Back to Journals » Journal of Multidisciplinary Healthcare » Volume 15
Factors Associated with Nutritional Risk in Patients with Pulmonary Tuberculosis and Structural Lung Disease: A Hospital-Based Cross-Sectional Study
Authors Wang X, Luo L, Zhang D, Wang J , Ning X, Lin Y, Ke X, Li G
Received 23 May 2022
Accepted for publication 2 August 2022
Published 26 August 2022 Volume 2022:15 Pages 1799—1807
DOI https://doi.org/10.2147/JMDH.S375441
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Xiufen Wang,1– 3 Li Luo,1– 3 Dandan Zhang,1– 3 Jinghua Wang,4,5 Xianjia Ning,4,5 Yi Lin,2,3,6 Xue Ke,1– 3 Guobao Li1– 3
1Department of the Third Pulmonary Disease, The Third People’s Hospital of Shenzhen, Shenzhen, Guangdong, People’s Republic of China; 2The Second Affiliated Hospital of Southern University of Science and Technology, Shenzhen, Guangdong, People’s Republic of China; 3National Clinical Research Center for Infectious Diseases, Shenzhen, Guangdong, People’s Republic of China; 4Center of Clinical Epidemiology, The Third People’s Hospital of Shenzhen, Shenzhen, Guangdong, People’s Republic of China; 5Laboratory of Epidemiology, Tianjin Neurological Institute, Tianjin, 300052, People’s Republic of China; 6Department of the Second Pulmonary Disease, The Third People’s Hospital of Shenzhen, Shenzhen, Guangdong, People’s Republic of China
Correspondence: Guobao Li; Xue Ke, Department of the Third Pulmonary Disease, The Third People’s Hospital of Shenzhen, The Second Affiliated Hospital of Southern University of Science and Technology & National Clinical Research Center for Infectious Diseases, 29 Bulan Road, Shenzhen, Guangdong Province, 518112, People’s Republic of China, Tel +86-755-61238942, Fax +86-755-61238928, Email [email protected]; [email protected]
Objective: Patients with tuberculosis have a high nutritional risk, and patients with tuberculosis and structural lung disease have a poor quality of life. However, few studies have investigated the nutritional risk of patients with tuberculosis and structural lung disease. This study aimed to evaluate nutritional risk in patients with pulmonary tuberculosis and structural lung disease and to identify factors associated with nutritional risk in this population.
Methods: We performed a cross-sectional study of patients diagnosed with pulmonary tuberculosis and structural lung disease admitted to The Third People’s Hospital of Shenzhen, China between January 1, 2019 and December 31, 2021. We assessed participants’ nutritional risk using the Nutritional Risk Screening 2002 tool, and analyzed the relationship between nutritional risk and sociodemographic factors, disease status, and laboratory test results.
Results: Of the 415 participants, 53.5% were at nutritional risk on admission to the hospital. Nutritional risk was significantly associated with being unmarried, destroyed lung, and red blood cell (RBC) and lymphocyte counts.
Conclusion: Patients with tuberculosis and structural lung disease had a high prevalence of nutritional risk. The main factors associated with nutritional risk were being unmarried, lung cavitation, and low RBC and lymphocyte counts. Patients hospitalized with pulmonary TB should be evaluated for nutritional risk. Moreover, unmarried patients and patients with lung cavitation or low RBC or lymphocyte counts should be closely monitored.
Keywords: structural lung disease, nutritional risk, risk factors, pulmonary tuberculosis, destroyed lung
Introduction
Tuberculosis (TB) is still one of the leading causes of death worldwide, especially in low- and middle-income countries. According to the World Health Organization (WHO), an estimated 9,900,000 people had active TB infection, and 1,300,000 people without human immunodeficiency virus infection died of TB in 2020; China is one of the eight countries with the highest TB burden.1
Malnutrition, which is prevalent in the low- and middle-income populations, is a risk factor for TB. According to the State of Food Security and Nutrition, 811 million people (almost one-tenth of the world’s population) worldwide had some form of malnutrition in 2020.2
TB and malnutrition are intrinsically linked: malnutrition can predispose to TB, and TB plays a role in malnutrition.3–6 Malnutrition is a major risk factor for the development of pulmonary TB and also a risk factor for extrapulmonary disease in patients with pulmonary TB.3 Previous studies have shown that hospitalized patients with TB have a high prevalence of malnutrition.5,6 Another study of 1181 patients with TB showed that malnutrition is closely related to the severity of TB.7 WHO and some researchers have recommended that attention be paid to the nutritional status of patients with TB.4,5,8 Structural lung disease (including secondary bronchiectasis, honeycomb lung, multiple cavities, and consolidation) occurs in patients with advanced pulmonary TB and further degrades patients’ quality of life.9 Therefore, in the treatment of TB, attention should be paid to patients’ nutritional status.
