Back to Journals » Research and Reports in Tropical Medicine » Volume 10
Factors associated with scabies outbreaks in primary schools in Ethiopia: a case–control study
Authors Ejigu K, Haji Y , Toma A , Tadesse BT
Received 7 May 2019
Accepted for publication 12 August 2019
Published 27 August 2019 Volume 2019:10 Pages 119—127
DOI https://doi.org/10.2147/RRTM.S214724
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Mario Rodriguez-Perez
Kefele Ejigu,1 Yusuf Haji,1 Alemayehu Toma,2 Birkneh Tilahun Tadesse3
1Department of Epidemiology and Biostatistics, School of Public Health, College of Medicine and Health Sciences, Hawassa University, Hawassa, Ethiopia; 2Department of Pharmacology, School of Medicine, College of Medicine and Health Sciences, Hawassa University, Hawassa, Ethiopia; 3Department of Pediatrics, School of Medicine, College of Medicine and Health Sciences, Hawassa University, Hawassa, Ethiopia
Correspondence: Birkneh Tilahun Tadesse
Department of Pediatrics, School of Medicine, College of Medicine and Health Sciences Hawassa University, PO Box 1560, Hawassa, Ethiopia
Tel +25 191 155 1807
Email [email protected]
Background: Scabies is a neglected tropical disease affecting more than 200 million people worldwide every year. Scabies in school adolescents and young adults could affect their school performance. The current study investigates the factors associated with an outbreak of scabies at primary schools in southern Ethiopia.
Method: A team of health professionals investigated an outbreak of scabies that occurred in primary schools from May 1 to 30, 2018. An unmatched case–control study was employed to assess factors which predisposed for the scabies outbreak. Cases of scabies were individuals having a skin lesion compatible with the WHO case definitions of scabies. Controls were from the same locality with no skin lesions. Data on sociodemographic and behavioral variables were collected using questionnaires. Data on clinical presentations of scabies among cases were recorded by two trained and experienced health professionals. Factors associated with scabies were assessed using bivariate and multivariate logistic regression, and strength of association was described using odds ratio (OR) and 95% confidence intervals (CIs).
Results: A total of 711 (237 cases and 474 controls) study subjects participated in the study. The mean age of study participants was 17.56±2.66 years. Poor knowledge about scabies, adjusted odds ratio (AOR)=4.32 (95% CI: 2.93, 6.36); male sex, AOR=2.69 (95% CI: 1.82, 3.96); and parental illiteracy, AOR =3.49 (95% CI: 2.06, 5.94) predicted scabies infestation. Additionally, socioeconomic variables like sharing clothes/beds or contact with others, AOR=3.12 (95% CI: 2.12, 4.59); low household annual income, AOR=2.13 (95% CI: 1.32, 3.44); and family size greater than five, AOR=1.77 (95% CI: 1.04, 3.01) were significantly associated with scabies infestation. Inaccessibility and poor utilization of water, AOR=1.64 (95% CI: 1.12, 2.40) and poor personal hygiene, AOR=1.69 (95% CI: 1.14, 2.51) were also factors independently associated with scabies.
Conclusion: Modifiable risk factors such as personal hygiene and literacy level were found to be independent predictors of scabies infestation. Access to and utilization of water were also important predictors. Strategies for poverty alleviation and awareness creation on personal hygiene and efficient use of water are recommended for effective prevention of scabies infestation in closed institutions.
