Back to Journals » International Journal of General Medicine » Volume 15
Factors Influencing Overall Survival for Patients with Fibrolamellar Hepatocellular Carcinoma: Analysis of the Surveillance, Epidemiology, and End Results Database
Authors Polychronidis G , Feng J, Murtha-Lemekhova A , Heger U, Mehrabi A, Hoffmann K
Received 21 September 2021
Accepted for publication 3 December 2021
Published 8 January 2022 Volume 2022:15 Pages 393—406
DOI https://doi.org/10.2147/IJGM.S338066
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Georgios Polychronidis,1 Jincheng Feng,2 Anastasia Murtha-Lemekhova,1 Ulrike Heger,1 Arianeb Mehrabi,1 Katrin Hoffmann1
1Department of General, Visceral and Transplant Surgery, Heidelberg University Clinic, Heidelberg, Germany; 2Department of Hepatobiliary Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China
Correspondence: Katrin Hoffmann
Department of General, Visceral and Transplant Surgery, University of Heidelberg, Im Neuenheimer Feld 420, Heidelberg, 69120, Germany
Email [email protected]
Background: The study aimed to develop a nomogram to predict overall survival (OS) for patients with FLC using a national database.
Methods: The Surveillance, Epidemiology, and End Results database of the National Cancer Institute was reviewed to identify FLC cases with histological confirmation between 2004 and 2014. Cox proportional hazards models were used to identify factors associated with OS. The validation of the nomogram was performed using concordance index (C-index) and calibration curves.
Results: Out of 170 cases with complete follow-up, 87 received surgery/ablation and 12 received transplantation with significantly higher OS than chemotherapy alone while transplantation combined with chemotherapy showed better survival than solely transplantation. The combination of surgery and chemotherapy showed worse OS than surgery alone. Survival was negatively influenced by T4 stadium (HR = 5.91), while young age and surgery were positive predictive factors. There was no influence of gender, ethnicity or nodal status on survival. The rate of AFP positivity was comparable with and without the presence of distal metastases.
Conclusion: FLC survival is greatly dependent upon appropriate surgical management irrespective of tumor stadium.
Keywords: fibrolamellar, hepatocellular, carcinoma, SEER
Background
Fibrolamellar hepatocellular carcinoma (FLC) is a rare type of primary liver tumor, mostly presenting in younger patients without underlying liver disease and cirrhosis.1–3 Due to its rarity, it has initially been classified as hepatocellular carcinoma (HCC), but the affected population and the underlying factors differ. National incidence also presents with marked variability, with the previously reported estimated age-adjusted incidence rate of less than 1 per 100,000 in the United States but with noticeably higher incidence in other countries such as Mexico (5.8% of liver tumors).4 The most commonly reported age span for the diagnosis of FLC is under 40 years of age.5 Diagnosis of FLC is mainly dependent on imaging since clinical symptoms are non-specific (abdominal pain is the most common symptom) and common biomarkers for liver disease are often negative. FLC presents most often as an enlarged single lesion with a centrally located scar in two-thirds of the cases. This makes the differentiation with other liver tumors (ie focal nodular hyperplasia) more difficult.6 Other entities which resemble FLC are adenomas or metastases of neuroendocrine tumors.7 The diagnosis is based on cross-imaging studies of CT and MRI, but a liver biopsy is recommended also for tumors that are deemed unresectable since there is a substantial risk of misdiagnosis.8–11
Timely resection of FLC is the main aspect of treatment; most patients are in need of extended liver resection since nodal and vascular infiltration have been reported as high as 60% and 43%, respectively.12–14 The option of liver transplantation has also been used for selected cases with good prognosis; however, there is little priority for patients with non-HCC liver tumors on the waiting list.15,16 A major prognosis burden is the fact that FLC presents high recurrence rates.17,18 Taking into account all these factors there is a need for better prognostic tools for FLC. Our goal was to assess the survival trends of FLC and construct a nomogram for overall survival (OS).
