Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 16
Factors Influencing the Length of Hospital Stay Among Patients with Chronic Obstructive Pulmonary Disease (COPD) in Macao Population: A Retrospective Study of Inpatient Health Record
Authors Li M , Cheng K, Ku K, Li J, Hu H , Ung COL
Received 18 February 2021
Accepted for publication 4 May 2021
Published 9 June 2021 Volume 2021:16 Pages 1677—1685
DOI https://doi.org/10.2147/COPD.S307164
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Meng Li,1,* Kun Cheng,2,* Keisun Ku,2 Junlei Li,1 Hao Hu,1 Carolina Oi Lam Ung1
1State Key Laboratory of Quality Research in Chinese Medicine, Institute of Chinese Medical Sciences, University of Macau, Macao SAR, China; 2Internal Medicine Department, Kiang Wu Hospital, Macao SAR, China
*These authors contributed equally to this work
Correspondence: Hao Hu; Carolina Oi Lam Ung
State Key Laboratory of Quality Research in Chinese Medicine, Institute of Chinese Medical Sciences, University of Macau, N22-2058, Macao SAR, China
Tel +853 8822 8538
Email [email protected]; [email protected]
Purpose: This study aims to identify the effects of patient and clinical therapy factors on the length of hospital stay (LOS) for admission due to chronic obstructive pulmonary disease (COPD) in Macao.
Patients and Methods: Health record of patients with COPD admitted to Kiang Wu Hospital from January 2017 to December 2019 was retrospectively analyzed. Demographic information, blood test results, clinical therapies, and LOS were described and analyzed by multivariable regression.
Results: A total of 1116 admissions were included with the average LOS being 12.28 (± 9.23) days. Among them, 735 (66.6%) were male with mean age 79.42 (± 10.35) years old, 697 were current or previous smokers (62.5%), and 360 (32.2%) had 3 or more comorbidities. During hospitalization, the most common treatments received were oxygen therapy (n=991,88.8%), antibiotics (n=828,74.2%), and systemic steroids (n=596,53.4%); only 120 (10.8%) had pulmonary rehabilitation (PR) and 128 (11.5%) received noninvasive ventilation (NIV). Inhaled medications were used during nearly 95% of hospitalization cases, while 2 and 3 types of inhaled medications were used during 230 (20.6%) and 582 (52.2%) hospitalization cases, respectively. Patient factors including age (B=0.178, 95% CI:0.535– 1.072), being female (B=− 1.147, 95% CI:-0.138– 0.056), being current (B=− 0.086, 95% CI:-0.124– 0.018) or previous smoker (B=0.072, 95% CI:0.004– 0.087), having 1, 2, 3 and over 3 comorbidities (B=0.126, 95% CI:0.034– 0.147; B=0.125, 95% CI:0.031– 0.144; B=0.116, 95% CI:0.028– 0.146, B=0.090, 95% CI:0.021– 0.166) and having low hemoglobin level (B=− 0.118, 95% CI:-0.629- − 0.214) exhibited significant associations with LOS. The use of NIV (B=0.080, 95% CI:0.022– 0.138), pulmonary rehabilitation (B=0.269, 95% CI:0.212– 0.327), two and three types of inhaled medications (B=0.109, 95% CI:0.003– 0.166, B=0.255, 95% CI:0.083– 0.237) were significantly associated with longer LOS (P< 0.05).
Conclusion: NIV, PR and combined inhaled medications, which are often used for AECOPD, are the main clinical therapies associated with longer LOS in Macao. Smoking cessation, early treatments of comorbidities may be crucial to avoiding AECOPD and reducing LOS and disease burden.