Patients with TB and structural lung disease are more likely to have nutritional risk, which may aggravate the primary disease. However, the factors associated with nutritional risk in patients with TB and structural lung disease remain unclear. This study aimed to evaluate the nutritional risk status of patients with pulmonary TB and structural lung disease and to analyze factors associated with nutritional risk using the Nutritional Risk Screening 2002 (NRS-2002) tool in order to improve the clinical outcomes of this population.
Patients and Methods
Study Design and Patient Selection
We performed a cross-sectional study of patients diagnosed with pulmonary TB and structural lung disease, admitted to The Third People’s Hospital of Shenzhen between January 1, 2019 and December 31, 2021. The inclusion criteria were: (1) a diagnosis of pulmonary TB; (2) structural lung disease diagnosed by computed tomography (CT), in addition to other imaging findings, such as secondary bronchiectasis, honeycomb lung, multiple lung bullae, lung consolidation, fibrous cavitary pulmonary TB, and tuberculous lung damage; and (3) provision of written informed consent. The etiology of the structural lung disease was not considered in this study. Patients were excluded if they met any of the following exclusion criteria: (1) mental illness or severe language impairment, cognitive dysfunction, or knowledge impairment, or other factors that could lead to poor compliance and inability to cooperate; (2) pregnant or lactating women; (3) transfer to another hospital or loss to follow-up during the study period; (4) incomplete clinical data; and (5) cancer, human immunodeficiency virus infection, or other wasting diseases.
Definition and Grouping
Pulmonary TB was diagnosed according to the Tuberculosis Classification Criteria WS196-2017 and Tuberculosis Diagnostic Criteria WS288-2017, published by the National Health and Family Planning Commission of China in 2017.
The nutritional risk status of the participants was assessed with the NRS-2002 tool on the day of admission. Based on their NRS-2002 score, the participants were divided into nutritional risk groups and without nutritional risk groups according to whether they were assessed as being at nutritional risk. The risk factors of the two groups were then compared.
Study Data
A questionnaire was used to collect data on participants’ sociodemographic characteristics, including age, sex, education, marital status, and medical insurance type. Disease-related data, including the use of antituberculous therapy and complications, condition of the lungs, and laboratory test results, were obtained from the medical records. The condition of the lungs, including the number of lobes affected, and the presence of bronchiectasis, pneumonia, atelectasis, bronchial stenosis, lung cavitation, and pneumothorax, was determined by CT. Laboratory markers included the levels of procalcitonin (PCT), interleukin 6, C-reactive protein (CRP), albumin, alanine transaminase, aspartate transaminase, urea, and creatinine; the estimated glomerular filtration rate (eGFR); and the red blood cell (RBC), white blood cell, lymphocyte, T cell, and platelet counts.
Participants with an NRS-2002 score ≥3 were assigned to the high nutritional risk group, and those with an NRS-2002 score <3 were assigned to the low nutritional risk group. Participants were divided into four age groups: <30, 30–45, 45–60, and ≥60 years. The level of education was divided into four levels according to the number of years of education: 1–6, 7–9, 10–12, and >12 years. Marital status was classified as never married, married, divorced, and widowed. Medical insurance type was classified as employee, resident, and self-paid. Body mass index (BMI) was defined as weight (kg) divided by the height squared (m2). Patients were divided into four groups according to BMI: underweight (<18.5 kg/m2), normal weight (18.5–24 kg/m2), overweight (24–28 kg/m2), and obesity (≥28 kg/m2).
Nutritional Risk Screening
We used the NRS-2002 tool, developed by the European Society for Parenteral and Enteral Nutrition, to assess participants’ nutritional risk. NRS-2002 assesses nutritional impairment (nutritional status, loss of weight, and dietary intake) and disease severity, with each category having a score ranging from 0 to 3. The risk score is increased by 1 if the patient’s age is ≥70 years, and a total score ≥3 indicates nutritional risk.10 This simple and effective tool is recommended for clinical nutritional risk screening in patients with pulmonary TB.11,12 Weight was measured with the patients standing on a scale.
Statistical Analysis
Categorical variables were reported as frequencies with percentages, and the statistical significance of differences between groups was assessed using the chi-square test. Continuous variables were reported as the mean and standard deviation or the median and interquartile range (IQR), and the differences between groups were compared using Student’s t-test or the Mann–Whitney U-test. Multivariable logistic regression models were used to identify the factors associated with nutritional risk. Nutritional risk was the dependent variable, and factors that were statistically significant in the univariate analysis were the independent variables. The nutrition risk was presented as odds ratio (OR) with 95% confidence interval (CI). P-values < 0.05 were considered to be statistically significant. Data analysis was performed using SPSS version 24.0 (IBM Corp., Armonk, NY, USA).