Methods
Data Source
A registry-based retrospective cohort study was performed using data from the SEER database for the years 2004–2014. SEER is supported by the Surveillance Research Program (SRP) in NCI’s Division of Cancer Control and Population Sciences (DCCPS). SEER Program provides information on comprehensive demographic and cancer statistics covering 28% of the US population.19 The characteristics and representativeness of this database have been discussed previously.20,21
Study Population
Between January 1, 2004, and December 31, 2014, 199 patients were diagnosed with FLC. This calendar year interval was chosen to provide a sufficient information on tumor characteristics and chemotherapy use. Cases were identified using a specific histologic code (International Classification of Diseases for Oncology [ICD-O] code 8171). Patients who had no confirmed pathology diagnosis (N=17), diagnosed at autopsy or by death certificate (N=1) and had another malignant primary tumor (N=11) were excluded. All cases were restricted to first primary FLC. A total of 170 patients were selected for further analysis.
The following variables were retrieved from the SEER database: sex, age (categorized as age <18, 18–39, 40–64, and >65 years), ethnicity (Caucasian, African-American, other (American Indian/Alaska native, Asian/Pacific islander)), year of diagnosis, insurance status, tumor multiplicity and size, tumor stage, AFP status, chemotherapy use and surgical management. Because all patient information in the SEER database is de-identified, this study was exempted from review by the Institutional Review Board.
Statistical Analysis
We conducted a univariate analysis for surgical approach, chemotherapy, and the combination of these approaches as a categorical outcome with a list of covariates. Univariate analysis was undertaken using validated dataset macros.22 The p-value was calculated by ANOVA for numerical covariates and chi-square test or Fisher’s exact for categorical covariates, where appropriate. The primary end point in this study was overall survival. OS was defined as the time from diagnosis to death, date of last follow-up. Kaplan–Meier methods and the Log rank test were utilized for the survival analysis. A Cox proportional hazards model was applied for multivariate survival analysis. Candidate variables with a p value <0.01 on univariate analysis for survival were subjected to multivariable Cox regression analysis. In addition, to adjust for the potential confounding effect due to sex, the multivariable model was fit where sex was included as a covariate.
The performance of the nomogram was measured by concordance index (C-index) and assessed by comparing nomogram-predicted versus observed Kaplan–Meier estimates of survival probability rates. Bootstraps with 1000 resample were used for internal validation purposes. Bootstrapping was used to obtain a relatively unbiased estimate. Statistical analyses were carried out with SAS version 9.4 for Windows (SAS Institute Inc., Cary, NC, USA) and R version 3.5.3 (https://www.r-project.org/). All tests of statistical significance were 2-sided, and statistical significance was established at P < 0.05.
Results
Demographics
Young adults aged under 40 years had the highest incidence (70%) with the majority of patients being Caucasian males. The 3-year incidence of the disease showed a marked reduction of 12.3% over the three periods of study. At baseline, most tumors had surpassed the size cut-off of 5cm (68.2%) while most patients had single lesions. Vascular infiltration was present at 39.4% of tumors and most tumors did not present with positive AFP values at baseline.
More than half of the patients received surgical treatment or transplantation, but we observed a trend towards less surgical treatment over the study period. Between 2004–2007 and 2008–2011, less patients received surgery or transplantation, but this was not statistically significant (chi-squared test p=0.104). Half of the patients received chemotherapy in this study cohort. The detailed demographics of the study sample are shown in Table 1.
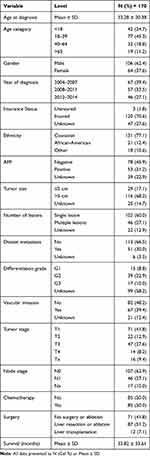 |
Table 1 Descriptive Statistics for All Variables |
Univariate Analysis
We performed a univariate analysis of covariates regarding surgical approaches in order to infer which factors affect surgical treatment in FLC. Chemotherapy was added as both a variable and a factor in separate analyses in order to ascertain the effect of chemotherapy on whether surgical treatment is chosen. In the analysis of surgery, the majority of patients presented with a single lesion (70.1%). Apart from tumor multiplicity, the tumor nodal status and size were of significance; however, most patients in the surgical treatment group presented with lesions larger than 5cm (75.9% vs 19.5% with ≤5cm) (Table 2).