Keywords: chronic obstructive pulmonary disease, Macao, length of hospital stay, blood test results, clinical therapy, inhaled medications, acute exacerbation
Introduction
Chronic obstructive pulmonary disease (COPD) is a major chronic disease characterized by persistent airflow limitation. Currently being the fifth leading cause of death worldwide, COPD is predicted to become the third by 2030.1 From 1990 to 2015, there was a 44.2% increase of COPD prevalence globally, and the number of patients reached 17.5 million in 2015.2 COPD also posed substantial economic and social burden on both patients and healthcare systems3,4 It has been reported that in China the direct medical costs of COPD ranged from 72 to 3565 USD per capita per year, accounting for 33.33% to 118.09% of local average annual income.5 Another study showed that the direct costs of COPD accounted for 56% of the total costs of respiratory diseases (about 6% of the total annual health care budget) in the European Union.6 Notably, nearly 35% to 84% of the COPD medical costs were due to acute exacerbations (AECOPD).7–9 It was found that hospitalized COPD patients were prone to decreased quality of life, high hospital costs, increased mortality, and a long length of hospital stay (LOS).10,11
LOS is a key indicator of COPD patients medical resource use and hospitalization costs.12,13 A meta-analysis reported that an increase in LOS was one of the significant risk factors for 30- and 90-day all-cause readmission.14 LOS may be affected by a range of factors such as age, history of tobacco smoking, Charlson index, cause of exacerbations, and therapies-related variables.15–17 Key therapies in COPD clinical practice included smoking cessation, vaccinations, pharmacological therapy, pulmonary rehabilitation (PR), oxygen therapy, and non-invasive ventilation (NIV).18,19 Regarding pharmacological therapy, the most common drug classes used for managing and preventing COPD symptoms and exacerbations were inhaled bronchodilators and/or corticosteroids (ICS), systemic steroids and antibiotics.18,20 Exploring these LOS-related factors can help inform clinical practice about how to reduce LOS, thereby alleviating the disease burden of COPD patients.
The analysis of characteristics of COPD inpatients and contributing factors to LOS is currently under-reported, especially among Asian populations. Therefore, this study aims to identify the effects of different patient and clinical therapy factors on LOS for COPD-related admissions. By addressing the gaps in COPD-related research in Macao, the study findings is expected to supplement the current demographic and clinical evidence for the study of COPD inpatients in Asia, thereby contributing to a more comprehensive reference for the management of COPD.
Methods
Data Collection
COPD is one of the top-ten causes of death in the past decade in Macao. Statistics showed that 14% local manpower was influenced by a second-hand cigarette in smoking permitted gambling casinos before October 2014, which potentially increased the morbidity and mortality of COPD in Macao.21,22 We retrospectively examined the COPD inpatient health record dated between 2017 and 2019 in Kiang Wu Hospital, which is one of the 3 major hospitals in Macao with 1928 staffs and 775 hospital beds (47% of the total in Macao).23
This dataset contained all COPD-related admissions to Kiang Wu Hospital from January 1, 2017 to December 31, 2019. All medical records were collected from the hospital’s electronic medical record system. Three inclusion criteria were applied: (1) patients were admitted with a main diagnosis of COPD (ICD-10 code J44), (2) all records were confirmed by the specialists as admission due to acute exacerbation, and (3) there was no missing data among all the included admission items. This study used data on hospitalization information per patient admission.
The study was approved by the ethical review committee at the Kiang Wu Hospital [Ref. No. 2019–015]. All data were collected retrospectively from the hospital electronic medical record system and fully anonymized in a standardized case report form to ensure data confidentiality. As such, written consent from the patients or their guardians was exempted and this study was carried out in full compliance with the Helsinki Declaration.
Variables and Measurements
The data was divided into 4 categories including demographic data, blood test results, clinical therapies, and LOS. Demographic data included patient’s age, gender, history of tobacco smoking and number of comorbidities. Blood test results included blood eosinophil count, hemoglobin, white blood cells (WBC) and creatinine. Eosinophil count is usually used as the biomarkers to predict and drive treatment choices in inflammatory lung disease like COPD.24,25 Hemoglobin, the main protein in red blood cells, can be used to evaluate the oxygen-carrying capacity and help diagnose anemia, and is also an independent prognostic predictor of premature mortality and a higher likelihood of hospitalization in COPD patients.26,27 WBC is the circulating leukocytes I whole blood and is also one of inflammatory biomarkers for the prediction of mortality in COPD.28 Measurement of creatinine is to assess renal function. Afzal, A et al found there was a significant relationship between the admission creatinine values and mortality of COPD.29 All blood tests were carried out at the time of admission to provide references for diagnosis and to assess COPD disease status and inform treatment plan.30 Regarding the clinical therapies for COPD in Macao, data about the usage of recommended therapies including inhaled medications, systemic steroids (prednisolone, dexamethasone, methylprednisolone), antibiotics, oxygen therapy, NIV and PR during hospitalization were extracted.18,31
Based on the use of inhaled COPD medications of COPD, the patients were assigned to 1 of the 4 groups: Group 1 included the patients who used only one type of inhaled medication, such as long-acting bronchodilators (long-acting beta2 agonist (LABA) only, long-acting muscarinic antagonist (LAMA) only or both), short-acting bronchodilators (short-acting beta-agonist (SABA) only, short-acting muscarinic antagonist (SAMA) only or both) or inhaled ICS only. Group 2 included the patients who received two types of inhaled medications, such as long-acting bronchodilators (LABA, LAMA or both) combined with short-acting bronchodilators (SABA, SAMA or both), long-acting bronchodilators (LABA, LAMA or both) combined with ICS, short-acting bronchodilators (SABA, SAMA or both) combined with ICS. Group 3 were the patients who used the combination of all 3 types of inhaled medications ((SABA, SAMA or both) and (LABA, LAMA or both) and ICS). Group 4 referred to the patients who did not use any inhaled medications during hospitalization. The LOS in this study referred to the number of days between admission and discharge.