Results
Demographic Characteristics and Nutritional Risk
A total of 415 patients with TB and structural lung disease were enrolled in the study, of whom the majority (71.1%) were men. Approximately one-third (35.9%) were aged between 45 and 60 years and 68.6% were married; the mean BMI was 18.99 kg/m2 (Table 1).
 |
Table 1 Demographic Characteristics and Nutritional Risk Among Patients with Structural Lung Disease |
Of the 415 participants, 53.5% were at nutritional risk. There was no significant difference in the nutritional risk according to sex or the mean age of participants, but there were significant differences according to age group (P = 0.049). There were also significant differences in nutritional risk according to educational level (P = 0.004), marital status (P = 0.006), or BMI (P < 0.001). Moreover, the nutritional risk group had higher hospitalization costs (P = 0.001) (Table 2).
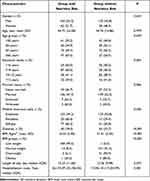 |
Table 2 Association of Clinical Features with Nutritional Risk Among Patients with of Structural Lung Disease in the Univariate Analysis |
Clinical Parameters and Nutritional Risk
Regarding comorbidities, the nutritional risk group had a significantly increased risk of anemia (19.4% vs 10.4%; P = 0.011) and hypoproteinemia (20.3% vs 6.2%; P < 0.001) and a significantly decreased risk of diabetes (18.0% vs 33.7%; P < 0.001). Regarding lesions, the nutritional risk group had a significantly greater number of bilateral lesions (64.9% vs 35.9%; P < 0.001), and a significantly greater risk of pulmonary infection (48.6% vs 32.1%; P < 0.001), destroyed lung (70.3% vs 43.0%; P < 0.001), or pneumothorax (10.8% vs 4.7%; P =0.021). For laboratory indicators, the nutritional risk group had significantly higher PCT levels (P = 0.001), eGFR (P = 0.006), and platelet counts (P < 0.001), significantly lower albumin levels (P < 0.001), and significantly lower lymphocyte (P < 0.001), Tc (P < 0.001), Th (P = 0.001), Ts (P = 0.001), and RBC (P < 0.001) counts. There was no significant difference between the two groups in the proportion of participants on antituberculous treatment before admission (Table 3).
 |
Table 3 Association of Laboratory Indicators with Nutritional Risk Among Patients with of Structural Lung Disease in the Univariate Analysis |
Multivariable Analysis
The prevalence of nutritional risk was 83% lower in married participants (OR: 0.17, 95% CI: 0.06–0.49; P = 0.001). The prevalence of nutritional risk was significantly higher in participants with lung cavitation than in those without lung cavitation (OR: 2.29, 95% CI: 1.10–4.75; P = 0.026). Moreover, low RBC and lymphocyte counts were associated with nutritional risk, with each additional unit of RBC and lymphocyte count reducing the nutritional risk by 65% (OR:0.35, 95% CI: 0.20–0.63; P < 0.001) and 59% (OR: 0.41, 95% CI: 0.23–0.73; P = 0.003), respectively (Table 4).
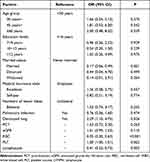 |
Table 4 Association of Structural Lung Disease with Nutritional Risk in the Multivariate Analysis |
Discussion
This study investigated the nutritional risk status of patients with pulmonary TB and structural lung disease and explored the factors associated with nutritional risk. More than half the participants (53.5%) were at nutritional risk on admission to hospital. Nutritional risk was unrelated to sex and use of antituberculous drugs before hospitalization. The hospitalization cost of patients with nutritional risk was significantly higher. Married participants had a significantly lower prevalence of nutritional risk, and participants with lung cavitation and low RBC or lymphocyte counts had a significantly higher prevalence of nutritional risk.
TB is a chronic wasting disease. Mycobacterium tuberculosis infection leads to metabolic disturbances and a reduced capacity of the body to repair damaged tissue, which affects recovery from the disease. Moreover, the disease has a relatively long course. Structural lung disease, which comprises irreversible damage to the lung parenchyma and interstitium due to any cause, further reduces the body’s resistance and increases nutrient consumption. Therefore, patients with pulmonary TB and structural lung disease are likely to have poor nutritional status. Reports on the prevalence of the nutritional risk in patients with TB vary greatly. In a study with a similar sample size, 50.1% of patients with TB had nutritional risk.11 However, in two other studies, 64.4% and 89.1% of patients had nutritional risk.12,13 Our study showed that the prevalence of nutritional risk in patients with TB and structural lung disease was 53.5%, which is similar to 50.1% but much lower than 89.1%. A possible explanation for the differences in nutritional risk in different studies is that the sample sizes and population characteristics differed, and that patients with structural lung disease have a poor quality of life and need more on nutritional support.