Keywords: scabies outbreak, primary school, Ethiopia
Background
Scabies is one of the most common dermatological problems and affects about 200 million people each year worldwide.1 The highest burden of scabies is recorded in hot tropical and pacific countries.2 Poverty and drought are associated with aggravation of endemic scabies disease in hot and tropical areas.3,4 Globally, scabies directly affects over 200 million people leading to about 21 in 1000 disability-adjusted life years.1,5 The effect of scabies on the skin alone is associated with 1.5 million years lived with disability while effects on other systems like the kidneys and the heart are even much more.5 With this wide variation of its burden worldwide, scabies causes significant morbidity around the world.3,6 Scabies mostly affects marginal age groups and resource-limited communities.7,8 For example, prevalence of scabies infestation was 17.8% in young children at Cameroon boarding schools, 5.88% at hospital settings in Bangui with the major risk factors being overcrowding and poverty. The closed institutions like schools, prisons, and refugee camps are vulnerable for scabies outbreak.7,9–11
The clinical features of scabies include itching and skin rash caused by sensitization (allergic reactions) to mite proteins or feces. Severe itching also known as “scabies rash” worsens at night. The scabies symptoms involve almost all parts of the body but mostly affected sites include interdigital spaces, wrist, elbow, armpit, penis, nipple, waist, buttock, abdomen, shoulder places, feet, and thigh. In children and infants, head, feet, neck palms and soles are involved.12–14
Patients having underlying immune-suppressive conditions easily progress to severe forms of scabies. A severe form of scabies, called Norwegian or crusted scabies, occurs during conditions that suppress the immune system.15,16 This form of scabies needs to be treated promptly as infected patients with Norwegian scabies exacerbate further transmission of disease and a potential risk for outbreak of scabies. In addition, scabies is often complicated by secondary bacterial infections leading to the development of skin sores and other more serious consequences such as septicemia, chronic heart, and kidney diseases.17–19
Scabies is a major health problem in Africa specifically in the sub-Saharan region including Ethiopia.20,21 About 86% of the children in Sierra Leone displacement camps are affected by scabies and is high in countries with unstable conditions and civil war. Ethiopia is one of the sub-Saharan African countries where scabies is endemic. In Ethiopia, 6.2% of the school-age children, 13.6% in under five children, and 5.6% of the orphan school children are affected by scabies.20,22
There are a variety of risk factors for scabies. It was reported that overcrowding, sex, level of education of parents or persons, income level, employment, place of residence, clothes or bed-sharing history, housing condition, personal hygiene, flooding, and contact history with patients having skin lesion were associated with scabies.21,23,24 The current study was done as part of a scabies outbreak investigation and management in southern Ethiopia. The outbreak was declared by the regional health bureau in the months of April and May 2018. This study was done as part of the outbreak investigation and the efforts to contain and treat the outbreak. The objectives of the study included describing clinical presentation of scabies and assessing factors associated with scabies among primary school students between the ages of 15 and 25 years in Damboya, Kembata-Tembaro Zone, Southern Nations, and Nationalities Peoples Regional (SNNPR) State, Ethiopia.
Methods and materials
Study area and setting
This study was conducted from May 1 to 30, 2018 at Damboya district in Kembata-Tembaro Zone, SNNP Region in Ethiopia. Damboya is located 216 km south of Addis Ababa. Damboya town is the administrative town of the district. The population of the district was estimated to be 109,804 in 2017. The district had 20 Kebeles (17 rural and 3 are urban). In the district, there are 32 schools of which 25 are primary and 4 are secondary schools. Among primary schools, 13 of them are first cycle (Grades 1–4) and 12 of them are second cycle (Grades 5–8) schools. In the district, there are four health centers and six health posts.21,23,24 Scabies was reported from all of the primary schools when an outbreak was declared in April–May 2018.
Study design and population
Unmatched case–control study (1:2 ratio) was conducted among adolescents and young adults in primary schools in Damboya district. The study population was adolescent and young adult students at primary schools (15–25 years old) in the study area who were attending class during the study period.
Cases and controls
Scabies cases
Scabies cases were adolescent and young adult students (15–25 years old) who were residents of Damboya with dermatologic manifestations of superficial burrows, intense itching (pruritus), especially at night and/or generalized pruritic skin rash with or without signs of secondary infection or/and dermatological problems clinically diagnosed as scabies by health professionals having special training on scabies.25
Controls
Controls were adolescent or young adult (15–25 years old) primary school students who were free from symptoms and signs of dermatological problems and were living in Damboya woreda.
Sample size calculation
The sample size was estimated using the following assumptions: confidence level was set at 95% and power of study at 80%. The case to control ratio was 1:2. Using exposure to infirmity as a risk factor to calculate sample size (odds ratio=1.62, 50% of the controls are exposed),25 a minimum sample size of 220 cases and 439 controls was obtained giving a total of 659 study subjects. After 10% non-response rate was considered and calculated, the final sample size was 725.
