Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Factors Influencing Skin Aging and the Important Role of Estrogens and Selective Estrogen Receptor Modulators (SERMs)
Authors Lephart ED , Naftolin F
Received 5 June 2022
Accepted for publication 19 July 2022
Published 19 August 2022 Volume 2022:15 Pages 1695—1709
DOI https://doi.org/10.2147/CCID.S333663
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Jeffrey Weinberg
Edwin D Lephart,1 Frederick Naftolin2
1Department of Cell Biology, Physiology and The Neuroscience Center, Brigham Young University, Provo, UT, USA; 2Department of Research and Development, e-Bio Corporation, Woodbridge, CT, USA
Correspondence: Edwin D Lephart, Department of Cell Biology, Physiology and The Neuroscience Center, LS 4005, College of Life Sciences, Brigham Young University, Provo, UT, 84602, USA, Tel +801 422-2006 ; +801 319 8173, Fax +1 801 422 0700, Email [email protected]
Abstract: The narrative for this overview focuses on updating the factors that influence skin aging and the important role estrogens and selective estrogen receptor modulators (SERMs) play in this process (mainly utilizing journal reports and reviews from the last four years). Estrogens have been known and studied for over a century. For many years, it has been recognized that estrogens are important in the maintenance of human skin. Women seek cosmetic and medical treatments to improve dermal health and physical characteristics to enhance their self-perception and inhibit skin aging, particularly in highly visible body areas. The goal: to retain estrogen’s positive benefits while aging and especially at/after menopause where estrogen-deficient skin contributes to the dramatic decline in skin health. In this overview, both background information and recent novel findings are included that cover aging (general mechanisms), skin aging, and factors that influence skin aging (intrinsic, extrinsic, skin microbiome and gut microbiome.) Plus, estrogen’s general role in maintaining skin health is presented through the classical estrogen receptors alpha (α) and beta (β) and non-classical (or non-genomic) estrogen receptor (G protein-coupled seven transmembrane receptor). More importantly, the various benefits of 17β-estradiol in skin health are examined (ie, skin collagen and elastin profiles that follow 17β-estradiol levels during aging and at/after menopause). Finally, a revision of information for estrogenic skin topical applications involving isoflavonoid compounds that act as SERMs, but are classified as endocrine disruptors, and a topical estrogen analog are explored to update the known and unknown characteristics of these treatments. Further study is warranted to understand the biological and molecular mechanisms by which estrogens support and enhance dermal health and wellbeing.
Keywords: estrogens, skin aging, estrogen receptors, SERMs, isoflavonoid, microbiome
Introduction
For many years, it has been recognized that estrogens are important in the maintenance of human skin. Women seek cosmetic and medical treatments to improve dermal health and physical characteristics to enhance their self-perception and inhibit skin aging, particularly in highly visible body areas. The goal: to retain estrogen’s positive benefits while aging and especially at/after menopause where estrogen-deficient skin results in the dramatic decline in skin health. For example, after menopause, there is a decrease in collagen content at a rate of 2% per postmenopausal year for up to 15 years in women not on hormone replacement therapy. Skin changes and the gut-skin axis associated with menopause have been reported elsewhere.1–4
The purpose of this narrative overview is to provide an updated board perspective on the factors that influence skin aging and the important role estrogens and selective estrogen receptor modulators (SERMs) play in this process by using descriptive text and elementary graphics to portray the literature results especially covering the last 4 years. Notably, sixty percent of the citations are from the last 4 years, while forty percent represent earlier background or supporting references. While journal reports/reviews have covered skin aging, estrogens, estrogen receptors along with 17beta-estradiol’s skin effects and the hormonal changes with aging (menopause), this overview updates these topics (in brief) and revises misleading information about SERMs. For example, SERMS such as topical isoflavonoid compounds that are classified as endocrine disruptors and an estrogen analog currently used in skin treatments. Principally, estrogen action and the gut microbiome in dermal health have been covered in detail recently by Lephart and Naftolin, 2022 (Dermatology and Therapy online, hardcopy in press).4
This review identifies previous journal articles and reviews (with emphasis over the last four years) from January 2018 through February 2022 using the keywords: estrogens, skin aging, SERMs, isoflavonoids; microbiome; and/or using different keyword combination (retrieved from February 25th, 2021, to March 15th, 2002). The following databases were utilized: PubMed maintained by the US National Library of Medicine at the National Institutes of Health (USA); Science Direct and Scopus by Elsevier and from Google Scholar. Also, background references (where appropriate) include the keywords: estrogens, SERMs, skin aging, microbiome, isoflavonoids, phytoestrogens, phytochemicals, polyphenols, and/or combinations (without a year-limit range for searching these topics).
Aging
Aging is accompanied by the progressive loss of anatomical structure and physiological function of the body (organs, tissues, and cells) leading to age-related diseases such as cardiovascular (atherosclerosis, stroke, etc.), musculoskeletal (sarcopenia and osteoporosis), neurodegenerative (dementia, etc.), immunological (arthritis, inflammaging and metainflammation), endocrine/metabolism diseases (diet, obesity and insulin resistance, etc.), gastrointestinal disorders (irritable bowel, celiac disease, etc.), and various cancers.5–8 Franceschi et al in 2018 and Li et al in 2021 outlined the hallmarks of aging, which include: 1) genomic instability, 2) reduced telomere length, 3) epigenetic influences, 4) loss of proteostasis or macromolecular damage 5) deregulated nutrient sensing and metabolic signaling pathways, 6) cellular senescence, 7) stem cell exhaustion, 8) stress and mitochondrial dysfunction and 9) altered intercellular communication.5,6 Other investigators have proposed that the underpinnings of aging and age-related diseases are based upon low-grade, unresolved, molecular inflammation or oxidative stress events.7 Notably, the 2020 US census revealed an aging population, where 28% of the US population is 55 years old or older and accounts for 57% of healthcare spending.9 All the age research viewpoints above provide insights into potential interventions that may affect the aging process and reduce age-related disease that in turn promote health and longevity.5–12
Skin Aging
Of all the organs, the skin is the most conspicuous to display signs of aging or dermal changes, which are due to chronological (intrinsic) and photoaging (extrinsic) mechanisms. However, it is important to emphasize the fact that the skin is the only organ to be exposed to the external environment especially in areas like the face, neck, hands, and arms.10–15
Extrinsic Skin Aging
While not systematically addressed in this review, extrinsic skin aging is an important collateral factor in cosmesis, appearance, diagnosis, and management of the skin of aging individuals. Chronologically aged skin includes the non-hormonal loss of skin homeostasis, and inflammatory/antioxidant actions.10–12 Whereas, photo-aging (exposure to ultra-violet light) and chronologically aged skin are involved in a cascade of cellular and molecular signaling mechanisms (mainly due to oxidative stress).10–12 These extrinsic aging factors may be independent of hormonal status and should be taken into account when considering the clinical management of the aging skin.11,12,14–19
Intrinsic Skin Aging