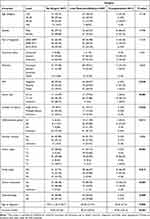 |
Table 2 Univariate Association with Study Cohort and Surgery |
Patients with distant metastases were treated more often with chemotherapy, although the database findings showed that 16 patients underwent resection and 2 underwent transplantation while distant metastasis was present. In the univariate analysis of chemotherapy, only age, N1, M1 and T were significantly associated with this treatment(Table 3).
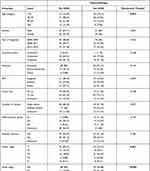 |
Table 3 Univariate Association with Study Cohort and Chemotherapy |
We performed a univariate association analysis of surgery and chemotherapy with the other covariates in our dataset. This revealed that with no chemotherapy only the tumor multiplicity, size and T stage are the only covariates with significant differences between surgical treatment groups. Patients receiving no chemotherapy and no surgical treatment were noticeably older than patients receiving resection or transplantation (mean age of 53.2±20.7 Vs 29.4±17.4 and 26.7±8.1 years of age, p< 0.001) (Table 2 and Supplementary Table 1). Another interesting finding in this cohort is that pediatric and adolescent patients did not receive chemotherapy when not being resected, whereas the opposite was the case for patients with liver resection/ablation. The positivity of AFP was comparable in patients with and without metastases (Supplementary Table 2).
Survival Analysis
We evaluated survival trends in the univariate analysis of both surgery and chemotherapy (Figures 1 and 2). The results have been in favor of surgery with a mean survival of 50 months. Chemotherapy alone did not show any influence on survival. Still, patients receiving both surgery and chemotherapy had a mean survival of 34 months, while patients with just resection and no chemotherapy showed a mean of 50.5 months. This finding was significant in both the chemotherapy and non –chemotherapy groups (Supplementary Table 1).
 |
Figure 1 Overall survival plot. |
 |
Figure 2 Survival plot depending on surgery, no resection and transplantation. |
The 5-year overall survival (OS) rate of all patients was 40.3% while for those who had undergone surgery it was 60.7%. The overall 10-year survival was 20.8%. We examined univariate associations between each variable of interest and the time-to-event survival using Cox proportional hazards models. Out of all patients’ characteristics, young age was the significant factor for better prognosis with an HR of 0.15 (95% CI 0.06–0.35) for patients under 18 years of age (Supplementary Table 3).
These results were incorporated in a multivariate Cox proportional hazards model and a Cox proportional hazards model with backward selection at a specified significance α level of 0.05.
There was no influence of gender, race, or nodal status on survival. Surgery and chemotherapy were the most significant independent prognostic factors for survival with an HR of 0.18 for resection, 0.14 for transplantation and 0.58 for chemotherapy (Table 4). Histologically undifferentiated tumors, distant metastases and age over 40 were independent negative factors of survival predictions. The factor with the highest impact on negative prognosis was the tumor stage with T4 showing an HR=5.91 (95% CI 1.19–29.33).
 |
Table 4 Univariable Analysis and Cox Proportional Hazards Regression Analysisa |
When utilizing a backward model of high significance with the last values as reference, the presence of distant metastases (HR=3.64; 95% CI 1.20–11.00) and the lack of surgical treatment (HR=4.64; 95% CI 1.40–15.37) were the most harmful prognostic factors. On the other hand, the age under 18 (HR 0.15; 95% CI 0.06–0.35) and under 35 (HR=0.30; 95% CI 0.14–0.64) as well as a T2 stage (HR=0.31; 95% CI 0.11–0.87) had the highest positive factor predictability. A good (G1) histological differentiation (HR=0.39; 95% CI 0.16–0.98) was also marginally an independent predictive factor of positive prognostic value while a G3 differentiation (HR=2.45; 95% CI 1.20–5.01) of negative one. Based on these findings, we created a survival nomogram with validation in 1-, 3- and 5-year survival (Figures 3 and 4). The nomogram showed that age was the largest predictor of OS, followed by surgery and tumor stage.