Sampling procedure and selection of participants
Selection of cases
An active case search was conducted in 9 out of 12 primary schools in Damboya woreda. Cases were enrolled consecutively. Three schools were excluded due to absence of target age group. The total registered adolescent and young adult age group students were 1076 in the target sites of which 845 individuals were screened voluntarily to participate in this study. We identified 310 students with dermatological problems from those screened students and 237 students were diagnosed with scabies. During screening, direct observation of their hands, palms, and interdigital spaces were observed to diagnose scabies. The diagnosis of scabies infestation was mainly clinical, made based on the presence of the typical rash and symptoms of unrelenting and worsening itch, particularly at night.25
The detailed clinical assessment (history and physical examination) was conducted in private rooms at each of primary school. After the clinical assessment was completed, study subjects completed a self-administered questionnaire translated to local language (Kambatisaa). Two experienced public health officers were trained for 2 days to clinically examine cases using a structured checklist before data collection. Agreement of both health professionals on the diagnosis of cases and controls was required for inclusion to the study.
Selection of controls
Selection of controls was conducted in a similar way as the cases. The controls were selected from students in the same school as the cases. Sociodemographic and behavioral data were collected similar to the cases.
Data collection and quality assurance
A pretested structured questionnaire was used for data collection. The questionnaire was primarily prepared in English and then translated into local language “Kembatisaa” and back-translated into English by another translator. The basic information included in the structured questionnaire included sociodemographic variables such as age, sex, ethnicity, residency, and educational status of father and mother, average household income, and family size. Knowledge of participants about scabies, personal hygiene, water access and utilization-related variables, contact history with others, sharing clothes with others, and handwashing practice were recorded for both cases and controls. Before data collection, a 2-day training was given to case screeners and supervisors by the investigators.
Definitions
Contact with scabies
Contact is defined as a person without signs and symptoms consistent with scabies who has had direct contact (particularly prolonged, direct skin-to-skin contact) with a suspected or confirmed case in the 2 months prior to the survey.25
Good knowledge of scabies
This was computed by combining knowledge questions on causes of scabies, mode of transmission, prevention, and treatment. Those with an average value or more were regarded as having good knowledge while those below an average value were classified as poor knowledge.
Data analysis
Data were described using frequency (percentage), median (range), and mean (standard deviation). Descriptive statistics were presented as tables and a figure. Association between sociodemographic and behavioral variables and presence of scabies infestation was assessed at two stages – a bivariate binary logistic regression where associations between individual independent predictors and scabies (yes/no) was assessed. On the second stage, a multivariate binary logistic regression was used to build a model including all the variables with a P-value <0.25 from the bivariate analysis. Adjusted odds ratio (AOR) and 95% confidence intervals (CI) were generated to show the presence and strength of association between independent variables and scabies (yes/no). A P-value of <0.05 was considered as statistically significant for the multivariate analysis. SPSS for Windows version 20 was used for data analysis.
Ethics approval
Ethical clearance was obtained from the institutional review board (IRB) of Hawassa University College of Medicine and Health science and support letter was obtained from Kembata Tembero zone. For participants older than 18 years, an individual permission was obtained from each study subject after informed consent about the importance of participation in the study was described. For study participants who are less than 18 years, both consent from parents/guardians and assent were taken. The data collectors explained all necessary information that study subjects wanted to know about the study. The study subjects who decided to participate voluntarily were included in the study. Study subjects who refused to participate in the study received the treatment for scabies. Written informed consent was collected from all study subjects. This study was conducted in accordance with the Declaration of Helsinki.
Results
The sociodemographic characteristics of study participants
This study was done as part of the outbreak investigation efforts of scabies in a rural district in southern Ethiopia. For this case–control study, a total of 711 participants were included in the study, giving a response rate of 98% (711/725) with a case to control ratio of 1:2 (235:474). More than half, 393 (55.3%) were male. The mean age of study participants was 17.56 years old (standard deviation (SD) 2.66). The average age of scabies cases was 17.40 years±2.23 years while the average age of controls was 17.64 years with SD of 2.34. The average family size was 6.27 individuals (SD=1.75). More than a third, 260/711 (36.6%) of mothers were illiterate (cases, 46.5% vs controls, 32%; P-value<0.001) (Table 1).