Statistical Analysis
All information was analyzed in a descriptive manner by using median (IQR), mean (SD), and proportions. Blood test results were categorized into below normal range, normal, and above normal ranges following the reference ranges for Macao’s blood tests. To investigate the factors influencing LOS, univariate analysis and multivariate were both applied. Unary linear regression, t-tests, and one-way ANOVA were used to explore the potential associations between LOS and independent variables (demographic information, blood tests results, and clinical therapies information). Furthermore, all the significant factors were entered into the final multivariate linear regression model as independents variables. Statistical analyses were performed in SPSS 23.0. (IBM Corporation, Armonk, NY, USA) with a significance level of 0.05.
Results
From 2017 to 2019, a total of 1116 COPD admissions were included in this study. Figure 1 summarised the key steps of data analysis.
 |
Figure 1 Key steps of data analysis in this study. |
Descriptive Analysis
Demographic Characteristics and Blood Test results
Demographic information including gender, age, history of tobacco smoking and number of comorbidities was summarized in Table 1. Among the patients, the average age was 79.42 (±10.35) years old and 66.6% of them were male. It was found that 37.5% cases never smoked, and 45.0% had quitted smoking accounting for the majority of the patients. Only 13.9% cases did not have any comorbidities while 32.2% had 3 or more comorbidities. Results of blood tests showed that 24.7% of cases were with blood eosinophils ≥300 cells/μL, 60.0% had hemoglobin below normal range, 94% had a higher level of WBC compared with normal range. For creatinine, 75.6% and 73.7% of male and female cases were within the normal range.
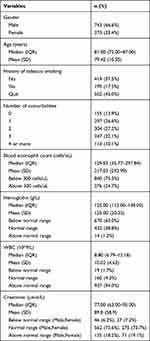 |
Table 1 Demographic Information and Blood Test Results (n=1116) |
Clinical Therapies and LOS
Among all the cases, 596 (53.4%) and 828 (74.2%) used systemic steroids and antibiotics, respectively, while 88.8% received oxygen therapy and 88.5% did not receive NIV. Only 10.8% received PR during hospitalization. Nearly 95% of the cases received inhaled medications during hospitalization, with 22% using only one type, 20.6% using two types, and 52.2% using a combination of all 3 types of inhaled medications. The average LOS was 12.28 (±9.23) days, whereas the median value was 11.0 days. A detailed summary is presented in Table 2.
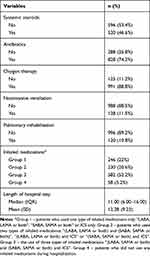 |
Table 2 Descriptive Analysis of Clinical Therapies and Length of Hospital Stay |
Multivariable Regression Analysis on LOS
LOS was a non-normal dependent variable (Skewness=3.373, Kurtosis=30.601), so log transformation was used (Skewness=−0.400, Kurtosis=0.234). Univariate analysis was firstly applied to identify potential factors. Then selected significant factors were included into the final multiple regression model.
Age (B =0.285 P<0.001), gender (t=6.173, P<0.001), history of tobacco smoking (F=22.145, P<0.001), number of comorbidities (F=6.559, P<0.001), blood eosinophil count (B =−0.071, P=0.024), hemoglobin (B =−0.212, P<0.001), use of systemic steroids (t=−2.807, P=0.038), oxygen therapy (t=−0.179, P<0.001), NIV (t=−7.668, P<0.001), PR (t=−14.955, P<0.001) and inhaled medications (F=17.290, P<0.001) during hospitalization were all associated with LOS. All these 10 factors were selected into the final multivariable regression model.