Up to 25 to 30 years of age the skin thickens in women, then all skin layers decrease progressively during aging.10–12,15 Chronological or intrinsic aging due to degenerative changes in the synthesis, abundance, and maintenance of skin components is an inevitable biological process.10–15 During skin aging, alterations in the shape of keratinocytes occur, and the epidermal-dermal junction (rete pegs) flattens, while the dermis decreases in thickness and declination of rebound is observed due to the loss of collagen, elastin, and hyaluronic acid.10,11 This is easily shown by pulling up the skin and seeing the time taken to return to its original shape (Skin Folding test).10 The loss of turgor is also seen as wrinkles, especially along natural lines of stress, gravity, and repeated mechanical muscle contraction (for example – smiling) around the eyes and mouth.10,11,15
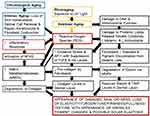
Aging skin displays fragmentation of collagen and elastin fibers by the action of matrix metalloproteinase (MMPs), and increased mitochondrial oxidative stress results in deletions of mitochondrial DNA via the c-Jun/AP-1 pathway in dermal fibroblasts (Figure 1).11–15 Skin blood flow and lipid deposition decrease with aging in the hypodermis.11,13,15 The flowchart in Figure 1 displays the chronological and photoaging cascade pathways that include several hallmarks of skin aging.11–13
Factors Influencing Skin Aging
Extrinsic – Photoaging Skin
Extrinsic or photoaging has many factors involved in the process of skin aging, which are displayed in Figure 2. They include not only sunlight exposure, but also environmental, gravity, air/water pollution, diet, exercise, stress, and lifestyle parameters that influence skin aging like tobacco use, sugar, and alcohol intake, and finally, drugs and medications.7,11,13–18,20,21
Intrinsic – Chronologically Aged Skin
Intrinsic or chronological factors influencing skin aging go beyond the passage of time, but also incorporate several factors like: genetics, metabolic, hormonal, immunological, cardiovascular, gastrointestinal, degenerative, and neoplastic diseases along with psychogenic (involving stress or affective disorders) (Figure 2).11,13–18,21
The Microbiota and Microbiome
Often the labels microbiota and microbiome are used interchangeably, but these two terms have subtle differences (see recent review for updated microbiome definitions).4,22 In general, microbiota refers to the microorganisms [by type belonging to different kingdoms (Prokaryotes: Bacteria, Archaea; Eukaryotes: Protozoa, Fungi, etc.)] found within a specific environment.22–26 For example, microbiota bacteria, archaea, viruses, and fungi are found common to both skin and gut.23–27
Conversely, the microbiome refers to the collection of genes (genomes) from all the microorganisms in a specific environment.22,23,26 Finally, the human gut microbiome is not only involved in skin health, but it is a potential controller of wellness and disease in many organs and tissue sites.23,25 It is beyond the scope of this overview to cover this topic in detail herein, but coverage is provided in other reviews.4,26–33
Skin Microbiome
The skin microbiome is determined at birth.24–30,32 As early as 1965 Marples, later Kearney et al in 1984 and Grice et al in 2008 reported that various skin structures comprise sub-habitats that may be associated with their own unique microbiota.34–36 Since that time and with the advancements of the Human Microbiome Project (HMP) using large-scale high-throughput analysis, it has been demonstrated that the skin microbiome is different from the gut microbiome.4,23,24,27,36 Recent reports suggest the skin microbiome has both intrinsic and extrinsic factors that influence skin aging (Figure 2).4,27,29,30,33,35 The intrinsic parameters shaping the skin microbiome include age, genetics, gender, immunity, hormones, sleep, stress, and metabolism.29,30 Whereas the extrinsic factors influencing the skin microbiome cover many of the extrinsic – photoaging items, but also include hygiene, beauty routine (cosmetics, etc.) and availability of nutrients for skin microorganisms.29,30,36
How the gut microbiome potentially influences skin aging has been recently reviewed using the estrobolome as one of the mechanisms for estrogen metabolism and the interchange between the gut-skin axis.4,27 For example, with aging, especially with the associated changes after menopause there are age-related disorders like osteoporosis and osteopenia.4 Finally, the exchange via the gut-skin axis is known to occur via metabolites and communication through the immune system. Also, ultraviolet B-light enhances vitamin D levels that increase the diversity of the gut microbiome that in turn influences the skin microbiome to enhance skin immune function, increase wound healing and inhibit inflammation.4
Gut Microbiome
Figure 2 displays the factors that influence skin aging via the gut microbiome. In general, most investigators view that before birth a sterile environment exists and the initial gut bacteria (founder species) depends on the mode of delivery.26,28,31–33 Other factors include early dietary intake such as breast vs bottle formula fed,28,31,33 and physical contact,25,26,33 that builds up through 6 months of age. In infancy, new strains outcompete old ones, and there is a rapid increase in diversity of the early microbiota that is somewhat unstable.26,33 Thereafter, there are shifts in response to diet with the introduction to solid foods (illness, antibiotics, etc.). By three years of age, the microbiome foundation is somewhat established.26,31–33 In adulthood, highly distinct microbiota are differentiated, and while the microbial community continues to change due to diet, hormones, environmental factors, etc.,37–48 this change occurs at a slower rate compared to childhood.33,36,40 In the elderly, there is a substantially different gut microbial community with lower diversity compared to younger adults,46–51 especially with the associated changes after menopause.4,46–49,52
Estrogens
Estrogens Affect Health and Homeostasis
Estrogens have been known and studied for a century. When estrogens were first studied, it was observed they caused maturation of the vaginal mucus membrane in a way analogous to estrogen’s action on the skin. For example, a thickening and production of protective keratinized cells of the upper layers of the membrane and a broadening and increased elasticity of the layer beneath are seen.10,53,54 This furnished an opaque integument that can stretch without tearing, rebounded after stretching and protected the tissues below it from outside influences such as bacterial infection. These qualities were the basis of the first definitions of estrogens.53,54
The identification and measurement of estrogens is no longer performed by traditional biological assays. The purification of estrogen in the 1920s to the discovery of estrogen receptors defining estrogen’s affinity, binding, and the molecular mechanisms of gene activation in cells/tissues has been reviewed.53,54 However, the qualities of estrogens are still measured to determine the “estrogenicity” of compounds. Biological determinations (steroid structure, binding affinity of estrogens to estrogen receptors alpha (ERα) and ER beta (β) for instance) have allowed the comparison of all compounds that affect cellular growth and function.53 Additionally, biological assays determine whether they are agonistic or antagonistic compared to the most potent biologically active natural estrogen, 17β-estradiol.53