 |
Table 1 Scio-demographic characteristics of adolescents and young adults in primary school students, Damboya district KembataTembaro, South Ethiopia, 2018 |
Factors associated with scabies
The sociodemographic variables such as low household annual income, male sex, larger family size, parental literacy were significantly associated with scabies. Similarly, poor knowledge about scabies, sharing clothing/bed or contact with scabies cases, water accessibility and utilization, and personal hygiene were associated with the disease in adolescent and young adult students (Table 2).
 |
Table 2 Bivariate analyses of factors associated with scabies among adolescents and young students in Damboya district, southern Ethiopia, 2018 |
Multivariate logistic regression analyses was conducted to identify independent risk factors for scabies. Covariates which were independent predictors of scabies infestation were poor knowledge about scabies (AOR=4.32; 95% CI: 2.93, 6.36), male sex (AO=2.69; 95% CI: 1.82, 3.96), parental illiteracy (AOR=3.49; 95% CI: 2.06, 5.94), low annual household income (AOR=2.13; 95% CI:1.32, 3.44), sharing clothes/beds or contact with scabies cases (AOR=3.12; 95% CI: 2.12, 4.59), average family size greater than five (AOR=1.531; 95% CI: 1.022, 2.294), poor personal hygiene (AOR=1.69, 95% CI:1.14, 2.51), access to water and utilization (AOR=1.64; 95% CI:1.12, 2.40) (Table 3).
 |
Table 3 Multivariate analyses of factors associated with scabies among adolescents and young adults at primary school students in Damboya district, south Ethiopia 2018 |
Clinical features
The most common clinical symptom identified among the cases was itching skin rash which accounted for 99.17% (235/237). The clinical examination findings revealed different types of skin rash among the cases. Nearly three-fourths, 73.41% (174/274) of cases had tiny red borrows or blisters on their skin. Some of the cases had discharge from scabies skin lesion. The discharge from skin lesion is either pus, 22.78% (54/237) or blood, 1.26% (3/237). Crusted skin lesions were observed in 2.95% (7/237) of the cases. Among patients that had taken medical treatment, 16.03% (34/237) used permethrin 5% lotion and 0.84% (2/237) benzyl benzoate lotion. Figure 1 summarizes the sites of the body affected by scabies among cases.
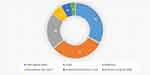 |
Figure 1 Body parts affected by scabies infestation among adolescents and young adults, Southern Ethiopia, 2018. |
Discussion
In the current study, poor knowledge about cause, prevention and treatment of scabies, parental illiteracy, low family income, large family size, male sex, sharing clothes/beds or contact with scabies cases, poor personal hygiene, and inaccessibility and poor utilization of water were assessed to the independent predictors of scabies infestation among adolescents and young adults attending a primary school in a resource-limited setting.
Scabies infection is a common problem in Ethiopia with a few outbreak investigation and management success stories.26 Outbreaks have been reported from several regions of the country including the southern region of Ethiopia.27,28 Scabies in Ethiopia and other similar settings where there are limited resources is strongly associated with low socioeconomic status and overcrowding which are in turn associated with significant level of poverty in a certain society.29
Knowledge about scabies could be associated with better personal protective measures from scabies infestation. Our findings are supported by previous reports that investigated the factors behind spreading of scabies infestation. The association between awareness of scabies infestation and risk of scabies was shown at Islamic boarding schools.30 Poor knowledge may be associated with practices of unfavorable personal behavior such as poor personal hygiene, sharing beds or clothes with others, and poor health care seeking for scabies treatment.
Overcrowding (family size of more than 5) was also associated with risk of scabies infestation. It was reported by former studies done elsewhere.31–33 This might be due to increased contact between individuals and shared clothing or beds as there might be inadequate resources in the household. Reports had similarly discussed that scabies spreads in crowded living conditions and institutions like schools.31–33
The finding of our study revealed that males are more affected than females by scabies. This result is similar to studies conducted in Cameroon at a boarding school11 and in Northern Ethiopia.34 However, the survey conducted in Fiji23 and in rural Nigeria35 reported that females were more affected. On the other hand, studies from Cameroon and Egypt among school children found no sex difference in the risk scabies.33 This might be due to differences in study populations and the study design. For example, one of the surveys included all age groups. Males tend to sleep together, share clothes, and have close contacts than females, and this might expose them to risk of scabies.