Table 3 summarized the results of univariate analysis and multivariable regression (F=24.401, p<0.001). Age (B=0.178, 95% CI: 0.535–1.072, P<0.001), gender (B=−1.147, 95% CI: −0.138–0.056, P<0.001), hemoglobin (B=−0.118, 95% CI: −0.629- −0.214, P<0.001), NIV (B=0.067, 95% CI: 0.009–0.124, P=0.023) and PR (B=0.232, 95% CI: 0.181–0.297, P<0.001) had statistically significant associations with LOS. For tobacco smoking, people who still smoked at admission (B=−0.086, 95% CI:-0.124–0.018, P=0.08) or people who quitted smoking prior to admission (B=0.072, 95% CI:0.004–0.087, P=0.030) had significant differences in LOS compared against people who never smoked. There were statistical differences between people who had comorbidities and people who did not have any comorbidity in LOS (B=0.126, 95% CI: 0.034–0.147, P=0.002; B=0.125, 95% CI: 0.031–0.144, P=0.002; B=0.116, 95% CI: 0.028–0.146, P=0.004; B=0.090, 95% CI: 0.021–0.166, P=0.011). Similarly, compared with patients who did not use any inhaled medications, COPD patients who used two or three types of inhaled medications had significant associations with longer LOS (B=0.109, 95% CI: 0.003–0.166, P=0.043, B=0.255, 95% CI: 0.083–0.237, P<0.001).
 |
Table 3 Significant Factors Associated with LOS |
Discussion
This is the first retrospective study investigating the influencing factors of LOS among COPD inpatients in Macao. Patients’ demographic information, blood test results, clinical therapies, and LOS were investigated separately. The average LOS was around 12 days. Age, gender, hemoglobin, history of smoking, the presence of comorbidities, and the use of NIV, PR and inhaled medications were found to be associated with an increase in LOS. The characteristics of LOS of COPD inpatients in Macao and the relationships between LOS and contributors are further discussed below.
The mean LOS among COPD patients in Macao (12.28 days) was higher than that in China (9.38 days),12 North West England (9.8 days),16 United States (5.9 days),32 and European countries (8.7 days).17 This may be partly explained by the older age of the patients in this study which found that an increase in log (age) by one-unit resulted in hospital day increase by 1.148 units. The average age of COPD inpatients was 79.42 years old in this study. This is considered high compared to the mean age of 69.9–70.4 years old among hospitalized COPD patients in the US,33 and 70.7 years old among COPD inpatients in European countries.17 In mainland China, a national cross-sectional study found that over 48% patients with severe COPD were only 70 years old or above.4
Having older COPD inpatients in Macao is not surprising considering the outlook of life expectancy and the aging population. The average life expectancy at birth in Macau in 2016–2019 was 83.8 years, and that for men and women was 80.8 years and 86.7 years, respectively.34 By 2025, the proportion of the elderly population is expected to reach 16.3% and Macau will become an aging society.35 Increasing age is prone to a progressive deterioration in lung function. Older COPD patients generally have a higher risk of acute exacerbations, a higher level of comorbid illness, longer LOS, and higher hospital cost.36 Poorer physical condition and frailty might make it harder for the patients to recover from an exacerbation.37,38 With the aging population in sight, the prevalence of severe COPD in older individuals resulting in hospitalization and negative impact on LOS will only be increasing.
Three out of 5 COPD inpatients in this study had at least one comorbidity which was similar to previous studies.39,40 The number of comorbidities was also associated with an increase in LOS reaffirming that comorbidities were important predictors of prolonged hospitalization.14,41–44 Such association might be contributed by pathophysiologic changes that compromised lung function leading to increased risks for other diseases or complications,45–47 while shared risk factors such as smoking, age, sex, and inactivity might also add to the complexity of the association between comorbidity and COPD. Collectively, the associations of age and comorbidities with LOS posed great implications to the healthcare provision drawing attention to the importance of an integrative and interdisciplinary approach for older patients.48,49 Pharmacists should be actively involved to improve adherence to pharmacotherapy and to optimize treatment outcome. Allied health care professionals such as physiotherapists are also important to support recovery and physical training.50
Regarding clinical interventions, 11.5% and 10.8% of the patients in this study used NIV and PR during hospitalization. The use prevalence of NIV and PR was consistent with what was reported about the COPD patients in UK (12.1% of COPD patients received NIV during hospitalization)51 and US (only 1.5% initiated pulmonary rehabilitation within 90 days of their hospital discharge).52 Both NIV and PR were associated with a longer LOS in this study. NIV is used to improve the blood oxygen and carbon dioxide exchange rate of the patient’s lungs to prevent endotracheal intubation and reduce mortality in severe cases.53,54 As such, NIV may be interpreted as an indirect indicator of more severe COPD, which could explain its association with a longer LOS.55
Special attention should be paid to the role of PR for COPD patients. Being a comprehensive clinical intervention for AECOPD managements,56 PR might benefit clinical outcome for the majority of the patients reducing dyspnea scale, LOS, risk of readmission and annual hospital day.57,58 The findings about PR being associated with a longer LOS in this study may be explained by the PR data being limited to each hospitalization. At present, various methods and therapies of PR have been used in COPD patients.59 Details of the PR design (components, frequency and duration) and the long-term benefits of PR for inpatients with COPD warrants further investigation in the future.
