Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 12
Factors Associated with Oral Candidiasis in People Living with HIV/AIDS: A Case Control Study
Authors Suryana K , Suharsono H, Antara IGPJ
Received 28 October 2019
Accepted for publication 20 December 2019
Published 14 January 2020 Volume 2020:12 Pages 33—39
DOI https://doi.org/10.2147/HIV.S236304
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bassel Sawaya
Ketut Suryana, 1 Hamong Suharsono, 2 I Gede Putu Jarwa Antara 1
1Department of Internal Medicine, Wangaya Hospital of Denpasar, Bali, Indonesia; 2Department of Biochemistry, Veterinary Faculty of Udayana, University of Denpasar, Bali, Indonesia
Corespondence: Ketut Suryana Akasia Street, Lane.VIII No. 22 Denpasar, Bali 80235, Indonesia
Tel +62 859 537 839 44
Email [email protected]
Background: Oral candidiasis (OC) is the most frequent opportunistic infection of the oral cavity caused by Candida species overgrowth. A wide variety of risk factor that contributes to yeast infection especially candidiasis. It might be acting as an early marker for people living with HIV/AIDS (PLWHA). There are some risk factors for PLWHA associated OC at Wangaya hospital in Denpasar, Bali, Indonesia.
Aim: To identify risk factors of OC in PLWHA at Wangaya Hospital in Denpasar, Bali, Indonesia.
Settings and Design: Case control study was conducted from March 1, 2016 and July 30, 2019, included 448 participants (207 cases and 241 controls). Consecutive recruitment was employed.
Methods: Cases were PLWHA (18 to 60 years old) with OC and controls without OC. Diagnosis of OC based on the clinical features which are the pseudomembranous candidiasis; oral thrush. An interviewer administered a structured questionnaire used to collect information on risk factors. Statistical analysis used: bivariate analysis was performed on all variables. Chi-square test with statistically significant was at a level of 0.05.
Results: The participants included 207 (46.20%) PLWHA with OC and 241 (53.80%) PLWHA who did not have OC. The majority participants, 293 (65.40%) were male. OC was associated with age [p = 0.03; OR = 0.66 (95% CI:0.45– 0.95)]; sex [p = 0.002; OR = 1.88 (95% CI:1.26– 2.80)]; Xerostomia [p = 0.000; OR = 4.15 (95% CI:2.76– 6.23)]; smoking [p = 0.000; OR = 6.83 (95% CI: 4.46– 10.44)]; alcohol consumption [p = 0.000; OR = 5.76 (95% CI: 3.74– 8.83)]; antibiotic usage [p = 0.000; OR = 4.49 (95% CI: 2.93– 6.90)]; CD4 count [p = 0.000; OR = 3.29 (95% CI:2.24– 4.86)]; HIV clinical stage [p = 0.000; OR = 3.58 (95% CI 2.39– 5.37)]. No significant association between prothesis with OC.
Conclusion: We found that age, sex, xerostomia, smoking, alcohol consumption, antibiotic usage, CD4 counts and advanced HIV Clinical stage (AIDS) were significant associated risk factors for OC in PLWHA.
Keywords: human immunodeficiency virus, acquired immunodeficiency syndrome, oral candidiasis, associated risk factors
Introduction
Oral Candidiasis (OC) is the most common opportunistic infection in PLWHA. It can be the early signs and more frequently observed in patients with cluster differentiation 4 (CD4) counts less than 200 cells/µL.1–3
Recently, the prevalence of fungal (Candida spp) oral infections was increased. It is also due to the increasing use of antibiotic and immunodeficiency condition which associated with HIV infection. A few studies reported that candidiasis is an asymptomatic oral colonization and that can cause the progression of oral lesions or become disseminated infections. The prevalence of Candida spp in the oral cavity healthy individuals is 40–60% while in the immunocompromized patients (HIV-infected patients) the prevalence was higher 62–93%.4–6 OC is the most frequent lesions in HIV-infected patients. It is considered that OC as early signs of HIV-related immunodeficiency and it is highly predictable of the progression of the disease. Other studies found that the prevalence of oral candidiasis varies between 20% and 70% in PLWHA, and although the prevalence was reduced after the Highly Active Antiretroviral Therapy (HAART) era. Ninety percent of PLWHA will progress OC. The predispose factors of OC are age, oral hygiene and smoking.7,8
The evolution of the commensal stage to hypha stages may be induced by microenvironmental changes and may be precipitated by local and systemic risk factors.3,9 There are some risk factors of OC in PLWHA such as: age, sex, xerostomia, smoking, alcohol consumption, antibiotic usage, CD4 counts and WHO clinical stage.10–12
The objective of this study is to identify risk factors of OC in PLWHA at Wangaya Hospital in Denpasar, Bali, Indonesia.