 |
Figure 3 Predictive survival nomogram. |
 |
Figure 4 Predicted 1-year (A), 3-year (B) and 5-year (C) survival based on the nomogram. |
Discussion
FLC is a disease with unique molecular characteristics affecting children and young adults, with a slow progression.6,23–26 Recent studies have identified a fusion gene that could provide with liquid biopsy alternatives or even therapeutic targets in the future.11,27,28 However, despite higher diagnostic rates and better understanding in its pathophysiology, FLC remains a disease with enigmatic prognosis. At present, surgical resection remains the main aim of treatment with a reported OS of 75–88 months.1 Our study showed that resection offers a median OS of 92 months with a 5-year overall survival rate of 60.7%. A very interesting finding proved the difference in survival among cases with a combination of chemotherapy and transplantation (27.44 months) versus cases treated with transplantation only (26.67 months), a finding that has not been described in extent before. Patients not undergoing surgery and not receiving treatment with chemotherapy showed a mean survival of 5.9 months, significantly lower than patients with chemotherapy. These were the only two groups with significant differences in patients’ mean age. This is an interesting finding that hints that patients who are not staged timely for chemotherapy tend to be older and survive much less. In a univariate association between chemotherapy and distant metastases, the only significant association was the age difference between patients who received chemotherapy while having a positive metastasis status (mean age 24.6 ±12.7 vs 38.1± 20.7; p=0.007); nonetheless, the survival of patients with distant metastases was comparable regardless of receiving chemotherapy treatment. This finding is significant when comparing metastasis and age in other cancer groups, where the mean age of oncological treatment is much higher.
The incidence of FLC has been previously described as low as 0.2/100,000 and previous publications on the SEER database have been reporting variable annual incidence.14,29,30 In our analysis, where patients were stratified in four categories we accounted for more than 30 new cases per year.
The already described factors predicting OS and DFS in FLC have been quite variable during the past years since most publications are based on case series with small sample size.31 Matching previous data, metastases and the differentiation of tumors were negative predictive factors. Furthermore, age and the lack of surgical treatment were factors with negative predictive value. Our most impressive finding was that larger tumors had a negative prognostic value, although one can see that most of these patients were surgically treated. This can be a case of small sample size (5 patients in total) but also a situation where recurrence occurred.
Concerning the diagnosis of FLC, multimodal imaging is of paramount importance since the clinical symptoms are inconstant and the diagnostic value of AFP is marginal. Our study confirmed that the levels of AFP are not indicative of disease or even of distant metastases. However, the null association of AFP with distant metastases at baseline in a univariate analysis has been demonstrated for the first time in this study. This finding may have more than the hypothesized limited clinical significance. A combination of this marker with other biomarkers should be tried in larger datasets.
The value of aggressive surgery has been demonstrated since the first publications on FLC and although even high-volume centers report a significant proportion of recurrent disease, the 5-year prognosis remains higher than this of not resected/ablated tumors. Our study was consistent with the findings of other analyses, which showed better survival for surgery/ablation, but in the present study, the effect of chemotherapy in combination with surgery showed actually a lower OS. This may be attributable to advanced disease, because due to age of diagnosis, one can postulate that the patients had few underlying conditions.
This study has a number of limitations apart from possible selection bias, with the database-derived factors being the most important ones. We had a relatively large sample size; however, due to the fact that other databases are not open for validation, we could not validate the analysis presented here. Another limitation is that some transplantation cases were not classified as patients who had received chemotherapy before or after transplantation, which would be a novel approach to this disease. Furthermore, the SEER does not contain data on recurrence, which would help specify our findings on the role of surgery or transplantation on survival. Finally, data on chemotherapy type/duration are required in order to assess its influence on survival.
Conclusions
The prognosis of FLC depends greatly on its surgical management. Surgery irrespective of combination with (neo-) adjuvant chemotherapy offers significantly better survival than systemic therapy alone.
Abbreviations
FLC, fibrolamellar hepatocellular carcinoma; HCC, hepatocellular carcinoma; OS, overall survival.
Data Sharing Statement
SEER data are available after registration. The authors will provide any additional data, including the analysis dataset at reasonable request.
Ethics Approval and Consent to Participate
N/A (The SEER database did not prerequisite any ethics approval since patient data are pseudonymised). Studies in which exclusively anonymous data are collected and evaluated are not required to be submitted to the ethics committee of the Medical Faculty of Heidelberg University.