Regarding inhaled medications, 94.8% of the patients used them and 78.2% used a combination that mostly involved a ICS and either a short-acting or a long-acting beta-agonist. While the benefits of inhaled medication in reducing bronchospasm and inflammation,20 relieving ongoing breathing problems,18 and improving the overall management of COPD18,60 have been well established, the use of inhaled medications was found to be associated with a longer LOS in this study. Such results should be interpreted carefully as the need for multiple pharmacotherapy during hospitalization might at best indicate the severity of COPD. To further evaluate the association between inhaled medications and LOS, future studies should focus on the medication use and adherence prior to admission.61
At last but not least, the negative impact of cigarette smoking to the LOS and the overall COPD management should be emphasized.62,63 In this study, only 17.5% patients smoked at admission which was a relatively low ratio compared to previous findings,4,64 and 45% patients already quitted smoking which was a higher ratio compared to another study.65 While current smokers and former smokers were found to have a longer LOS,66–68 non-smokers in this study were found to have longer LOS when compared with current smokers in this study. Upon an in-depth investigation, the average age of the smoking population in this study was lower (73.4 vs 79.8 years old) and the smokers in this study might have a milder condition and so a shorter LOS. Nevertheless, smoking cessation at an early stage of COPD has been shown to improve prognosis largely69 and any efforts to prevent cigarette smoking and support smoking cessation should be reinforced continuously.
There were some limitations in this study. Firstly, due to the limitation of data, the severity of the disease, the dosage and duration of medications used by the patients during hospitalization and the cost factor was not involved in this research, which may collectively affect LOS. Furthermore, future studies may supplement the current findings by taking into account other factors that might indicate the COPD disease stages such as intensive care unit admission data, detailed information about NIV, the laboratory measurement data, oxygen saturation and the use of supplemental oxygenation data, etc. Pulmonary function test indicators such as baseline spirometry, FEV1/FVC ratio, were not considered in this study due to a limited number of tested patients, which prevented a full glimpse of the patients’ overall lung status. Despite the data limitation, this current study has provided valuable analytical results about the influencing factors of LOS among COPD patients in Macao which may serves a foundation to support future research on reducing disease burden and improving COPD management.
Conclusion
COPD patients treated with NIV, PR, 2 or 3 types of inhaled medications during hospitalization had longer LOS in Macao, which may be associated with more severe acute exacerbations before admission. Patients with COPD should avoid risk factors that cause acute exacerbations, such as smoking, more comorbidities, and low hemoglobin level. Early smoking cessation, use of inhaled medications and timely clinical treatments of other diseases such as anemia are effective interventions for COPD patients to reduce LOS and long-term disease burden.
Acknowledgments
The authors would like to acknowledge Kiang Wu Hospital and Macau Medicinal Administration Association for their support in data collection.
Author Contributions
ML, KC, COLU, and HH conceived and designed this study. KK and JL acquired and collected the data. ML, KC, JL performed data analysis and drafted the first version of the manuscript. All authors have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi:10.1164/rccm.201701-0218PP
2. Soriano JB, Abajobir AA, Abate KH; Collaborators GCRD. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5(9):691. doi:10.1016/S2213-2600(17)30293-X
3. Zhou M, Wang H, Zhu J, et al. Cause-specific mortality for 240 causes in China during 1990–2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet. 2016;387(10015):251–272. doi:10.1016/S0140-6736(15)00551-6
4. Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] Study): a National Cross-Sectional Study. Lancet. 2018;391(10131):1706–1717. doi:10.1016/S0140-6736(18)30841-9
5. Zhu B, Wang Y, Ming J, Chen W, Zhang L. Disease burden of COPD in China: a systematic review. Int J Chron Obstruct Pulmon Dis. 2018;13:1353. doi:10.2147/COPD.S161555
6. European respiratory society on behalf of the forum of international respiratory societies(FIRS). The global impact of respiratory disease, second edition; 2017. Available from: https://www.who.int/gard/publications/The_Global_Impact_of_Respiratory_Disease.pdf.
7. Izquierdo J. The burden of COPD in Spain: results from the confronting COPD survey. Respir Med. 2003;97:S61–S69. doi:10.1016/S0954-6111(03)80026-4
8. Britton M. The burden of COPD in the UK: results from the confronting COPD survey. Respir Med. 2003;97:S71–S79. doi:10.1016/S0954-6111(03)80027-6
9. Bi-jun S, Tao Z, Jun C, Yong W, Hui L. Direct economic burden of 803 patients with chronic obstructive pulmonary disease and influencing factors in Ningbo. Dis Surveill. 2015;30(4):300–304.