Methodology
Materials and Methods
Study Design
A case-control study was conducted at Wangaya Hospital in Denpasar, Bali, Indonesia between March 1, 2016 and July 30, 2019 was recruited.
Participants Size
The total number of participants were 448, divided into 2 groups 207 participants as cases and 241 participants as controls. Consecutive sampling technique was recruited continuously. The participants who met the inclusion criteria were enrolled in the study until the required number of participants were completed. The participants in this study were PLWHA, who were confirmed with OC diagnosis were based on the nature of clinical presenting features which are the pseudomembranous candidiasis.13–15 Oral candidiasis is commonly known as oral thrush.16
The participant age ranges from 18 to 60 years in the study period from March 1, 2016 to July 30, 2019. The exclusion criteria were PLWHA who refused to join in this study. The minimum proportion of exposure between the case and control groups is 20%. It is known that the proportion of exposure in the control group is 10%. Type I errors are set at 5% and type II errors at 20%. The proportion of exposure in the control group is 0.1. The difference in the minimum proportion of exposure that is considered significant is set at 0.17. The sample size is 73 samples with an estimated loss to follow up of 10%, the minimum sample is 80 samples each group.
Variables and Data Sources
The main variables studied were PLWHA who visited Wangaya Hospital routinely. An interviewer administered a structured questionnaire used (consist of demography data, clinical and laboratory data) to collect information on risk factors. After the questionnaire is completed, it will be checked by the expertise team to verify the data. All participants were confirmed with HIV infection by serologic test (Rapid test). Participants with a confirmed diagnosis of OC were included in the cases group; those without clinical features of OC were as a control group. The following variables were obtained from the case and control groups: age, sex, xerostomia, smoking, alcohol consumption, prothesis, antibiotic usage, CD4 counts and WHO clinical stage. Xerostomia or dry mouth is defined as the subjective feeling of oral dryness perceived by the participant.17–19
Smoking individuals were identified as those who had smoked ≥ 100 cigarettes over their life time and smoked at the time of the study. Smoking has short and long-term effects on inflammatory aspects and immune responses in the oral cavity.3
Alcohol consumption was considered when the patient consumed alcohol on a daily basis.10
Statistical Analysis
Descriptive test was performed to describe the participant characteristics. Kolmogorov–Smirnov test was used to test the data distribution. Variables with numerical or continuous scales and normally distributed are displayed in the form of mean and standard deviation. If the data is not normally distributed, it is displayed in median and range. For categorical scale variables, the data is displayed in the form of relative frequency (number and percent). Clinical and laboratory data were compared between the case and the control groups. Bivariate analysis was performed on all variables of this study using the Chi-square test. Statistical significance was at a level of 0.05. The results of this analysis were expressed as an Odds Ratio (OR) with a 95% confident interval (CI). Variables with p < 0.05.
Ethical Clearance
The study procedure was approved by Ethical Committee of Wangaya Hospital in Denpasar Bali Indonesia with register number: 03/RSUDW/Litbang/2016. The study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all the participants.
Results
Characteristics of Patients
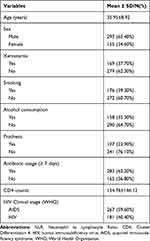
A total of 448 PLWHA were included in this study. Age varied from 18 to 60 years (mean 35.95 ± 8.92). The majority of participants, 293 (65.40%) were male. Xerostomia was found in 169 (37.70%); 176 (39.30%) were smoking. Alcohol consumption was found in 158 (35.30%) participants, prothesis were found among 107 (23.90%); 283 (63.20%) were found using antibiotic ≥ 7 days. CD4 lymphocytes counts 154.76±146.12 and WHO HIV clinical stage: AIDS was found 267 (59.60%) (Table 1).