There are four natural estrogens [estrone, estradiol, estriol (mainly present during pregnancy) and estetrol (only produced during pregnancy, which was discovered in 1965)].10,53,54 These compounds are named according to the number of hydroxyl side-groups in the molecule.53 All the natural estrogens arise by the enzymatic removal of a carbon atom from androgen precursor molecules, a process termed aromatization.50 The enzyme is termed aromatase, a product of the CYP19A1 gene.10,53,54 Recall, 17β-estradiol is the most potent steroid hormone produced by the ovaries until menopause (Figure 3).10,53,54 All estrogens and their estrogenic analogs, such as tamoxifen,56 phenytoin,57 DT56a58 and even some androgens59 act through binding to ERα and ERβ.53 Since the majority of cells express ERs, estrogen actions influence almost all tissues and are responsible for: a) homeostatic regulation, b) cell proliferation and apoptosis, c) liver protein expression, d) lipid metabolism, e) energy balance, f) glucose metabolism, g) immune and cardiovascular alterations, h) gonadotrophin feedback and gametogenesis, i) brain-neuronal development/memory processing and repair/neurodegeneration, j) bone growth, etc.,7,15,53,60 including estrogen’s positive (agonist) or negative (antagonist) actions on skin, especially in women.10,15,61
 |
Figure 3 (A) Upper Panel: 17β-Estradiol hormone action via the nuclear estrogen receptors ERα and ERβ and membrane bound estrogen receptors (G protein-coupled seven transmembrane receptor), see red rectangular box. While not yet identified in the mitochondria of skin cells, ERα and ERβ are present in mitochondria in the cardiovascular and nervous system and through ERβ, 17β-estradiol activates antioxidant and anti-apoptotic actions through ERβ.67–69 (B) Lower Panel: the benefits of 17β-estradiol in skin health (blue rectangular box, a through z). |
Estrogen Receptors (Nuclear, Membrane-Bound, and Mitochondria) are the Active Agents in Estrogen Action
The estrogen receptors are the primary effectors in estrogen action. When the nuclear estrogen receptors bind (ligand) compounds such as the natural estrogens, the receptors are stereo-chemically rearranged, which allows them to pair as receptor dimers.53 As the dimers enter the nucleus they are further regulated by tissue-specific co-trans-activation factors, and then, next to estrogen response elements (ERE) to stimulate gene expression.53 Depending on the original ligand, the outcomes of the binding to the ERE are agonistic (stimulatory) of or antagonistic (inhibitory) on gene action, compared to that of estradiol binding.53,59–61
The products of the genes affected by nuclear estrogen receptor dimers are proteins and peptides such as enzymes, inflammatory peptides, and neurotransmitters.47,53 The presence (expression) of estrogen receptors in the majority of tissues and the presence of EREs in the majority of genes make it possible for estrogen action to occur in almost all cells in the body including the skin.50,51,57,58
The nuclear estrogen receptors (ER), ERα and ERβ, are members of the superfamily of nuclear hormone receptors (Figure 3A).10,15,53 For example, human ERβ is homologous to ERα, particularly in the DNA-binding domain (97% amino acid identity), but share little homology in the other domains (such as in the ligand-binding region), where one would predict that 17β-estradiol would display different affinities for the ERs, but surprisingly, it has almost equal high affinity for ERα and for ERβ.5,61
There are differences in the affinity and residence times of estrogens and the SERMs.59–61 For example, estrone has less affinity for ERα and ERβ and is more rapidly dissociated from the ERs than 17β-estradiol.53 The result is a seven-fold greater effect of 17β-estradiol than estrone in estrogen agonist actions.10,15,59
There is tissue-specific expression in humans of the ERs; ERβ is more widely expressed in skin compared to ERα, and this is especially the case in the human scalp (Figure 3A).10,15,61 ERβ activation has been shown to promote wound healing1–3,10,61,62 independent of estrogen’s anti-inflammatory properties63 and promote tissue repair in human skin tissues via dermal structural and biomechanical mechanisms.53,60–65
Estrogen Receptor Ligands
Recall, certain compounds (estrogenic or otherwise) that bind to the ERs in a targeted and tissue-specific manner regarding function (agonist or antagonist) are termed SERMs.59,61 The natural estrogens compete with SERMs for receptor binding.59,61 The outcome is the overall estrogen agonist-antagonist balance. Because the expression of co-transactivating factors is tissue-specific, the biological outcomes differ in different tissues.59,61,65 For example, saturating the system with administered tamoxifen results in a blockade of natural estrogen action on the regulation of gonadotropins by the pituitary-hypothalamic axis and inhibition of breast cancer cell division, while inducing growth of the uterine endometrial cells.56,57 Because of the plethora of cell types in the body and their different co-transactivating factors, it is necessary to test individual tissues for the outcome of administration of each SERM.59–61,66
Membrane-Bound Estrogen Receptors
A group of estrogen receptors, generally of the ERα family, are tethered to the outer surface of the cell membrane.67 When they bind estradiol or another SERM, the result is the activation of intracellular metabolic pathways.67 Skin cells express these G protein-coupled seven-transmembrane tethered ER, termed GPER and are also known as GPER1 or GPR30 (Figure 3A).10,15,68 While there is less known about the GPR30 in skin, a few reports have shown positive influences on dermal health. For example, it has been shown to induce rapid cytoskeletal changes in human dermal fibroblasts and protect against ultraviolet B-induced injury in epidermal stem cells.68,69 Also, GPER activation shields against epithelial barrier disruption by Staphylococcus aureus α-toxin.70 These actions are immediate and do not depend on binding to the EREs.69
Mitochondrial Estrogen Receptors
Estrogens regulate mitochondrial dynamics,71 which has been reported in the cardiovascular and nervous system.71–73 While not yet identified in the mitochondria of skin cells, ERα and ERβ are present in mitochondria elsewhere and through ERβ, estrogen stimulates manganese superoxide dismutase to reduce damage from reactive oxygen species that inhibits apoptosis.53 Thus, 17β-estradiol activates antioxidant and anti-apoptotic actions through ERβ.71–73
Summary of Estrogens and Their Mechanism of Action
The terms “estrogen or estrogenic” have broadened to include any natural or synthetic compound that binds to estrogen receptors.10,15,59 The outcome of binding is to activate, block or inhibit the receptors that regulate the gene products or protein synthesis.53,60,61,65 However, compounds are classified as estrogenic even if binding to the ERs is 10- or more than 100-times lower compared to 17β-estradiol.53,59 It is well established that in the absence of agonist action on the ERs in skin there is atrophy due to lack of protein synthesis.10,65 Conversely, administration of agonistic SERMs, such as the natural estrogens, DT56a (Femarelle) or isoflavonoids compounds like equol, has been shown to restore atrophic skin.10,61,65,74 For example, while it is not known which ERs mediate the rescue of atrophic skin. However, it is known that ERβ predominates in dermal cells and the expression of ERβ declines after menopause with the loss of collagen and elastin.10,15,61,65 To support this proposition, Ahn et al in 2020 showed that estrogenic actions promote tissue repair in mice and human skin via ERβ.64
Benefits of 17Beta-Estradiol in Skin Health
Normal estrogen levels before menopause have numerous cutaneous benefits that are displayed in Figure 3B. Estrogen is known to stimulate collagen, elastin, and erzin.1–4,10 These dermal components provide the interlinking of skin cells for cutaneous integrity, and elastic recoil properties associated with youthful skin.10,61,65,75–81 Estrogens also enhance moisture/hydration (via hyaluronic acid, mucopolysaccharides, and sebum production) where skin turgor, dermal thickness, and keratinocyte and fibroblast proliferation are enhanced.1–3,10,61,65,75–79 Additional positive influences of estrogen include increased cell viability and extracellular matrix components such as fibrillin and tissue inhibitor of matrix metalloproteinases (MMPs) also known as TIMPs.1–3,10,61,75–78 Estrogens also act as antioxidants to counteract reactive oxygen species (ROS) and oxidative stress (OS).1,2,10,81–84 This is accomplished by stimulating nuclear factor erythroid 2-related factor (Nrf2), which performs as a master controlling-factor that increases the expression of other antioxidants and detoxifying enzymes.10,81–84 Finally, estrogens: a) increase blood flow, b) enhance the expression of ERβ after menopause, c) stimulates transforming factor-beta (TGFβ), d) counteracts androgen hormone actions and photo-aging, and e) enhances facial attractiveness and perceived age in women.10,15,61,65,75,78,85