10. Holguin F, Folch E, Redd SC, Mannino DM. Comorbidity and mortality in COPD-related hospitalizations in the United States, 1979 to 2001. Chest. 2005;128(4):4. doi:10.1378/chest.128.4.2005
11. Soler-Cataluna J, Martínez-García MÁ, Sánchez PR, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. doi:10.1136/thx.2005.040527
12. Li M, Wang F, Chen R, et al. Factors contributing to hospitalization costs for patients with COPD in China: a retrospective analysis of medical record data. Int J Chron Obstruct Pulmon Dis. 2018;13:3349. doi:10.2147/COPD.S175143
13. Dang-Tan T, Zhang S, Tavares RV, et al. The burden of illness related to chronic obstructive pulmonary disease exacerbations in Québec, Canada. Can Respir J. 2017;2017:1–10. doi:10.1155/2017/8184915
14. Alqahtani JS, Njoku CM, Bereznicki B, et al. Risk factors for all-cause hospital readmission following exacerbation of COPD: a systematic review and meta-analysis. Eur Respir Rev. 2020;29(156):156. doi:10.1183/16000617.0166-2019
15. Luo L, Lian S, Feng C, Huang D, Zhang W. Data mining-based detection of rapid growth in length of stay on COPD patients. IEEE. 2017;254–258.
16. Agboado G, Peters J, Donkin L. Factors influencing the length of hospital stay among patients resident in Blackpool admitted with COPD: a Cross-Sectional Study. BMJ Open. 2012;2(5):5. doi:10.1136/bmjopen-2012-000869
17. Ruparel M, López-Campos JL, Castro-Acosta A, Hartl S, Pozo-Rodriguez F, Roberts CM. Understanding variation in length of hospital stay for COPD exacerbation: European COPD audit. ERJ Open Res. 2016;2(1):00034–2015. doi:10.1183/23120541.00034-2015
18. Global initiative for chronic Obstructive Lung Disease (GOLD) Committee. Global strategy for the diagnosis, management, and prevention of COPD; 2020. Available from: https://goldcopd.org/2021-gold-reports/.
19. Mirza S, Clay RD, Koslow MA, Scanlon PD. COPD guidelines: a review of the 2018 GOLD report. Mayo Clin Proc. 2018;93(10):1488–1502. doi:10.1016/j.mayocp.2018.05.026
20. Montuschi P. Pharmacological treatment of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2006;1(4):409–423. doi:10.2147/copd.2006.1.4.409
21. Government of Macao Special Administrative Region. Statistics and census service survey on manpower needs and wages – gaming industry; 2014. Available from: http://www.dsec.gov.mo/Statistic.aspx?NodeGuid=6289ca07-25cc-450b-8499-4e34765c1769.
22. Ng WI, Smith GD. Effects of a self-management education program on self-efficacy in patients with COPD: a mixed-methods sequential explanatory designed study. Int J Chron Obstruct Pulmon Dis. 2017;12:2129. doi:10.2147/COPD.S136216
23. Wikipedia. Kiang Wu Hospital; 2020. Available from: https://en.wikipedia.org/wiki/Kiang_Wu_Hospital.
24. Austin D, Pouliquen I, Yancey S, Bleeker E. A blood eosinophil count of greater than 150 cells/uL predicts sputum eosinophilia ≥ 3% in patients with severe asthma with other markers of inflammatory lung disease. In: B101 Phenotyping of Asthma in the Era of Biomarkers and Omics. American Thoracic Society; 2016:A4338–A4338.
25. Kostikas K, Brindicci C, Patalano F. Blood eosinophils as biomarkers to drive treatment choices in asthma and COPD. Curr Drug Targets. 2018;19(16):1882–1896. doi:10.2174/1389450119666180212120012
26. Yohannes AM, Ershler WB. Anemia in COPD: a systematic review of the prevalence, quality of life, and mortality. Respir Care. 2011;56(5):644–652. doi:10.4187/respcare.01002
27. Beutler E, Waalen J. The definition of anemia: what is the lower limit of normal of the blood hemoglobin concentration? Blood. 2006;107(5):1747–1750. doi:10.1182/blood-2005-07-3046
28. Celli BR, Locantore N, Yates J, et al. Inflammatory biomarkers improve clinical prediction of mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185(10):1065–1072. doi:10.1164/rccm.201110-1792OC
29. Afzal A, Heyes K, Baksi S, Khalid S. P254 Association of Low Serum Creatinine and Mortality in COPD. BMJ Publishing Group Ltd; 2019.