This study also revealed that lower household monthly income and poor parental education increased the odds of being infested with scabies. This is in line with the understanding that poor and disadvantaged communities are at risk of scabies infestation. For example, prevalence studies conducted in Egypt33 revealed that fathers’ and mothers’ education was one of the risk factors for scabies among primary school children. In resource-poor communities of rural Nigeria, poverty-related variables such as illiteracy and lower household incomes were accounted for seven times higher chance of having scabies infestation.35 This finding can be attributed to the impact of parental education on the social standings of a family, as educated fathers may have more income and their families have access for better medical consultations.
Another important risk factor for scabies is personal behavior such as sharing clothes, beds, and any close contact with a person having itching skin lesion in the previous 2 months and poor personal hygiene. The finding is supported by studies done in Ethiopia and Egypt31–33 where personal contact, bed sharing, or poor handwashing practices increased the risk of infestation by scabies among school children. Similarly, a study in Brazil36 revealed that poor educational levels, low household income, poor housing, and sharing clothes and towels with other family members or persons are all determinant factors for infestation by scabies.
This study also identified water inaccessibility and poor utilization as risk factors for infestation by scabies. Our results are in line with previous studies conducted in northern Ethiopia.34 The reasons for inadequacy of water for household consumption including keeping personal hygiene could be because of the season of the year when the data collection for the current study was done. The dry season is the time when there is limited availability of water sources in the study area.
Implication for the policy
Our study determined various risk factors for scabies among adolescents and young adults (15–25 years) who are a productive age group of the population. This may affect their school performance, quality of life, and intellectual disabilities.37 In addition, patients with scabies may suffer from many health problems leading to anxiety, depression, anger, and embarrassment, which lead to social isolation and absenteeism at work and school. We, therefore, recommend policymakers to give emphasis to poverty reduction, change behavioral factors of this age group to increase their school performance and productivity.
Limitation of the study might be the absence of laboratory confirmation of parasites, burrow scraping, and microscopic examination among cases since we relied on only clinical assessment. Another limitation could be recall bias as participants were asked retrospectively about risk factors. As cases and controls were selected from schools not from the populations in rural areas, there might be limitations to generalizability of our findings. Moreover, the lack of prevalence data of scabies in the study population and details of the student population can be considered as a limitation of our study.
Conclusion
Poor knowledge on scabies causes, prevention, diagnosis, parental illiteracy, overcrowding, and low-income level of the households are risk factors associated with scabies infestations. Moreover, poor personal hygiene, behavioral factors like sharing of cloths/beds and contact with scabies cases are also determinant factors of scabies. Water accessibility and utilization are found to be risk factors for scabies among adolescent and young adults in Damboya district. Health education and sensitization about scabies prevention and control with emphasis on keeping personal hygiene, avoidance of bed/cloth sharing, overcrowding in schools, and poverty reduction in the district are recommended.
Abbreviations
AOR, adjusted odds ratio; CI, confidence interval; COR, crude odds ratio; DALYS, disability-adjusted life years; HUCMH, Hawassa University College of Medicine and Health Sciences; IRB, Institutional Review Board; OR, odds ratio; SD, standard deviation; SNNPR, Southern Nations Nationalities and Peoples Region; WHO, World Health Organization; YLDS, years lived with disability.
Data availability
Data related to this study can be accessed based on request to the authors at [email protected].
Acknowledgment
We would like to thank Hawassa University, College of Medicine and Health Sciences for the support during data collection. Our thanks also go to Kambata Tambaro Zone and Damboya District Health Offices for their cooperation in conducting the study. Finally, we would like to thank our data collectors and participants of the study without whom our study could not have been possible.
No specific funding to conduct the study; however, several activities including data collection were supported by the Kambata Tambaro Zone and Damboya District Health Office technically and financially.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. WHO. Neglected tropical diseases: scabies. Available from:https://www.who.int/neglected_diseases/diseases/scabies/en/. Accessed July 27, 2019.
2. CDC. Scabies epidemiology and risk factors. Available from: https://www.cdc.gov/parasites/scabies/epi.html. Accessed July 27, 2019.