Skin Collagen and Elastin Profiles with Changes in 17Beta-Estradiol Levels During Aging
During reproductive years, the major estrogen produced by the ovaries is 17β-estradiol, which peaks in the late 20s (Figure 4A and B).10,15,85 During a similar interval, expression of skin collagen and elastin also reach their maximum levels.10,15,85
As estrogen levels begin to decline, around 30–35 years of age, the skin thins and folds or wrinkles appear due to the loss of collagen and elastin fibers in the dermal layer (Figure 4A).7–80,85 After 35 years of age, 17β-estradiol levels continue to decline and by 45 years of age there are high and low erratic swings in ovarian estrogen production as the follicles respond to gonadotrophins signals until they become exhausted with the onset of menopause (which the average in the USA is 51 years of age ± 4 years).10,86 Concurrent with the loss of 17β-estradiol production from the ovaries at menopause estrogen production in the body occurs at peripheral adipose tissue sites.7,15,58,62,72,82 While skin cells can produce estrogens locally, the aromatase enzyme activity is 30-times lower compared to premenopausal ovarian tissue.15,53,86 Moreover, the negative androgenic impact on skin cells via the 5α-reductase type I enzyme can counteract the positive estrogenic influence, especially after menopause.10,13,66 The outcome is the lack of estradiol and/or agonist SERM actions, which account for the dramatic loss in skin health parameters such as collagen and elastin causing atrophy, wrinkles, poor wound healing/barrier function/hydration.1,2,10,65,75,87 (Figure 4A) The deterioration of the skin is accentuated by solar exposure, the presence of free radicals in the environment and cigarette smoking.10,15,65,75 There also are adverse effects on many physiological functions (cardiovascular, bone, immune, brain health, etc.), which are reviewed elsewhere.1,2,17,61,65,75,88,89
Update and Revision of Information on Estrogenic Skin Treatments Selective Estrogen Receptor Modulators (SERM’s)
The discovery and development of synthetic SERMs for clinical practice has been reviewed by Maximov et al in 2013.90 In the 1970s tamoxifen was one of the first compounds to be tested, but the first public description of the clinical concept of SERMs as useful medicines for women’s health was at the First International Chemoprevention meeting in New York, NY in 1987.90 Since that time, it is now clear that ERα activation is a major factor in maintenance of reproductive cancers (for example, breast and prostate), whereas, ERβ activation appears to be chemoprotective at these tissue sites.10,13,89
SERM’s Use to Replace the Need for Progestins in Women Who Have a Uterus and Wish to Avoid Endometrial Activation During Hormone Treatment
Menopausal estrogen treatment (ET) has been a mainstay for the post-menopausal women, including the maintenance of skin health.10,53,65 However, the possibility of endometrial activation, hyperplasia, bleeding, and cancer was a problem until an estrogen-antagonist progestin compound was implemented to the regimen.89 Progestins such as progesterone block expression of estrogen receptors in the uterus, thereby avoiding endometrial activation during ET.10,15,89 The combination of ET plus progestin is termed menopausal hormone treatment (MHT).10,89–92
Since oral formulations of progesterone were subject to inactivation by gut metabolism a synthetic progestin, medroxyprogesterone acetate (MPA) was widely utilized.10,89,93 When micronized progesterone became available, it was adopted, but because of side effects such as drowsiness and mood effects it did not attain great commercial success.89,93 During the period of the Women’s Health Initiative (WHI) it became clear that MPA is associated with breast cancer, and it is no longer preferred for MHT.89,92
SERM’s and Menopausal Hormone Treatment (MHT)
Two strategies have been developed to employ SERMs that furnish estrogen action, while avoiding endometrial activation.
Stand-alone SERM treatment – Several SERMs furnish protection against the lack of estrogen in the post-menopause. These act through tissue-specific receptor-mediated estrogen action without increasing estrogen receptor expression or agonist actions at the endometrial level. These compounds may be synthesized or derived from plants.10,15,91 Of current interest is estetrol, which is a natural estrogen that spares the endometrium, while being an estrogen receptor agonist in the tissues, including the skin, that deteriorate following the loss of estradiol in the post-menopausal period.55 Plant SERMs, generally containing genistein and/or daidzein and its metabolite, equol, serve the same stand-alone SERM treatment goal.10,91,94,95
Estradiol plus bazedoxifene – The antagonist SERM bazedoxifene has been successfully combined with micronized estradiol to furnish estrogen agonist action on all target tissues with the exception of the endometrium.94
Plant SERMs affecting skin health have been reviewed as potential estrogenic dermal treatments to selectively harness the positive effects of estrogen stimulation, while avoiding negative effects such as endometrial activation and blood clotting.7,10,15,65,81,91
Polyphenolic/Isoflavonoid Compounds as SERM’s
Plant estrogens have received a mixed reputation in Western medicine.10,91,95 However, many reports point out that plant SERMs may have diverse and tissue-dependent estrogen agonistic and antagonistic effects.95
Plant-derived polyphenols and flavones bind preferentially, but not exclusively, to ERβ.10,59,91 Their biological actions throughout the body depend on ER and co-transactivation factor expression.53 Although they have been classified as endocrine disruptors (binding to ERβ at lower levels compared to 17β-estradiol), this is a very narrow view, and these compounds are coming to the forefront as agonist-antagonist SERMs that are especially suited for MHT.7,10,91 ERβ-agonists such as DT56a,74 liquiritigenin,96 and equol10,13,91 are especially promising. This is because they are agonistic in the skin, but are not agonistic when bound to ERα, thereby avoiding hyperplasia of the breast and endometrium and venous thromboembolism.89 In fact, like 17β-estradiol, phytoestrogens have been shown to decrease nonalcoholic fatty liver disease,97 which was associated in one study with equol production status.98 From in vitro and clinical studies, equol has similar actions to 17β-estradiol by stimulating collagen, elastin and TIMP expression, while inhibiting elastase and MMPs.10,13,91,99
For example, in a randomized, single-center 12-week clinical study women that applied a topical equol treatment twice per day displayed significant increases (across eight skin parameters) over baseline values (Figure 5).10 The significant increases ranged from 52 to over 75% covering skin firmness to hydration (Figure 5). Remarkably, 76% of the women in this study were 3 years post-menopausal, but still exhibited positive changes in dermal health. Therefore, the results of this clinical study provide corroborating evidence to the in vitro reports of equol’s positive influence on skin components and biomarkers.10,13,91 Moreover, along this topic of SERMs like equol, similar compounds have been reported to have anti-microbial actions that are just beginning to be explored.100
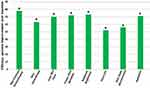 |
Figure 5 Efficacy of a topical equol treatment in a randomized, single-center 12-week clinical study in women. Demographics: N = 59 (30 Caucasian, 29 Asian); Ages 40–70; Mean age 56.1 ± 7.8 (45 out of the 50 or 76% of the women were amenorrheic for at least 3 years); Glogua Aging II and III (moderate to advanced wrinkling). The women applied 0.2% equol twice per day.10 For each parameter there was a significant increase (percent improvement over baseline), *p < 0.05. Copyright © 2021. Springer Nature. Adapted from Lephart E, Naftolin F. Menopause and the skin: old favorites and new innovation in cosmeceuticals for estrogen-deficient skin. Dermatol Ther. 2021;11(9):53–69.10 |