Research with anonymous data does not fall under the consultation and submission obligation of Section 15 (1) of the Professional Code for Doctors in Baden-Württemberg.
Consent for Publication
The authors have consented to publication after having read the final manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by China Scholarship Council [File No.201708080137].
Disclosure
The authors have declared no conflicts of interest.
References
1. Mavros MN, Mayo SC, Hyder O, Pawlik TM. A systematic review: treatment and prognosis of patients with fibrolamellar hepatocellular carcinoma. J Am Coll Surg. 2012;215(6):820–830. doi:10.1016/j.jamcollsurg.2012.08.001
2. Craig JR, Peters RL, Edmondson HA, Omata M. Fibrolamellar carcinoma of the liver - a tumor of adolescents and young-adults with distinctive clinicopathologic features. Cancer. 1980;46(2):372–379. doi:10.1002/1097-0142(19800715)46:2<372::AID-CNCR2820460227>3.0.CO;2-S
3. Edmondson HA. Differential diagnosis of tumors and tumor-like lesions of liver in infancy and childhood. AMA J Dis Child. 1956;91(2):168–186. doi:10.1001/archpedi.1956.02060020170015
4. Arista-Nasr J, Gutierrez-Villalobos L, Nuncio J, Maldonaldo H, Bornstein-Quevedo L. Fibrolamellar hepatocellular carcinoma in Mexican patients. Pathol Oncol Res. 2002;8(2):133–137.
5. Abdul-Al HM, Wang G, Makhlouf HR, Goodman ZD. Fibrolamellar hepatocellular carcinoma: an immunohistochemical comparison with conventional hepatocellular carcinoma. Int J Surg Pathol. 2010;18(5):313–318. doi:10.1177/1066896910364229
6. Lalazar G, Simon SM. Fibrolamellar carcinoma: recent advances and unresolved questions on the molecular mechanisms. Semin Liver Dis. 2018;38(1):51–59. doi:10.1055/s-0037-1621710
7. Liu S, Chan KW, Tong J, Wang Y, Wang B, Qiao L. PET-CT scan is a valuable modality in the diagnosis of fibrolamellar hepatocellular carcinoma: a case report and a summary of recent literature. Qjm-An Int J Med. 2011;104(6):477–483. doi:10.1093/qjmed/hcr040
8. Torbenson M. Fibrolamellar carcinoma: 2012 update. Scientifica. 2012;2012:1–15. doi:10.6064/2012/743790
9. Malouf G, Falissard B, Azoulay D, et al. Is histological diagnosis of primary liver carcinomas with fibrous stroma reproducible among experts? J Clin Pathol. 2009;62(6):519–524. doi:10.1136/jcp.2008.062620
10. Malouf GG, Job S, Paradis V, et al. Transcriptional profiling of pure fibrolamellar hepatocellular carcinoma reveals an endocrine signature. Hepatology. 2014;59(6):2228–2237. doi:10.1002/hep.27018
11. Simon EP, Freije CA, Farber BA, et al. Transcriptomic characterization of fibrolamellar hepatocellular carcinoma. Proc Natl Acad Sci USA. 2015;112(44):E5916–E25. doi:10.1073/pnas.1424894112
12. Ang CS, Kelley RK, Choti MA, et al. Clinicopathologic characteristics and survival outcomes of patients with fibrolamellar carcinoma: data from the fibrolamellar carcinoma consortium. GCR. 2013;6(1):3–9.