30. NHS. Overview of blood tests. Available from: https://www.nhs.uk/conditions/blood-tests/.
31. Chronic Obstructive Pulmonary Disease Group RDB, Chinese Medical Association. Chinese guidelines for the diagnosis and treatment of chronic obstructive pulmonary diseases (revised 2013). Chin J Med Sci. 2014;6(2):67–80.
32. Perera PN, Armstrong EP, Sherrill DL, Skrepnek GH. Acute exacerbations of COPD in the United States: inpatient burden and predictors of costs and mortality. Chronic Obstr Pulm Dis. 2012;9(2):131–141. doi:10.3109/15412555.2011.650239
33. Jinjuvadia C, Jinjuvadia R, Mandapakala C, Durairajan N, Liangpunsakul S, Soubani AO. Trends in outcomes, financial burden, and mortality for acute exacerbation of chronic obstructive pulmonary disease (COPD) in the United States from 2002 to 2010. Chronic Obstr Pulm Dis. 2017;14(1):72–79. doi:10.1080/15412555.2016.1199669
34. Health Bureau of Macao Special Administrative Region. Average life expectancy of the population; 2020. Available from: https://www.ssm.gov.mo/portal/.
35. Region GoMSA. Macau Population Policy Research Report. 2015.
36. Patil SP, Krishnan JA, Lechtzin N, Diette GB. In-hospital mortality following acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med. 2003;163(10):1180–1186. doi:10.1001/archinte.163.10.1180
37. Connolly MJ, Lowe D, Anstey K, Hosker H, Pearson M, Roberts C. Admissions to hospital with exacerbations of chronic obstructive pulmonary disease: effect of age related factors and service organisation. Thorax. 2006;61(10):843–848. doi:10.1136/thx.2005.054924
38. George P, Stone R, Buckingham R, Pursey N, Lowe D, Roberts C. Changes in NHS organization of care and management of hospital admissions with COPD exacerbations between the national COPD audits of 2003 and 2008. QJM. 2011;104(10):859–866. doi:10.1093/qjmed/hcr083
39. Franssen FM, Rochester CL. Comorbidities in patients with COPD and pulmonary rehabilitation: do they matter? Eur Respir Rev. 2014;23(131):131–141. doi:10.1183/09059180.00007613
40. Negewo NA, McDonald VM, Gibson PG. Comorbidity in chronic obstructive pulmonary disease. Respir Investig. 2015;53(6):249–258. doi:10.1016/j.resinv.2015.02.004
41. Baty F, Putora PM, Isenring B, Blum T, Brutsche M. Comorbidities and burden of COPD: a Population Based Case-Control Study. PLoS One. 2013;8(5):e63285. doi:10.1371/journal.pone.0063285
42. de Miguel-díez J, Morgan JC, García J-G. The association between COPD and heart failure risk: a review. Int J Chron Obstruct Pulmon Dis. 2013;8:305. doi:10.2147/COPD.S31236
43. Wang Y, Stavem K, Dahl FA, Humerfelt S, Haugen T. Factors associated with a prolonged length of stay after acute exacerbation of chronic obstructive pulmonary disease (AECOPD). Int J Chron Obstruct Pulmon Dis. 2014;9:99. doi:10.2147/COPD.S51467
44. Inabnit LS, Blanchette C, Ruban C. Comorbidities and length of stay in chronic obstructive pulmonary disease patients. Chronic Obstr Pulm Dis. 2018;15(4):355–360. doi:10.1080/15412555.2018.1513470
45. Agustí A, Edwards LD, Rennard SI, et al. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS One. 2012;7(5):e37483. doi:10.1371/journal.pone.0037483
46. Vestbo J, Anderson W, Coxson HO, et al. Evaluation of COPD longitudinally to identify predictive surrogate end-points (ECLIPSE). Eur Respir J. 2008;31(4):869–873. doi:10.1183/09031936.00111707
47. Barr RG, Bluemke DA, Ahmed FS, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;362(3):217–227. doi:10.1056/NEJMoa0808836
48. Janssen DJ, Spruit MA, Uszko-Lencer NH, Schols JM, Wouters EF. Symptoms, comorbidities, and health care in advanced chronic obstructive pulmonary disease or chronic heart failure. J Palliat Med. 2011;14(6):735–743. doi:10.1089/jpm.2010.0479
49. Lanken PN, Terry PB, DeLisser HM, et al. An official American thoracic society clinical policy statement: palliative care for patients with respiratory diseases and critical illnesses. Am J Respir Crit Care Med. 2008;177(8):912–927. doi:10.1164/rccm.200605-587ST
50. Taylor RS, Sagar VA, Davies EJ, et al. Exercise‐based rehabilitation for heart failure. Cochrane Database Syst Rev. 2014;(4). doi:10.1002/14651858.CD003331.pub4
51. Jayadev A, Stone R, Steiner MC, McMillan V, Roberts CM. Time to NIV and mortality in AECOPD hospital admissions: an observational study into real world insights from national COPD audits. BMJ Open Respir Res. 2019;6(1):e000444. doi:10.1136/bmjresp-2019-000444
52. Stefan M, Priya A, Pekow P, et al. The association between pulmonary rehabilitation and rehospitalizations in patients with COPD. In: A94 Expanding Our Horizons; Leading Research in Pulmonary Rehabilitation: 2020. American Thoracic Society; 2020:A2503–A2503.