3. Karimkhani C, Colombara DV, Drucker AM, et al. The global burden of scabies: a cross-sectional analysis from the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17:1247–1254. doi:10.1016/S1473-3099(17)30483-8
4. Romani L, Steer AC, Whitfeld MJ, Kaldor JM. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis. 2015;15:960–967. doi:10.1016/S1473-3099(15)00132-2
5. Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527–1534. doi:10.1038/jid.2013.446
6. Karimkhani C, Dellavalle RP, Coffeng LE, et al. Global skin disease morbidity and mortality: an update from the Global Burden of Disease Study 2013. JAMA Dermatol. 2017;153:406–412. doi:10.1001/jamadermatol.2016.5538
7. Kouotou EA, Nansseu JR, Sieleunou I, Defo D, Bissek AC, Ndam EC. Features of human scabies in resource-limited settings: the Cameroon case. BMC Dermatol. 2015;15:12. doi:10.1186/s12895-015-0031-0
8. Yotsu RR, Kouadio K, Vagamon B, et al. Skin disease prevalence study in schoolchildren in rural Cote d’Ivoire: implications for integration of neglected skin diseases (skin NTDs). Clin Infect Dis. 2018;12:e0006489.
9. Kuhne A, Gilsdorf A. [Infectious disease outbreaks in centralized homes for asylum seekers in Germany from 2004-2014]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2016;59:570–577. doi:10.1007/s00103-016-2332-9
10. Kobangue L, Guerendo P, Abeye J, Namdito P, Mballa MD, Gresenguet G. [Scabies: epidemiological, clinical and therapeutic features in Bangui]. Bull Soc Pathol Exot. 2014;107:10–14. doi:10.1007/s13149-014-0324-7
11. Kouotou EA, Nansseu JR, Kouawa MK, Zoung-Kanyi Bissek AC. Prevalence and drivers of human scabies among children and adolescents living and studying in Cameroonian boarding schools. Parasit Vectors. 2016;9:400. doi:10.1186/s13071-016-1690-3
12. Nair PA, Vora RV, Jivani NB, Gandhi SS. A study of clinical profile and quality of life in patients with scabies at a rural tertiary care centre. J Clin Diagn Res. 2016;10:Wc01–Wc05. doi:10.7860/JCDR/2016/20938.8703
13. Olsen JR, Gallacher J, Finlay AY, Piguet V, Francis NA. Quality of life impact of childhood skin conditions measured using the Children’s Dermatology Life Quality Index (CDLQI): a meta-analysis. Br J Dermatol. 2016;174:853–861. doi:10.1111/bjd.14361
14. Perez-Crespo M, Ramos-Rincon JM, Albares-Tendero MP, Betlloch-Mas I. Dermatoses in Latin American Immigrant Children Seen in a Universitary Hospital of Spain. J Immigr Minor Health. 2016;18:16–20. doi:10.1007/s10903-014-9999-5
15. Nagsuk P, Moore R, Lopez L. A case report of crusted scabies in an adult patient with Down syndrome. Dermatol Online J. 2015;21.
16. Razon FM, Weidman-Evans E. Insight into scabies. Jaapa. 2017;30:1–3. doi:10.1097/01.JAA.0000511790.29560.a2
17. Liu JM, Hsu RJ, Chang FW, et al. Increase the risk of intellectual disability in children with scabies: a nationwide population-based cohort study. Medicine (Baltimore). 2017;96:e7108. doi:10.1097/MD.0000000000007108
18. Lokuge B, Kopczynski A, Woltmann A, et al. Crusted scabies in remote Australia, a new way forward: lessons and outcomes from the East Arnhem scabies control program. Med J Aust. 2014;200:644–648.
19. Thomas J, Peterson GM, Walton SF, Carson CF, Naunton M, Baby KE. Scabies: an ancient global disease with a need for new therapies. BMC Infect Dis. 2015;15:250. doi:10.1186/s12879-015-0983-z
20. Walker SL, Lebas E, De Sario V, et al. The prevalence and association with health-related quality of life of tungiasis and scabies in schoolchildren in southern Ethiopia. PLoS Negl Trop Dis. 2017;11:e0005808. doi:10.1371/journal.pntd.0005808
21. Ayalew A, Enbiale W. Investigation of a scabies outbreak in drought-affected areas in Ethiopia. Tropicalmed. 2018;3:114.