Risk Factors Associated with Oral Candidiasis
Among 448 PLWHA, there were 207 (46.20%) participants with OC (cases) and 241 (53.80%) participants who did not have OC (controls).
The bivariate analysis showed the risk factors which are statistically significantly associated with oral candidiasis were: age, sex, xerostomia, smoking, alcohol consumption, antibiotic usage ≥ 7 days, CD4 counts < 104.5 cell/μL and HIV clinical stage (WHO). No significant association was found with prothesis (Table 2).
 |
Table 2 Risk Factors Associated with Oral Candidiasis; Bivariate Analysis (N=448) |
The increasing age of PLWHA (≥ 34 years) was significantly associated with OC (P = 0.03; OR= 0.66; 95% CI: 0.45–0.95). Male was found to have a significant association with OC (p = 0.002; OR = 1.88; 95% CI: 1.26–2.80). Xerostomia had also a strong association with OC (p = 0.000; OR= 4.15; 95% CI: 2.76–6.23). Smoking habit made greater or almost 7 fold for OC (p = 0.000; OR= 6.83; 95% CI: 4.46–10.44). Alcohol consumption had six times risk to have OC (p = 0.000; OR= 5.76; 95% CI: 3.74–8.83). Using antibiotic for more than 7 days made a four greater to have OC (p = 0.000; OR= 4.49; 95% CI: 2.93–6.90). The CD4 counts (< 108 cell/μL) had three times greater for OC (p = 0.000; OR=3.29; 95% CI: 2.24–4.86) and AIDS (Advanced HIV clinical stage) had a greater risk for OC (p = 0.000; OR= 3.58; 95% CI: 2.39–5.37).
Discussion
Oral candidiasis is a clinical predictor of HIV infection progression and it has been suggestive that OC represents a marker of immunological state of PLWHA.1,14 Oral candidiasis is one of the seven cardinal lesions associated with HIV besides necrotizing ulcerative gingivitis, necrotizing ulcerative periodontitis, linear gingival erythema, hairy leukoplakia, Kaposi’s sarcoma and non-Hodgkin lymphoma.14,20,21
We found that increasing age (≥ 34 years) was identified as a statistically significant risk factor for OC among PLWHA (P = 0.03; OR= 0.66; 95% CI: 0.45–0.95). In other study, Takahashi et al (2015) revealed increasing age (p = 0.007; Adjusted OR = 1.02; 95% CI: 1.00–1.03) was significantly associated with OC.12 Goulart et al (2018) found that age was the risk factor for oral colonization bay Candida spp; colonization was associated to participants aged 45 to 59 years (p= 0.0273; PR: 1.90; 95% CI: 1.57–6.31) and 60 years or more (p=0.0273; PR: 4.43; 95% CI: 1.57–34.18), colonization risks increased with age.22 Owotade et al (2013) reported that age was the risk factor for oral Candidiasis (P=0.034; OR= 0.95; 95% CI: 0.90–0.99).23
This study found the difference between sex; male was statistically significant higher (p=0.002; OR=1.88; 95% CI: 1.26–2.80). In another study, Rao et al (2016) demonstrated the difference between sex was statistically significant in OC. There was a significant difference in the occurrence of oral candidiasis 18.8%, male 10.3% female (P=0.00).24 In the other hand, Awoyeni et al (2017); reported that female was statistically significantly higher (p=0.042; OR=0.37; 95% CI: 0.14–0.91).25
Xerostomia is a subjective complaint of dry mouth, which may be caused by decreased saliva production. As we know, one of the salivary functions is to maintain the bacterial activity. Decreasing of this can cause significant morbidity. When this function alters, there is an increasing risk for having diseases such as candidiasis.17,18,26 Hypofunction of salivary gland may alter the oral microbiota and increase the risk of candidiasis and there was a significant negative correlation between salivary flow rate and candida colony-forming units in the patient with xerostomia.19
In our study, xerostomia has a strong association to have OC (P=0.000; OR= 4.15; 95% CI: 2.76–6.23). Shinozaki et al (2012) revealed patients with xerostomia exhibited significantly decreased whole salivary flow rate, increased rate of oral mucosa symptoms and higher numbers of candida, the cause of oral candidiasis.18