Conversely, of all the phytoestrogens consumed by humans, the isoflavonoids have gone through the most critical scrutiny regarding safety concerns.91 Recent reviews that examined isoflavonoids and the health of postmenopausal women concluded that a) adding soy foods or phytoestrogens to the diet can contribute to the health of postmenopausal women,91,101–103 b) isoflavones or phytoestrogens do not increase the risk of breast, endometrial or colorectal cancer,91,102,103 and c) the research evidence does not support classifying isoflavones as endocrine disruptors.104
There are many reasons for plant SERMs to be exploited as the skin alternatives to synthetic SERMs not only for dermal applications, but in conditions as osteoporosis, menopausal symptoms, and prostate health.60,91,101–106
Topical Use of Estrogen and Estrogen Analogs
There are other indications for treatment of aging skin without the use of systemic estrogen or other SERMs. The most common contraindication is the presence of estrogen-sensitive cancer.89 In those cases, the goal beyond hormone replacement therapy or the use of SERMs is to limit the extracutaneous effects.89,107 However, a novel strategy has evolved in which the active agent delivers its positive effects in the dermal layers, but then is metabolized to an inactive state once it reaches the blood circulation. One such preparation involves the topical administration of an estrogen analog, methyl estradiol propanoate (MEP).108 The proposed mechanism for its metabolism is the cleavage of the active side chain of MEP by blood esterase(s). This produces an inactive residue that is proposed to be harmlessly cleared from the blood.108,109 Clinical studies on postmenopausal women with MEP have been promising.108,109
For example, in a single-site vehicle-controlled double-blinded study examining 79 female subjects aged 53–80 years that applied the MEP treatment twice per day for 14 weeks displayed significant improvement in facial skin dryness, laxity, and dullness (Figure 6).108 Subjects also noted improvements in facial lines and atrophy after the 14-week MEP treatment.108
 |
Figure 6 Efficacy of a topical methyl estradiol propanoate (MEP) treatment in a single-site vehicle-controlled double-blinded study in women. Demographics: N = 79 female subjects aged 53–80 years; Glogua Aging II–IV (moderate to severe wrinkling). The women applied the MEP treatment twice per day for 14 weeks. The results displayed significant improvement in facial skin dryness, laxity, and dullness over vehicle-control values, *p < 0.05. Data from Draelos.108 |
In a second clinical study that examined 14 female subjects aged 53–68 years who were amenorrheic for 1–10 years (mean interval, 5 years) that applied a MEP night cream (at 2%) for a total of 20 weeks displayed improvements in: a) skin texture (53%), b) keratoses (21%), and c) skin laxity (15%).109 Subjects also reported improvement in wrinkles, texture, color and hydration.109 The investigators from both MEP studies suggested that the MEP treatment has the potential to reactive dormant estrogen receptors.108,109 However, the authors of both studies did not state which ER was implicated. This is an interesting proposition since a previous study demonstrated that skin ERα abundance is not altered by menopause, while dermal ERβ expression declined by 15% after menopause,110 suggesting a decline in estrogen hormone action.
However, further studies are necessary to elucidate the mechanism by which MEP provides the presumed reactivation of dormant estrogen receptors. Because cleavage of the MEP side-group may take place in the presence of the specific esterase(s) in epidermal/dermal layers of the skin111–113 and not in the bloodstream that would alter the mechanism of action, especially the estrogen binding characteristics.
Summary and Conclusion
Several factors influence skin aging and the homeostasis of dermal health such as intrinsic, extrinsic aging and the gut-skin axis via the microbiome. Estrogens are paramount in maintaining dermal health and have many anti-aging characteristics by binding to estrogen receptors in the skin. Especially where skin collagen and elastin profiles are known to follow 17β-estradiol levels during aging and at/after menopause. Finally, the classification of isoflavonoid compounds as endocrine disruptors is unwarranted. In fact, isoflavonoids act as SERMs that bind to ER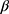 in the skin to enhance dermal health. Whereas the topical estrogen analog (MEP) has presumed mechanisms of action as a skin treatment.
in the skin to enhance dermal health. Whereas the topical estrogen analog (MEP) has presumed mechanisms of action as a skin treatment.
Abbreviations
AP-1, activator protein – 1; CYP, cytochrome P450; ER, estrogen receptor; ERE, estrogen response element; ET, menopausal estrogen treatment; g, gram; GPER, G protein-coupled seven-transmembrane estrogen receptor; HA, hyaluronic acid; MEP, methyl estradiol propanoate; MHT, menopausal hormone treatment; MMP, matrix metalloproteinase; MPA, medroxyprogesterone acetate; Mol, mole; MW, molecular weight; Nf2, nuclear factor erythroid 2-related factor; NF
Acknowledgment
We thank Janet Faye for her helpful comments and editorial assistance.
Funding
This work was supported, in part, by funding from TTO/Life Science College 19-2215 at Brigham Young University.
Disclosure
Edwin Lephart has no funding or sponsor conflicts of interest in the decision of the data/research presented in the report and regarding the publication of this manuscript. Edwin Lephart is an inventor on polyphenolic patents (US and worldwide) on various human health applications. Frederick Naftolin has nothing to disclose.
References
1. Zouboulis CC, Blume-Peytavi U, Kosmadaki M, Roo E, Vexiau-Robert D. Skin, hair and beyond: the impact of menopause. Climacteric. 2022;1–9. doi:10.1080/13697137.2022.2050206
2. Rzepecki AK, Murase JE, Juran R, Fabi SG, McLellan N. Estrogen-deficient skin: the role of topical therapy. Internat J Womens Dermatol. 2019;5(2):85–90. doi:10.1016/j.ijwd.2019.01.001
3. Reus TL, Brohem CA, Schuck DC, et al. Revisiting the effects of menopause on skin: functional changes, clinical studies, in vitro models and therapeutic alternatives. Mech Aging Dev. 2020;185:111193. doi:10.1016/j.mad.2019.111193
4. Lephart E, Naftolin F. Estrogen action and gut microbiome metabolism in dermal health. Dermatol Ther. 2022;12:1535–1550. doi:10.1007/s13555-022-077759-1
5. Li Z, Zhang Z, Ren Y, et al. Aging and age-related diseases: from mechanisms to therapeutic strategies. Biogerontology. 2021;22(2):165–187. doi:10.1007/s10522-021-09910-5
6. Franceschi C, Garagnani P, Parini P, et al. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nature Rev Endocrinol. 2018;14(10):576–590. doi:10.1038/s41574-018-0059-4
7. Chung HY, Cesare M, Anton S, et al. Molecular inflammation: underpinnings of aging and age-related disease. Ageing Res Rev. 2009;8(1):18–30. doi:10.1016/j.arr.2008.07.002
8. Dumic I, Nordin T, Jecmenica M, et al. Gastrointestinal tract disorders in older age. Can J Gastroenterol Hepatol. 2019;19. doi:10.1155/2019/6757524
9. Meola A. The aging US population is creating many problems-especially regarding elderly healthcare issues. Business Insider. Available from: https://www.businessinsider.com/aging-population-healthcare.
10. Lephart E, Naftolin F. Menopause and the skin: old favorites and new innovation in cosmeceuticals for estrogen-deficient skin. Dermatol Ther. 2021;11(9):53–69. doi:10.1007/s13555-020-00468-7
11. Bonté F, Girard D, Archambault J-C, et al. Chapter 10, skin changes during aging, 9n. In: Harris JR, Korolchuk VI, editors. Biochemistry & Cell Biology of Aging: Part II Clinical Sciences, Subcellular Biochemistry 91. Singapore Pte Ltd: Springer Nature; 2019:249–280.