13. Lafaro KJ, Eng OS, Raoof M, et al. A prognostic nomogram for patients with resected fibrolamellar hepatocellular carcinoma. Hepatobiliary Surg Nutr. 2019;8(4):338–344. doi:10.21037/hbsn.2019.05.03
14. Mayo SC, Mavros MN, Nathan H, et al. Treatment and prognosis of patients with fibrolamellar hepatocellular carcinoma: a national perspective. J Am Coll Surg. 2014;218(2):196–205. doi:10.1016/j.jamcollsurg.2013.10.011
15. Atienza LG, Berger J, Mei X, et al. Liver transplantation for fibrolamellar hepatocellular carcinoma: a national perspective. J Surg Oncol. 2017;115(3):319–323. doi:10.1002/jso.24515
16. Grossman EJ, Millis JM. Liver transplantation for non-hepatocellular carcinoma malignancy: indications, limitations, and analysis of the current literature. Liver Transpl. 2010;16(8):930–942. doi:10.1002/lt.22106
17. Groeschl RT, Miura JT, Wong RK, et al. Multi-institutional analysis of recurrence and survival after hepatectomy for fibrolamellar carcinoma. J Surg Oncol. 2014;110(4):412–415. doi:10.1002/jso.23658
18. Stipa F, Yoon SS, Liau KH, et al. Outcome of patients with fibrolamellar hepatocellular carcinoma. Cancer. 2006;106(6):1331–1338. doi:10.1002/cncr.21703
19. Surveillance, Epidemiology, and End Results (SEER) program (www. seer.cancer.gov) research data (1973–2015), National Cancer Institute, DCCPS, surveillance research program, surveillance systems branch, released April 2018, based on the November 2018 submission. Available from: https://seer.cancer.gov/.
20. Doll KM, Rademaker A, Sosa JA. Practical guide to surgical data sets: Surveillance, Epidemiology, and End Results (SEER) database. JAMA Surg. 2018;153(6):588–589. doi:10.1001/jamasurg.2018.0501
21. Nathan H, Pawlik TM. Limitations of claims and registry data in surgical oncology research. Ann Surg Oncol. 2007;15(2):415–423. doi:10.1245/s10434-007-9658-3
22. Liu Y, Nickleach DC, Zhang C, Switchenko JM, Kowalski J. Carrying out streamlined routine data analyses with reports for observational studies: introduction to a series of generic SAS ((R)) macros. F1000Res. 2018;7:1955. doi:10.12688/f1000research.16866.1
23. Pinna AD, Iwatsuki S, Lee RG, et al. Treatment of fibrolamellar hepatoma with subtotal hepatectomy or transplantation. Hepatology. 1997;26(4):877–883. doi:10.1002/hep.510260412
24. Dinh TA, Vitucci ECM, Wauthier E, et al. Comprehensive analysis of the cancer genome atlas reveals a unique gene and non-coding RNA signature of fibrolamellar carcinoma. Sci Rep. 2017;7. doi:10.1038/srep44653
25. Graham RP, Jin L, Knutson DL, et al. DNAJB1-PRKACA is specific for fibrolamellar carcinoma. Modern Pathol. 2015;28(6):822–829. doi:10.1038/modpathol.2015.4
26. Graham RP, Yeh MM, Lam-Himlin D, et al. Molecular testing for the clinical diagnosis of fibrolamellar carcinoma. Modern Pathol. 2018;31(1):141–149. doi:10.1038/modpathol.2017.103
27. Darcy DG, Chiaroni-Clarke R, Murphy JM, et al. The genomic landscape of fibrolamellar hepatocellular carcinoma: whole genome sequencing of ten patients. Oncotarget. 2015;6(2):755–770. doi:10.18632/oncotarget.2712
28. Honeyman JN, Simon EP, Robine N, et al. Detection of a recurrent DNAJB1-PRKACA chimeric transcript in fibrolamellar hepatocellular carcinoma. Science. 2014;343(6174):1010–1014. doi:10.1126/science.1249484
29. Eggert T, McGlynn KA, Duffy A, Manns MP, Greten TF, Altekruse SF. Epidemiology of fibrolamellar hepatocellular carcinoma in the USA, 2000–10. Gut. 2013;62(11):1667–1668. doi:10.1136/gutjnl-2013-305164
30. Eggert T, McGlynn KA, Duffy A, Manns MP, Greten TF, Altekruse SF. Fibrolamellar hepatocellular carcinoma in the USA, 2000–2010: a detailed report on frequency, treatment and outcome based on the Surveillance, Epidemiology, and End Results database. United European Gastroenterol J. 2013;1(5):351–357. doi:10.1177/2050640613501507
31. Lemekhova A, Hornuss D, Polychronidis G, et al. Clinical features and surgical outcomes of fibrolamellar hepatocellular carcinoma: retrospective analysis of a single-center experience. World J Surg Oncol. 2020;18(1):93. doi:10.1186/s12957-020-01855-2
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