One of the predisposing factors for oral candidiasis is the local factor. This local factor includes epithelial changes, loss of vertical dimension, poor-fitting dentures, smoking and poor oral hygiene.27
Smoking is one of the systemic risk factors for OC. Smoking is associated with an increased risk of oral candidiasis, with possible pathomechanisms include mucosal injury, epithelial alterations from smoke that facilitates Candida colonization, cigarette smoke directly promotes candida growth, suppression of local or systemic immunologic defenses. Smoking patients have a greater chance of suffering from Candida infections. Non-smoking individuals were identified as those who had not smoked equal/more than 100 cigarettes in their lifetime.10,28,29
In our study, smoking habit makes six times greater for oral candidiasis (P = 0.000; OR = 6.83; 95% CI: 4.46–10.44). In a cross-sectional study held in USA, it was found smoking was an independent risk factor for oral candidiasis in HIV patient, current smokers had a 2.5 times greater risk for oral candidiasis compared to non-smokers (OR= 2.5; 95% CI: 1.3–4.8).3 In fact that over 40% of persons with HIV are current smokers, which increases HIV-associated infection.28 Heavy smokers also have six times greater to have oral candidiasis in patients with multiple oral leukoplakia compared to lighter smokers.28 This may explain the effect of smoking increased the risk of oral candidiasis.
In this study, alcohol consumption had five times the risk to have OC (P=0.000; OR= 5.76; 95% CI: 3.74–8.83). Takahashi et al, 2015, reported the significant association between alcohol consumption and candida esophagitis (p=0.038).12 Petruzzi et al, 2013 also found the chronic consumption of alcohol was a risk factor for OC (P=0.011; OR=2.38; 95% CI: 1.22–4.67).30
Prolong therapy with broad-spectrum antibiotics, this alters the local flora in oral cavity and makes a suitable environment for candida to grow.31,32
In our study revealed using antibiotic for more than 7 days made a seven greater to have oral candidiasis (P= 0.000; OR= 4.49; 95% CI: 2.93–6.90). Hung et al, 2005 reported that on the basis of multivariate analysis, antibiotic treatment and lower CD4+ counts less than 200 cells/mm3 were independent risk factors for the development of oropharyngeal candidiasis (p=0.0012; OR=6.095; 95% CI=2.038–18.229).33 Our study revealed that CD4 counts less than 108 cell/μL had three times greater for OC (P=0.000; OR=3.29; 95% CI: 2.24–4.86). A lot of studies documented a relation between low CD4 counts to oral candidiasis. Low CD4 counts make the risk of oral candidiasis in HIV patient increases due to low immune status or immunosuppression.34,35 As per Bodhade et al, 2011, a cross-sectional study in India revealed OC was found to be significantly correlated to a reduced CD4 cell counts less than 200 (p=0.000; OR=3.1; 95% CI: 1.9–4.9).36 Rao et al, in 2016, in India also showed CD4 counts ≤ 200 cell/μL had a twice higher risk developing OC than CD4 counts more than 200 cell/μL (p=0.000; OR=2.446; 95% CI: 1.932–3.098).24 Nanteza et al, in 2014, with their descriptive and analytical cross-sectional study in Uganda also showed there was a significant association between oral candidiasis and CD4 counts less than 350 cells/μL (P<0.001; OR=2.69; 95% CI: 1.608–4.502).37 A study in Brazil also concluded CD4 less than 350 cell/μL had a higher risk for developing OC compared more than 500 (p<0.001; OR=3.82; 95% CI: 2.16–6.77).30 Takahashi et al, in 2015, reported the significant association between CD4 cell counts less than 100/µL with Candidiasis (p< 0.001).12 Sani et al, in 2017, found a significantly higher prevalence of candida infection, 7 (53.8%) out of 13 were observed among patients with CD4 counts ≤ 200 cells/μL followed by 13 (27.7%) out of 47 among patients with CD4 200–500 cells/μL and 16.7% (10 out of 60) among patients with CD4 > 500 cells/μL, respectively (p<0.001).38 Other studies have reported a