12. Karim PL, Nopriyati IAA. Anatomy and histology of intrinsic aging skin. Bioscientia Medicina. 2021;5(11):1065–1077. doi:10.32539/bsm.v5i11
13. Lephart ED. Skin aging and oxidative stress: Equol’s anti-aging effects via biochemical and molecular mechanisms. Ageing Res Rev. 2016;31(9):36–54. doi:10.1016/j.arr.2016.08.001
14. Zhang S, Duan E. Fighting against skin aging: the way from bench to bedside. Cell Transplant. 2018;27(5):729–738. doi:10.1177/0963689717725755
15. Lephart ED. A review of the role of estrogen in dermal aging and facial attractiveness in women. J Cosmet Dermatol. 2018;17(3):282–288. doi:10.1111/jocd.12508
16. Huang AH, Chien AL. Photoaging: a review of the current literature. Curr Dermatol Rep. 2020;9(1):22–29. doi:10.1007/s13671-020-00288-0
17. Dyer JM, Miller RA. Chronic skin fragility of aging: current concepts in pathogenesis, recognition, and management of dermatoporosis. J Clin Aesthet Dermatol. 2018;11(1):13–18.
18. Sparavigna A. Role of the extracellular matrix in skin aging and dedicated treatment- state of the art. Plast Aesthet Res. 2020;7:14. doi:10.20517/2347-9264.2019.73
19. Lee A-Y. Skin pigmentation abnormalities and their possible relationship with skin aging. Internat J Mole Sci. 2021;22:3727. doi:10.3390/ijms22073727
20. Sant Anna Addor FA. Beyond photoaging: additional factors involved in the process of skin aging. Clin Cosmet Invest Dermatol. 2018;11(9):437–443. doi:10.2147/CCID.S177448
21. Woodby B, Penta K, Pecorelli A, et al. Skin health from the inside out. Annu Rev Food Sci Technol. 2020;11(3):235–254. doi:10.1146/annurev-food-032519-051722
22. Berg G, Rybakova D, Berg G, et al. Microbiome definition re-visited: old concepts and new challenges. Microbiome. 2020;8:103. doi:10.1186/s40168-020-00875-0
23. Mazur M, Tomczak H, Lodyga M, et al. The microbiome of the human skin and its variability in psoriasis and atopic dermatitis. Adv Dermatol Allergol. 2021;38(2):205–209. doi:10.5114/ada.2021.106197
24. Sinha S, Lin G, Ferenczi K. The skin microbiome and the gut-skin axis. Clin Dermatol. 2021;39(5):829–839. doi:10.1016/j.clindermatol.2021.08.021
25. Kho ZY, Lal SK. The human gut microbiome a potential controller of wellness and disease. Front Microbiol. 2018;9. doi:10.3389/fmicb.2018.01835
26. Gilbert J, Blaser MJ, Caporaso JG, et al. Current understanding of the human microbiome. Nature Med. 2018;24:392–400. doi:10.1038/nm.4517
27. De Pessemier B, Grine L, Debaere M, et al. Gut-skin axis: current knowledge of the interrelationship between microbial dysbiosis and skin conditions. Microorganisms. 2021;9:353. doi:10.3390/microorganisms9010353
28. Hoashi M, Meche L, Mahal LK, et al. Human milk bacterial and glycosylation patterns differ by delivery mode. Reprod Sci. 2016;23(7):902–907. doi:10.1177/1933719115623645
29. Moskovicz V, Gross A, Mizrahi B. Extrinsic factors shaping the skin microbiome. Microorganisms. 2020;8:1023. doi:10.3390/microorganisms8071023
30. Skowron K, Bauza-Kaszewska J, Kraszewska Z, et al. Human skin microbiome: impact of intrinsic and extrinsic factors of skin microbiota. Microorganisms. 2021;9:543. doi:10.3390/microorganisms9030543
31. Sekirov I, Russell SL, Antunes CM, et al. Gut microbiome in health and disease. Physiol Rev. 2010;90(3):859–904. doi:10.1152/physrev.00045.2009
32. Khanna S, Pardi DS. Clinical implications of antibiotic impact on gastrointestinal microbiota and Clostridium difficile infections. Expert Rev Gastroenterol Hepatol. 2016;10(10):1145–1152. doi:10.1586/17474124.2016.1158097
33. Sprockett D, Fukami T, Relman DA, et al. Role of priority effects in the early-life assembly of the gut microbiota. Nat Rev Gastroenterol Hepatol. 2018;15(4):197–205. doi:10.1038/nrgastro.2017.173
34. Marples M. The Ecology of the Human Skin, Charles C Thomas. Springfiled, IL: Bannerston House; 1965.
35. Kearnery JN, Harnby D, Gowland G, et al. The follicular distribution and abundance of resident bacteria on human skin. J Gen Microbiol. 1984;130(4):797–801. doi:10.1099/00221287-130-4-797
36. Grice EA, Kong HH, Renaud G, et al. A diversity profile of the human skin microbiota. Genome Res. 2008;18(7):1043–1050. doi:10.1101/gr.075549.107
37. Ellis SR, Nguyen M, Vaughn AR. The skin and gut microbiome and its role in common dermatological conditions. Microorganisms. 2019;7:550. doi:10.3390/microorganisms7110550
38. Sfriso R, Claypool J. Microbial reference frames reveal distinct shifts in the skin microbiota after cleansing. Microorganisms. 2020;8:16344. doi:10.3390/microorganisms8111634
39. Ju W. The gut microbiome and its impact on the brain. In: 4.1 Pressbooks, Neuroscience: Canadian 1st Edition Open Textbook. University of Toronto; 2020.
