.3892/etm.2014.1609
12. Takesue Y, Kusachi S, Mikamo H, et al. Antimicrobial susceptibility of common pathogens isolated from postoperative intra-abdominal infections in Japan. J Infect Chemother. 2018;24(5):330–340. doi:10.1016/j.jiac.2018.02.011
13. Tang H, Lu W, Yang Z, et al. Risk factors and long-term outcome for postoperative intra-abdominal infection after hepatectomy for hepatocellular carcinoma. Medicine (Baltimore). 2017;96(17):e6795.
14. Rungsakulkij N, Vassanasiri W, Tangtawee P, et al. Preoperative serum albumin is associated with intra-abdominal infection following major hepatectomy. J Hepatobiliary Pancreat Sci. 2019;26(11):479–489. doi:10.1002/jhbp.673
15. Xiao H, Xiao Y, Quan H, Liu W, Pan S, Ouyang Y. Intra-abdominal infection after radical gastrectomy for gastric cancer: incidence, pathogens, risk factors and outcomes. Int J Surg. 2017;48:195–200.
16. Jia L, Lu J, Ma X. [Analysis of risk factors of intra-abdominal infection after surgery for colorectal cancer]. Zhonghua Wei Chang Wai Ke Za Zhi. 2016;19(4):409–413. Chinese.
17. Sánchez-Velázquez P, Pera M, Jiménez-Toscano M, et al. Postoperative intra-abdominal infection is an independent prognostic factor of disease-free survival and disease-specific survival in patients with stage II colon cancer. Clin Transl Oncol. 2018;20(10):1321–1328. doi:10.1007/s12094-018-1866-8
18. Tu RH, Lin JX, Desiderio J, et al. Does intra-abdominal infection after curative gastrectomy affect patients’ long-term prognosis? A multi-center study based on a large sample size. Surg Infect (Larchmt). 2019;20(4):271–277. doi:10.1089/sur.2018.246
19. Sanders JM, Tessier JM, Sawyer R, et al. Does isolation of enterococcus affect outcomes in intra-abdominal infections? Surg Infect (Larchmt). 2017;18(8):879–885. doi:10.1089/sur.2017.121
20. Würstle S, Hapfelmeier A, Wöhrle C, et al. Changes in pathogen spectrum and antimicrobial resistance development in the time-course of acute necrotizing pancreatitis. J Gastroenterol Hepatol. 2019;34(12):2096–2103. doi:10.1111/jgh.14748
21. Sartelli M, Weber DG, Ruppé E, et al. Antimicrobials: a global alliance for optimizing their rational use in intra-abdominal infections (AGORA). World J Emerg Surg. 2016;11:33.
22. Bassetti M, Eckmann C, Giacobbe DR, Sartelli M, Montravers P. Post-operative abdominal infections: epidemiology, operational definitions, and outcomes. Intensive Care Med. 2020;46(2):163–172. doi:10.1007/s00134-019-05841-5
23. Pearson-Stuttard J, Blundell S, Harris T, Cook DG, Critchley J. Diabetes and infection: assessing the association with glycaemic control in population-based studies. Lancet Diabetes Endocrinol. 2016;4(2):148–158. doi:10.1016/S2213-8587(15)00379-4
24. Kim EJ, Ha KH, Kim DJ, Choi YH. Diabetes and the risk of infection: a national cohort study. Diabetes Metab J. 2019;43(6):804–814. doi:10.4093/dmj.2019.0071
25. Abu-Ashour W, Twells LK, Valcour JE, Gamble JM. Diabetes and the occurrence of infection in primary care: a matched cohort study. BMC Infect Dis. 2018;18(1):67. doi:10.1186/s12879-018-2975-2
26. Andersen SB, Baunbæk Egelund G, Jensen AV, Petersen PT, Rohde G, Ravn P. Failure of CRP decline within three days of hospitalization is associated with poor prognosis of community-acquired pneumonia. Infect Dis (Lond). 2017;49(4):251–260.
27. Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754. doi:10.3389/fimmu.2018.00754
28. Miguel-Bayarri V, Casanoves-Laparra EB, Pallás-Beneyto L, et al. Prognostic value of the biomarkers procalcitonin, interleukin-6 and C-reactive protein in severe sepsis. Med Intensiva. 2012;36(8):556–562. doi:10.1016/j.medin.2012.01.014
29. Reinhart K, Bauer M, Riedemann NC, Hartog CS. New approaches to sepsis: molecular diagnostics and biomarkers. Clin Microbiol Rev. 2012;25(4):609–634.
30. Bartoletti M, Antonelli M, Bruno Blasi FA, et al. Procalcitonin-guided antibiotic therapy: an expert consensus. Clin Chem Lab Med. 2018;56(8):1223–1229. doi:10.1515/cclm-2018-0259
31. Zhang J, Qu D, Ren XX. Value of procalcitonin in predicting the severity and prognosis of neonates with septicemia. Zhonghua Yi Xue Za Zhi. 2018;98(16):1267–1272.
32. Cabral L, Afreixo V, Meireles R, et al. Checking procalcitonin suitability for prognosis and antimicrobial therapy monitoring in burn patients. Burns Trauma. 2018;6:10. doi:10.1186/s41038-018-0112-5
33. Branche A, Neeser O, Mueller B, Schuetz P. Procalcitonin to guide antibiotic decision making. Curr Opin Infect Dis. 2019;32(2):130–135. doi:10.1097/QCO.0000000000000522
34. Biron BM, Ayala A, Lomas-Neira JL. Biomarkers for sepsis: What is and what might be? Biomark Insights. 2015;10(Suppl 4):7–17.
35. Farmer D, Tessier JM, Sanders JM, et al. Age and its impact on outcomes with intra-abdominal infection. Surg Infect (Larchmt). 2017;18(2):77–82. doi:10.1089/sur.2016.184
36. Rojek-Jarmula A, Hombach R, Krzych LJ. APACHE II score predicts mortality in patients requiring prolonged ventilation in a weaning center. Anaesthesiol Intensive Ther. 2016;48(4):215–219. doi:10.5603/AIT.a2016.0036
37. Liu X, Shen Y, Li Z, et al. Prognostic significance of APACHE II score and plasma suPAR in Chinese patients with sepsis: a prospective observational study. BMC Anesthesiol. 2016;16(1):46. doi:10.1186/s12871-016-0212-3
38. Godinjak A, Iglica A, Rama A, et al. Predictive value of SAPS II and APACHE II scoring systems for patient outcome in a medical intensive care unit. Acta Med Acad. 2016;45(2):97–103. doi:10.5644/ama2006-124.165
39. Vincent JL, Russell JA, Jacob M, et al. Albumin administration in the acutely ill: what is new and where next? Crit Care. 2014;18(4):231. doi:10.1186/cc13991
40. Strul J, Braham DL. Is albumin administration beneficial in patients with sepsis? Br J Hosp Med (Lond). 2016;77(11):666. doi:10.12968/hmed.2016.77.11.666
41. Caironi P, Gattinoni L. The clinical use of albumin: the point of view of a specialist in intensive care. Blood Transfus. 2009;7(4):259–267.
42. Artero A, Zaragoza R, Camarena JJ, Sancho S, González R, Nogueira JM. Prognostic factors of mortality in patients with community-acquired bloodstream infection with severe sepsis and septic shock. J Crit Care. 2010;25(2):276–281. doi:10.1016/j.jcrc.2009.12.004
43. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304–377.
44. Delaney AP, Dan A, McCaffrey J, Finfer S. The role of albumin as a resuscitation fluid for patients with sepsis: a systematic review and meta-analysis. Crit Care Med. 2011;39(2):386–391. doi:10.1097/CCM.0b013e3181ffe217
45. Caironi P, Tognoni G, Masson S, et al. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med. 2014;370(15):1412–1421. doi:10.1056/NEJMoa1305727
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