significant association between a CD4 counts equal or less than 200 cell/µL and oral candidiasis colonization.23 Delgado et al, 2009, found the isolation of Candida was significantly higher in patients with virological failure (83/147; p=0.0002) and CD4+ T-lymphocyte counts, 200 cell/mm3 (30/83; p=0.0003).39 Ohmit et al, 2003, reported that the odds of oral candidiasis were increased 7-fold for HIV-infected women with CD4 counts < 200 cells/mm3 compared within non-HIV infected women (p<0.001; OR=7.07; 95% CI = 2.59–19.27). The odds were increased > 2-fold for patients with CD4+ 200–500 cells/mm3 (p=0.026; OR=2.95; 95% CI=1.14–7.65).40
Due to the severity of HIV progression (WHO HIV clinical stage) makes an increasing susceptibility of infection and depends on patients’ degree of immunosuppression This severity was described by WHO HIV clinical stage. In our study, WHO HIV clinical stage II-IV had a strong association for OC compared with WHO stage I (P=0.000; OR= 3.58; 95% CI: 2.39–5.37).41 Nanteza et al, in 2014, showed clinical WHO stage 3 had three times higher to develop OC compared to stage 1 (p=0.025; OR=3.803; 95% CI: 1.182–12.240).37 Putranti et al, in 2018, also showed oral candidiasis mostly appeared in clinical stage 4. A study held in India revealed an oral lesion was considered to be marker of progression of HIV into the final clinical stage of AIDS.42
Conclusion
The age, sex, xerostomia, smoking, alcohol consumption, using antibiotic, low CD4 counts and severe clinical stage (AIDS) are the risk factors that associated with oral candidiasis in HIV patients at Wangaya Hospital in Denpasar Bali Indonesia. Based on update overview of candidiasis, it is reasonable to consider that the older age, male, xerostomia, smoking, alcohol consumption, taking antibiotic more than a week, low CD4 counts and severity of clinical stage (AIDS) may predispose to the development of the clinical manifestations of candidiasis.
Limitation of the Study
In this study, the diagnosis of oral candidiasis was solely based on clinical findings, no definitive diagnostic test was performed. It might be given a measurement bias into the study.
Acknowledgments
We would like to thank the Director of Wangaya Hospital, all of the participants and their family, the Wangaya HIV Study Group staff, all of our colleagues who supported this study. We would also like to thank Aditya Permana and Yoel Purnama, Valentina Tjandra Dewi, I Gede Supriadhiana for collecting and reporting data.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declared that there is no conflict of interest related to this study.
References
1. Saravani S, Nosratzehi T, Kadeh H, Mir S. Oral manifestations and related factors of HIV positive patients in south-east of Iran. J Dent Mater Tech. 2017;6:11–18.
2. Caceres NA, Vieira MMC, Vieira IF, Monteleone VF, Neto LJM, Bonafe S. Opportunistic infections in AIDS patients. iMedPub Journals. 2015;7:1–17.
3. Chattopadhyay A, Patton LL. Smoking as a risk factor for oral Candidiasis in HIV-infected adults. J Oral Pathol Med. 2013;42:302–308. doi:10.1111/jop.2013.42.issue-4
4. Samaranayake L. Commensal oral Candida in Asian cohorts. Int J Oral Sci. 2009;1:2–5. doi:10.4248/ijos.08006
5. Costa CR, Cohen AJ, Fernandes OF, et al. Asymptomatic oral carriage of Candida species in HIV-infected patients in the highly active antiretroviral therapy era. Rev Inst Med Trop Sao Paulo. 2006;48:257–261. doi:10.1590/S0036-46652006000500004
6. Felix DH, Wray D. The prevalence of oral candidiasis in HIV infected individuals and dental attenders in Edinburgh. J Oral Pathol Med. 1993;22:418–420. doi:10.1111/jop.1993.22.issue-9
7. Ramos-Gomez FJ, Flaitz C, Catapano F, Murray P, Milnes AR, Dorenbaum A. Classifications, diagnostic criteria, and treatment recommendations for orofacial manifestations in HIV-infected pediatric patients. collaborative workgroup on oral manifestations of pediatric HIV infection. J Clin Pediatr Dent. 1999;23:85–96.