40. Lephart ED, Naftolin F. Goodman’s medical cell biology, chapter 15. In: The Microbiome. London, UK: Academic Press, Elsevier; 2021. doi:10.1016/B978-0-12-817927-7.09993-2
41. Garcia-Gomez E, Gonzalez-Pedrajo B, Camacho-Arroyo I. Role of sex steroid hormones in bacterial-host interactions. BioMed Res Internat. 2013;2013:1–10. doi:10.1155/2013/928290
42. Fuhrman BJ, Feigelson HS, Flores R, et al. Associations of the fecal microbiome with urinary estrogens and estrogen metabolites in postmenopausal women. J Clin Endocrinol Metab. 2014;99(12):4632–4640. doi:10.1210/jc.2014-2222
43. David LA, Maurice C, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi:10.1038/nature12820
44. Wu GD, Compher C, Chen EZ, et al. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut. 2016;65(1):63–72. doi:10.1136/gutjnl-2014-308209
45. Orlich MJ, Siapco G, Jung S, et al. Vegetarian diets and the microbiome. In: Mariotti F, editor. Vegetarian and Plant-Base Diets in Health and Disease Prevention. London, UK: Academic Press-Elsevier; 2017:429–461. doi:10.1016/B978-0-12-803968-7.00024-1
46. Vieira AT, Castello PM, Ribeiro DA, et al. Influence of oral and gut microbiome in the health of menopausal women. Front Microbiol. 2017;8. doi:10.3389/fmicb.2017.01884
47. Shin J-H, Park Y-H, Sim M, et al. Serum levels of sex steroid hormones is associated with diversity and profiles of human gut microbiome. Res Microbiol. 2019;170(4–5):192–201. doi:10.1016/j.resmic.2019.03.003
48. Mayneris-Perxachs J, Arnoriaga-Rodriguez M, Luque-Cordoba D, et al. Gut microbiome steroid sexual dimorphism and it impact on gonadal steroids: influences of obesity and menopausal status. Microbiome. 2020;8:136. doi:10.1186/s40168-010-00913-x
49. Rettedal EA, Ilesanmi-Oyelere BL, Roy NC, et al. The gut microbiome is altered in postmenopausal women with osteoporosis and osteopenia. JBMR Plus. 2021;5:e10452. doi:10.1002/jbm4.10452
50. Tanes C, Bittinger K, Gao Y, et al. Role of dietary fiber in the recovery of the human gut microbiome and its metabolome. Cell Host Microbe. 2021;29(3):394–407. doi:10.1016/j.chom.2020.12.012
51. van der Merwe M. Gut microbiome changes induced by diet rich in fruits and vegetables. Internat J Food Sci Nutr. 2012;72(5):665–669. doi:10.1080/09637486.2020.1852537
52. Hussain T, Murtaza G, Kalhoro DH, et al. Relationship between the gut microbiome and host-metabolism: emphasis on hormones related to reproductive function. Animal Nutr. 2021;7(1):1–10. doi:10.1016/j.aninu.2020.11.005
53. Santen RJ, Simpson E. History of estrogen: its purification, structure, synthesis, biological actions, and clinical implications. Endocrinol. 2019;160(3):605–625. doi:10.1210/en.2018-00529
54. Blakemore J, Naftolin F. Aromatase: contributions to physiology and disease in women and men. Physiology. 2016;31(4):258–269. doi:10.1152/physiol.00054.2015
55. Fruzzetti F, Fidecicchi T, Guevara MMM, et al. Estetrol: a new choice for contraception. J Clin Med. 2021;10:5625. doi:10.3390/jcm10235625
56. Webb P, Lopez GN, Uht RM, et al. Tamoxifen activation of the estrogen receptor/AP-1 pathway: potential origin for the cell-specific estrogen-like effects of antiestrogens. Mol Endocrinol. 1995;9(4):443–456. doi:10.1210/mend.9.4.7659088
57. Fadiel A, Song J, Tivon D, et al. Phenytoin is an estrogen receptor α-selective modulator that interacts with helix 12. Reprod Sci. 2015;22(2):146–155. doi:10.1177/1933719114549853
58. Nachtigall MJ, Jessel RH, Flaumenhaft R, et al. The selective estrogen receptor modulator DT56a (Famarelle) does not affect platelet reactivity in normal or thrombophilic postmenopausal women. Menopause. 2011;18(3):285–288. doi:10.1097/gme.0b013e3181f2f01a
59. Kuiper GG, Carlsson B, Grandien K, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinol. 1997;138(3):863–870. doi:10.1210/endo.138.3.4979
60. Ceccarelli I, Bioletti L, Peparini S, et al. Estrogens and phytoestrogens in body functions. Neurosci Biobehav Rev. 2022;132:648–663. doi:10.1016/j.neubiorev.2021.12.007
61. Thornton MJ. Estrogens and skin aging. Dermatoendocrinology. 2013;5(2):264–270. doi:10.4161/derm.23872
62. Horng H-C, Chang W-H, Yeh -C-C, et al. Estrogen effects on wound healing. Internat J Mol Sci. 2017;18(11):2325. doi:10.3390/ijms18112325
63. Campbell L, Emmerson E, Davies F, et al. Estrogen promotes cutaneous wound healing via estrogen receptor beta independent of its anti-inflammatory activities. J Exp Med. 2010;207(9):1825–1833. doi:10.1084/jem.20100500
64. Ahn S, Chantre CO, Ardona HAM, et al. Biomimetic and estrogenic fibers promote tissue repair in mice and human skin via estrogen receptor beta. Biomaterials. 2020;255:120149. doi:10.1016/j.biomaterials.2020.120149
65. Wilkinson HN, Hardman MJ. A role for estrogen in skin ageing and dermal biomechanics. Mech Ageing Dev. 2021;197:111513. doi:10.1016/j.mad.2021.111513
66. Pomari E, Valle LD, Pertile P, et al. Intracrine sex steroid synthesis and signaling in human epidermal keratinocytes and dermal fibroblasts. FASEB J. 2015;29(2):508–524. doi:10.1096/fj.14-251363
67. Carnesecchi J, Malbouyres M, de Mets R, et al. Estrogens induce rapid cytoskeleton reorganization in human dermal fibroblasts via the non-classical receptor GPR30. PLoS One. 2015;10(3):e0120672. doi:10.1371/journal.pone.0120672
68. Arnai J-F, Lenfant F, Metivier R, et al. Membrane and nuclear estrogen receptor alpha actions: from tissue specificity to medical implications. Physiol Rev. 2017;97(3):1045–1087. doi:10.1152/physrev.00024.2016
69. Zhang Y, Li L, Xu Y, et al. Protective mechanism of GPR30 agonist G1 against ultraviolet B-induced injury in epidermal stem cells. Artif Cells Nanomed Biotechnol. 2019;47(1):4165–4171. doi:10.1080/21691401.2019.1687497
70. Triplett KD, Pokhrel S, Castleman MJ, et al. GPER activation protects against epithelial barrier disruption by Staphylococcus aureus alpha-toxin. Sci Rep. 2019;9:1343. doi:10.1038/s41598-018-37951-3
71. Kalkhoran SB, Kararigas G. Oestrogenic regulation of mitochondrial dynamics. Internat J Mol Sci. 2022;23:1118. doi:10.3390/ijms23031118
72. Torrens-Mas M, Pons D-G, Sastre-Serra J, Oliver J, Roca P. Sexual hormones regulate the redox status and mitochondrial function in the brain. Pathological implications. Redox Biol. 2020;13:101505. doi:10.1016/j.redox.2020.101505
73. Tsialtas I, Georgantopoulos A, Karipidou ME, et al. Anti-apoptotic and antioxidant activities of the mitochondrial estrogen receptor beta in N2A neuroblastoma cells. Internat J Mol Sci. 2021;22:7620. doi:10.3390/ijms22147620
74. Sanchez-Borrego R, Mendoza N, Llaneza P. A prospective study of DT56a (Femarelle) for the treatment of menopause symptoms. Climacteric. 2015;18(6):813–816. doi:10.3109/13697137.2015.1065247
75. Fore J. A review of skin and the effects of aging on skin structure and function. Ostomy Wound Manag. 2006;52(9):24–35.
76. Nikolakis G, Stratakis CA, Kanaki T, et al. Skin steroidogenesis in health and disease. Rev Endocrinol Metab Disord. 2016;17(3):247–258. doi:10.1007/s11154-016-9390-z
77. Farage MA, Miller KW, Maibach HI. Degenerative changes in aging skin. In: Farage MA, Miller KW, Haibach HI, editors. Textbook of Aging Skin. Berlin: Springer; 2010:225–235.
78. Stevenson S, Thornton J. Effects of estrogens on skin aging and the potential role of SERMs. Clin Intervention Aging. 2007;2(3):283–297. doi:10.2147/CIA.S798
79. Fujimura T, Haketa K, Hotta M, et al. Loss of skin elasticity precedes to rapid increase of wrinkle levels. J Dermatol Sci. 2007;47(3):233–239. doi:10.1016/j.jdermsci.2007.05.002
80. Quan C, Yan Y, Qin Z, Lin Z, Quan T. Ezrin regulates skin fibroblast size/mechanical properties and YAP-dependent proliferation. J Cell Commun Signal. 2018;12:549–560. doi:10.1007/s12079-017-0406-6
81. Kim Y-S, Kim T-H, Park ED, Fadiel A, Naftolin F. Ezrin expression and activation in hypertrophic and keloid scar. Gynecol Reprod Endocrinol Metab. 2020;1:29.