53. Ambrosino N, Vagheggini G. Non-invasive ventilation in exacerbations of COPD. Int J Chron Obstruct Pulmon Dis. 2007;2(4):471.
54. Rai S, Panda B, Upadhyay K. Noninvasive positive pressure ventilation in patients with acute respiratory failure. Med J Armed Forces India. 2004;60(3):224–226. doi:10.1016/S0377-1237(04)80050-9
55. Kaul S, Pearson M, Coutts I, Lowe D, Roberts M. Non-invasive ventilation (NIV) in the clinical management of acute COPD in 233 UK hospitals: results from the RCP/BTS 2003 national COPD audit. Chronic Obstr Pulm Dis. 2009;6(3):171–176. doi:10.1080/15412550902902646
56. Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13–e64. doi:10.1164/rccm.201309-1634ST
57. Katajisto M, Laitinen T. Estimating the effectiveness of pulmonary rehabilitation for COPD exacerbations: reduction of hospital inpatient days during the following year. Int J Chron Obstruct Pulmon Dis. 2017;12:2763. doi:10.2147/COPD.S144571
58. Hui KP, Hewitt AB. A simple pulmonary rehabilitation program improves health outcomes and reduces hospital utilization in patients with COPD. Chest. 2003;124(1):94–97. doi:10.1378/chest.124.1.94
59. Zeng Y, Jiang F, Chen Y, Chen P, Cai S. Exercise assessments and trainings of pulmonary rehabilitation in COPD: a literature review. Int J Chron Obstruct Pulmon Dis. 2018;13:2013. doi:10.2147/COPD.S167098
60. Gruffydd-Jones K, Loveridge C. The 2010 NICE COPD guidelines: how do they compare with the GOLD guidelines? Prim Care Respir J. 2011;20(2):199–204. doi:10.4104/pcrj.2011.00011
61. George J, Kong DC, Stewart K. Adherence to disease management programs in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2007;2(3):253.
62. Hu G, Zhou Y, Tian J, et al. Risk of COPD from exposure to biomass smoke: a metaanalysis. Chest. 2010;138(1):20–31. doi:10.1378/chest.08-2114
63. Taylor JD. COPD and the response of the lung to tobacco smoke exposure. Pulm Pharmacol Ther. 2010;23(5):376–383. doi:10.1016/j.pupt.2010.04.003
64. Halpern MT, Stanford R, Borker R. The burden of COPD in the USA: results from the confronting COPD survey. Respir Med. 2003;97:S81–S89. doi:10.1016/S0954-6111(03)80028-8
65. Tønnesen P. Smoking cessation and COPD. Eur Respir Rev. 2013;22(127):37–43. doi:10.1183/09059180.00007212
66. Rezaei S, Akbari Sari A, Arab M, Majdzadeh R, Shaahmadi F, Mohammadpoorasl A. The association between smoking status and hospital length of stay: evidence from a hospital-based cohort. Hosp Pract. 2016;44(3):129–132. doi:10.1080/21548331.2016.1178579
67. Azagba S, Sharaf MF, Liu CX. Disparities in health care utilization by smoking status in Canada. Int J Public Health. 2013;58(6):913–925. doi:10.1007/s00038-013-0452-7
68. Wacker M, Holle R, Heinrich J, et al. The association of smoking status with healthcare utilisation, productivity loss and resulting costs: results from the population-based KORA F4 study. BMC Health Serv Res. 2013;13(1):278. doi:10.1186/1472-6963-13-278
69. Bai J, Chen X, Liu S, Yu L, Xu J. Smoking cessation affects the natural history of COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:3323. doi:10.2147/COPD.S150243
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