8. Lourenco AG. Figueiredo LT oral lesions in HIV infected individuals from Ribeirao Preto, Brazil. Med Oral Patol Oral Cir Bucal. 2008;13:E281–E286.
9. Feller L, Khammissa RAG, Chandran R, Altini M, Lemmer J. Oral candidosis in relation to oral immunity. J Oral Pathol Med. 2014;563–569. doi:10.1111/jop.12120
10. Moura MDG, Grossman SMC, Fonseca LMS, Jorge MLR, Mesquita RA. Risk factors for oral candidiasis in Brazilian HIV-infected adult patients. Braz J Oral Sci. 2010;9:470–474.
11. Shirazi JH, Ali MI, Rashid A, Ahmed S, Nayab A. Prevalence and risk factors assessment of Candida albicans in tertiary health care institutions of Pakistan. Int J Curr Microbiol App Sci. 2015;4:1067–1072.
12. Takahashi Y, Nagata N, Shimbo T, et al. Long-term trends in esophageal candidiasis prevalence and associated risk factors with or without HIV infection: lessons from an endoscopic study of 80.219 patients. PLoS One. 2105;10:e0133589. doi:10.1371/journal.pone.0133589
13. Fourie J, Khammissa RAG, Ballyram R, Wood NH, Lemmer J, Feller L. Oral candidosis: an update on diagnosis, aetiopathogenesis and management. S Afr Dent J. 2016;71:317–318.
14. Jha R, Kaur T, Sharma A. Oral manifestations of HIV-AIDS: a diagnostic and management dilemma. J Res Med Dent Sci. 2014;2:96–101. doi:10.5455/jrmds.20142118
15. Rathod P, Punga R, Dalal V, Rathod D. Oral Candidiasis-widely prevalent, frequently missed. Int J Sci Study. 2015;3:193–198.
16. Shiva KKL, Shankare GVS, Basavarajaiah DM. Incidence of oral candidiasis among HIV infected patients-cohort prospective study. Int J Sci Res Publ. 2013;3:1–6.
17. Nadig SD, Ashwathappa DT, Manjunath M, Krishna S, Annaji AG, Shivaprakash PK. A relationship between salivary flow rates and Candida counts in patients with xerostomia. J Oral Maxillofac Pathol. 2017;21:316–321. doi:10.4103/jomfp.JOMFP_231_16
18. Shinozaki S, Moriyama M, Hayashida J, et al. Close association between oral Candida species and oral mucosal disorders in patients with xerostomia. Oral Dis. 2012;18:667–672. doi:10.1111/j.1601-0825.2012.01923.x
19. Guggenheimer J, Moore PA. Xerostomia. Etiology, recognition and treatment. J Am Dent Assoc. 2003;134:61–69. doi:10.14219/jada.archive.2003.0018
20. Denny CE, Ramapuram J, Bastian TS, et al. Oral lesions in HIV/AIDS patients on a highly active antiretroviral therapy. World J Orthod. 2016;7:95–99. doi:10.5005/jp-journals-10015-1373
21. Garcia-Cuesta C, Bagán JV. Current treatment of oral candidiasis: a literature review. J Clin Exp Dent. 2014;6:576–582. doi:10.4317/jced.51798
22. Goulart LS, de Souza WWR, Vieira CA, de Lima JS, de Olinda RA, de Araujo C. Oral colonization by Candida species in HIV-positive patients: association and antifungal susceptibility study. Einstein. 2018;16:1–6. doi:10.1590/s1679-45082018ao4224
23. Owotade FJ, Patel M, Ralephenya TRMD, Vergotine G. Oral Candida colonization in HIV-positive women: associated factors and changes following antiretroviral therapy. J Med Microbiol. 2013;62:126–132. doi:10.1099/jmm.0.047522-0
24. Rao UKM, Ranganathan K, Kumarasamy N. Gender differences in oral lesions among persons with HIV disease in Southern India. J Oral Maxillofac Pathol. 2016;16:388–394. doi:10.4103/0973-029X.102492
25. Awoyeni A, Olaniran O, Odetoyin B, et al. Isolation and evaluation of Candida species and their association with CD4+ T cells counts in HIV patients with diarrhea. Afr Health Sci. 2017;17:322–329. doi:10.4314/ahs.v17i2.5