82. Ruiz-Larrean MB, Martin C, Martinez R, et al. Antioxidant activities of estrogens against aqueous and lipophilic radicals: differences between phenol and catechol estrogens. Chem Phys Lipids. 2000;105(2):179–188. doi:10.1016/S0009-3084(00)00120-1
83. Chang S-H, Chang C-H, Yang M-C, et al. Effects of estrogen on glutathione and catalase levels in human erythrocyte during menstrual cycle. Biomed Rep. 2015;3(2):266–268. doi:10.3892/br.2014.412
84. Savoia P, Raina G, Camillo L, et al. Anti-oxidative effects of 17β-estradiol and genistein in human skin fibroblasts and keratinocytes. J Dermatol Sci. 2018;92(1):62–67. doi:10.1016/j.jdermsci.2018.07.007
85. Phuoung C, Maibach HI. Biological effects of estrogen on skin. In: Farage MA, Miller KW, Haibach HI, editors. Textbook of Aging Skin. Berlin: Springer; 2010:763–774.
86. Stanczyk FZ. Production, clearance, and measurement of steroid hormones. Glob Libr Womens Med. 2009. doi:10.3843/glowm.10278
87. Reilly DM, Lozano J. Skin collagen through the life stages importance for skin health and beauty. Plast Aesthet Res. 2021;8:2. doi:10.20517/2347-9264.2020.153
88. Monteleone P, Mascagni G, Giannini A, et al. Symptoms of menopause – global prevalence, physiology and implications. Nature Rev Endocrinol. 2018;14(4):199–215. doi:10.1038/nrendo.2017.180
89. Naftolin F, Friedenthal J, Nachtigall R, et al. Cardiovascular health and the menopause woman: the role of estrogen and when to begin and end hormone treatment. F1000Res. 2019;8:F1000 Faculty Rev–1576. doi:10.12688/f1000research.155548.1
90. Maximov PY, Lee TM, Jordan VC. The discovery and development of selective estrogen receptor modulators (SERMs) for clinical practice. Curr Clin Pharm. 2013;8:135–155. doi:10.2174/1574884711308020006
91. Lephart ED. Phytoestrogens (resveratrol and equol) for estrogen-deficient skin- controversies/misinformation versus anti-aging in vitro and clinical evidence via nutraceutical-cosmetics. Internat J Mol Sci. 2021;22:11218. doi:10/3390/ijms222011218
92. Chlebowski RT, Anderson GL, Aragaki AK, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the women’s health initiative randomized clinical trials. JAMA. 2020;324(4):369–380. doi:10.1001/jama.2020.9482
93. Chlebowski RT, Aragaki AK, Pan K. Breast cancer prevention: time for change. JCO Oncol Pract. 2021;17(2):709–716. doi:10.1200/OP.21.00343
94. Pickar JH, Boucher M, Morgenstern D. Tissue selective estrogen complex (TSEC): a review. Menopause. 2018;25(9):1033–1045. doi:10.1097/GME.0000000000001095
95. Bedell S, Nachtigall M, Naftolin F. The pros and cons of plant estrogens for menopause. J Steroid Biochem Mol Biol. 2014;139:225–236. doi:10.1016/j.jsbmb.2012.12.004
96. Mersereau JE, Levy N, Staub RE, et al. Liquiritigenin is a plant-derived highly selective estrogen receptor beta agonist. Mol Cell Endocrinol. 2008;283(1–2):49–57. doi:10.1016/j.mce.2007.11.020
97. Li H, Jia E, Hong Y, et al. Phytoestrogens and NAFLD: possible mechanism of action. Mini Rev Med Chem. 2020;20:578–583. doi:10.2174/1389557520666200103114123
98. Akahane T, Kaya D, Noguchi R, et al. Association between equol production status and nonalcoholic steatohepatitis. Int J Mol Sci. 2021;22:11904. doi:10.3390/ijms222111904
99. Lephart ED. 4’,7-Isoflavandiol (equol) enhances human dermal fibroblast renewal and has effects similar to 17β-estradiol in stimulating collagen and elastin expression. Cell cycle and RT-PCR analysis without Phenol Red. Cosmetics. 2021;8:5. doi:10.3390/cosmetics8010005
100. Garg A, Singh B, Sharma R, et al. Selective estrogen receptor modulators (SERMs): mechanistic insights against microbial infection. Curr Mol Med. 2020;20(2):102–115. doi:10.2174/1566524019666191014112133
101. Messina M. Soy foods, isoflavones, and the health of postmenopausal women. Am J Clin Nutr. 2014;100(Suppl. S1):423S–430S. doi:10.3945/ajcn.113.071464
102. Messina M. Soy and health update: evaluation of the clinical and epidemiologic literature. Nutrients. 2016;8:754. doi:10.3390/nu8120754
103. Rowe IJ, Baber RJ. The effects of phytoestrogens on postmenopausal health. Climacteric. 2021;24:57–63. doi:10.1080/13697137.2020.1863356
104. Messina M, Mejia SB, Cassidy A, et al. Neither soyfoods nor isoflavones warrant classification as endocrine disruptors: a technical review of the observational and clinical data. Critical Rev Food Sci Nutr. 2021. doi:10.1080/10408398.2021.1895054
105. Yoshikata R, Myint KZY, Ohta H, et al. Effects of equol-containing supplement on advanced glycation end products, visceral fat and climacteric symptoms in postmenopausal women: a randomized controlled trial. PLoS One. 2021;16(9):e0257332. doi:10.1371/journal.pone.0257332
106. Desmawati D, Sulastri D. Phytoestrogens and their health benefits. Macedonian J Med Sci. 2019;7(3):495–499. doi:10.3889/oamjms.2019.086
107. Svoboda RM, Del Rosso JQ, Zeichner JA, et al. Revisiting the beneficial effects of estrogen on skin: a comprehensive review of the literature and a look to the future. SKIN. 2018;2(5):308–316. doi:10.25251/2.3.4
108. Draelos ZD. A double-blind randomized pilot study evaluating the safety and efficacy of topical MEP in the facial appearance improvement of estrogen deficient females. J Drugs Dermatol. 2018;17(1):1186–1189.
109. Cohen JL. Evaluation of efficacy of a skin care regimen containing methyl estradiolpropanoate(MEP) for treating estrogen deficient skin. J Drugs Dermatol. 2018;18(12):1226–1230.
110. Inoue T, Miki Y, Abe K, et al. The role of estrogen-metabolizing enzymes and estrogen receptors in human epidermis. Mol Cell Endocrinol. 2011;344:35–40. doi:10.1016/j.mce.2011.06.015
111. Kazem S, Linssen EC, Gibbs S. Skin metabolism Phase I and Phase II enzymes in native and reconstructed human skin: a short review. Drug Discov Today. 2019;24(9):1889–1910. doi:10.1016/j.drudis.2019.06.002
112. Oesch F, Fabian E, Landsiedel R. Xenobiotica-metabolizing enzymes in the skin of rat, mouse, pig, Guinea pig, man, and in human skin models. Arch Toxic. 2018;92(8):2411–2456.
113. Barker CL, Clothier RH. Human keratinocyte cultures as models of cutaneous esterase activity. Toxicol In Vitro. 1997;11(5):637–640. doi:10.1016/S0887-2333(97)00037-4
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.