22. Accorsi S, Barnabas GA, Farese P, et al. Skin disorders and disease profile of poverty: analysis of medical records in Tigray, northern Ethiopia, 2005-2007. Trans R Soc Trop Med Hyg. 2009;103:469–475. doi:10.1016/j.trstmh.2008.11.028
23. Romani L, Whitfeld MJ, Koroivueta J, et al. The epidemiology of scabies and impetigo in relation to demographic and residential characteristics: baseline findings from the skin health intervention Fiji Trial. Am J Trop Med Hyg. 2017;97:845–850. doi:10.4269/ajtmh.16-0753
24. Ramos JM, Molés-Poveda P, Tessema D, et al. Skin problems in children under five years old at a rural hospital in Southern Ethiopia. Asian Pac J Trop Biomed. 2016;6:625–629. doi:10.1016/j.apjtb.2016.05.009
25. Federal Ministry of Health. Intrem Guideline for Multi Sectorial Scabies Outbreak Emergency Response, FMOH: Ethiopia; 2015.
26. Word Health Organisation. Ethiopia - Scabies outbreak response in Amhara regional state. Available from: https://wwwafrowhoint/news/ethiopia-scabies-outbreak-response-amhara-regional-state.
27. Wochebo W, Haji Y, Asnake S. Scabies outbreak investigation and risk factors in Kechabira district, Southern Ethiopia: unmatched case control study. BMC Res Notes. 2019;12:305. doi:10.1186/s13104-019-4317-x
28. Sara J, Haji Y, Gebretsadik A. Scabies outbreak investigation and risk factors in East Badewacho District, Southern Ethiopia: unmatched case control study. Dermatol Res Pract. 2018;2018:7276938. doi:10.1155/2018/7276938
29. Hay RJ, Steer AC, Engelman D, Walton S. Scabies in the developing world–its prevalence, complications, and management. Clin Microbiol Infect. 2012;18:313–323. doi:10.1111/j.1469-0691.2012.03798.x
30. Talukder K, Talukder MQ, Farooque MG, et al. Controlling scabies in madrasahs (Islamic religious schools) in Bangladesh. Public Health. 2013;127:83–91. doi:10.1016/j.puhe.2012.09.004
31. Sara J, Haji Y, Gebretsadik A. Scabies outbreak investigation and risk factors in East Badewacho District, Southern Ethiopia: unmatched case control study. Hindawi Dermatol Res Pract. 2018;2018:10.
32. El Sherbiny NA, Abd El Raheem TA, Nasif GA, Hassan M, Hassan NS, Zeiada AN. Epidemiological study of scabies in primary schools, Fayoum Governorate- Egypt. J Primary Health Care Gen Pract. 2017;1.
33. Hegab DS, Kato AM, Kabbash IA, Dabish GM. Scabies among primary schoolchildren in Egypt: sociomedical environmental study in Kafr El-Sheikh administrative area. Clin Cosmet Investig Dermatol. 2015;8:105–111. doi:10.2147/CCID.S78287
34. Yassin ZJ, Dadi AF, Nega HY, Derseh BT, Asegidew W. Scabies outbreak investigation among “Yekolo Temaris” in Gondar Town, North Western Ethiopia, November 2015. Electronic J Biol. 2017;13:203–209.
35. Ugbomoiko US, Oyedeji SA, Babamale OA, Heukelbach J. Scabies in resource-poor communities in Nasarawa State, Nigeria: epidemiology, clinical features and factors associated with infestation. Trop Med Infect Dis. 2018;3.
36. Feldmeier H, Jackson A, Ariza L, et al. The epidemiology of scabies in an impoverished community in rural Brazil: presence and severity of disease are associated with poor living conditions and illiteracy. J Am Acad Dermatol. 2009;60:436–443. doi:10.1016/j.jaad.2008.11.005
37. Jin-gang A, Sheng-xiang X, Sheng-bin X, et al. Quality of life of patients with scabies. J Eur Acad Dermatol Venereol. 2010;24:1187–1191. doi:10.1111/j.1468-3083.2010.03618.x
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