26. Cherian AP, Jeftha A. Xerostomia and salivary flow rates in HIV patients. SADJ. 2017;72:62–67.
27. Akpan A, Morgan R. Oral candidiasis. Postgrad Med J. 2002;78:455–459. doi:10.1136/pmj.78.922.455
28. Lifson AR, Lando HA. Smoking and HIV: prevalence, health risks, and cessation strategies. Curr HIV/AIDS Rep. 2012;9:223–230. doi:10.1007/s11904-012-0121-0
29. Chiu CT, Li CF, Li JR, et al. Candida invasion and influences in smoking patients with multiple oral leucoplakias – a retrospective study. Mycoses. 2010;54(5):e377–e383. doi:10.1111/j.1439-0507.2010.01927.x
30. Petruzzi MNMR, Cherubini K, Salum FG, Figueiredo MAZ. Risk factors of HIV-related oral lesions in adults. Rev Saude Publica. 2013;47:52–59.
31. Mushi MF, Mtemisika CI, Bader O, et al. High oral carriage of non-albicans Candida spp. among HIV-infected individuals. Int J Infect Dis. 2016;49:185–188. doi:10.1016/j.ijid.2016.07.001
32. Martins N, Ferreira I, Barros L, Silva S, Henriques M. Candidiasis: predisposing factors, prevention, diagnosis and alternative treatment. Mycopathologia. 2014;177:223–240. doi:10.1007/s11046-014-9749-1
33. Hung CC, Yang YL, Lauderdale TL, et al. Colonization of human immunodeficiency virus-infected outpatients in Taiwan with Candida species. J Clin Microbiol. 2005;43:1600–1603. doi:10.1128/JCM.43.4.1600-1603.2005
34. Wu CJ, Lee HC, Yang YL, et al. Oropharyngeal yeast colonization in HIV infected outpatients in southern Taiwan: CD4 count, efavirenz therapy and intravenous drug use matter. Clin Microbiol Infect. 2012;18:485–490. doi:10.1111/j.1469-0691.2011.03655.x
35. Kumar S, Gowda S. Casavarajaiah. Incidence of oral candidiasis among HIV infected patients-cohort prospective study. Int J Sci Res Publ. 2013;3:1–6.
36. Bodhade AS, Ganvir SM, Hazarey VK. Oral manifestations of HIV infection and their correlation with CD4 count. J Oral Sci. 2011;53:203–211. doi:10.2334/josnusd.53.203
37. Nanteza M, Tusiime JB, Kalyango J, Kasangaki A. Association between oral candidiasis and low CD4 + count among HIV positive patients in Hoima regional referral hospital. BMC Oral Health. 2014;14:1–6. doi:10.1186/1472-6831-14-143
38. Sani NM, Yusuf IB, Mujahid NS. Prevalence of oropharyngeal candidiasis among HIV patients attending ART Clinic, Infectious Disease Hospital (IDH) Kano-Nigeria. J Adv Med Med Res. 2017;24:1–6.
39. Delgado ACD, Pedro RDJ, Aoki FH, et al. Clinical and microbiological assessment of patients with a long-term diagnosis of human immunodeficiency virus infection and Candida oral colonization. Clin Microbiol Infect. 2009;15:364–371. doi:10.1111/j.1469-0691.2009.02707.x
40. Ohmit SE, Sobel JD, Schuman P, et al. Longitudinal study of mucosal Candida species colonization and Candidiasis among Human Immunodeficiency Virus (HIV)-seropositive and at-risk HIV-seronegative women. JID. 2003;7:188–227.
41. Anwar KP, Malik A, Subhan KH. Profile of candidiasis in HIV infected patients. Iran J Microbiol. 2012;4:204–209.
42. Putranti A, Asmarawati TP, Rachman BE, Hadi U. Nasronudin. Oral candidiasis as clinical manifestation of HIV/AIDS infection in airlangga University hospital patients Oral candidiasis as clinical manifestation of HIV/AIDS infection in airlangga University hospital patients. IOP Conf Ser Earth Environ Sci. 2018;125:1–6. doi:10.1088/1755-1315/125/1/012063
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.