Marital status was an independent predictor of nutritional risk, suggesting the importance of assessing nutritional support in unmarried patients. Unmarried people may have poorer diets than married people, which may increase their nutritional risk. This may be because patients with structural lung disease have a lower quality of life than patients with simple TB,7 and a spouse can provide psychological support and improve the patient’s compliance with treatment, which reduces the possibility of nutritional risk. However, a previous study found no association between marital status and low calorie and protein intake in patients with TB.14 The exact relationship between nutritional risk and marital status requires further research.
The present study found lung cavitation to be an independent predictor of nutritional risk. This may be because lung cavitation is associated with prolonged illness and heavy financial burden, in addition to being a serious condition;15,16 thus, long-term drug treatment may cause patients to have poor appetite, adverse economic conditions, and inadequate nutrition.
A previous study suggested that the nutritional risk in TB is associated with a high CRP level and low lymphocyte count.11 However, in this study, no significant association was found between nutritional risk and the CRP level. The specific reasons for this discrepancy need to be explored. However, consistent with the previous study, our results showed that nutritional risk associated with a low lymphocyte count. This may be because when the lymphocyte count decreases, the body’s immune system function decreases, and the body becomes more susceptible to complications such as infection, in turn leading to decreased nutrient consumption.17 Moreover, the degree of malnutrition in patients with TB is associated with the degree of immune impairment.17
There are some limitations to this study. First, this was a single-center study, and thus, the participants may not be representative of all patients with TB and structural lung disease. Larger, multicenter studies are needed to confirm the study findings. Second, the study was a cross-sectional study, and we did not follow-up patients to determine changes in nutritional risk or their clinical outcomes. The relationship between nutritional risk and prognosis needs further research. Third, there are few published studies on nutritional risk in patients with TB and structural lung disease, which made it difficult to make comparisons with other studies. However, our research may serve as a reference for comparison with future studies.
Conclusion
To the best of our knowledge, this is the first report on nutritional risk in patients with TB and structural lung disease. Our findings suggest that patients with TB and structural lung disease have a high prevalence of nutritional risk. The main risk factors were being unmarried, lung cavitation, and low RBC and lymphocyte counts. Patients hospitalized with pulmonary TB should be evaluated for nutritional risk so that nutritional interventions can be applied to patients with a high nutritional risk to improve their quality of life and reduce the cost of hospitalization. In addition, the nutritional status of unmarried patients, and patients with lung cavitation or low RBC and lymphocyte counts, should be monitored so that nutritional risk can be prevented or promptly treated in this population.
Abbreviations
TB, tuberculosis; CT, computed tomography; WHO, World Health Organization; PCT, procalcitonin; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; RBC, red blood cell; BMI, body mass index; OR, odds ratio; CI: confidence interval.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
The study was approved by the Ethics Committee of The Third People’s Hospital of Shenzhen (contract number: 2022-051-02); this study complies with the Declaration of Helsinki. The written informed consent was obtained from all participants during recruitment.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by Shenzhen Fund For Guangdong Provincial High-level Clinical Key Specialties (No:SZGSP010), Guangdong Clinical Research Center for Infectious Diseases (Tuberculosis) Project (No: 2020B1111170014), and Clinical Research Project of The Third People’s Hospital of Shenzhen (No: G2022004).
Disclosure
The authors declare that they have no competing interests.
References
1. World Health Organization. Global tuberculosis report; 2021. Available from: https://www.who.int/publications/i/item/9789240037021.
2. FAO, IFAD, UNICEF, WFP and WHO The state of food security and nutrition in the world 2021. Transforming food systems for food security, improved nutrition and affordable healthy diets for all; 2021. Available from: https://data.unicef.org/resources/sofi-2021/.
3. Gao N, Guo Y, Zhang X, Shang X. Nutritional risk status and its influencing factors in patients with pulmonary tuberculosis. Chin J Nurs. 2014;49(5):552–555.
4. Lazzari TK, Forte GC, Silva DR. Nutrition status among HIV-positive and HIV-negative inpatients with pulmonary tuberculosis. Nutr Clin Pract. 2018;33(6):858–864. doi:10.1002/ncp.10006
5. Zachariah R, Spielmann MP, Harries AD, Salaniponi FM. Moderate to severe malnutrition in patients with tuberculosis is a risk factor associated with early death. Trans R Soc Trop Med Hyg. 2002;96(3):291–294. doi:10.1016/S0035-9203(02)90103-3
6. World Health Organization. Guideline: nutritional care and support for patients with tuberculosis. Geneva: world Health Organization; 2013. Available from: https://www.who.int/publications/i/item/9789241506410.
7. Zeng H, Fan Y, Liu H, Luo X, Qiugui H, Liu Z. Analysis of quality of life investigation in patients with tuberculosis and structural lung change. Chin J Antitubere. 2015;37(5):504–507.
8. Kondrup J, Allison SP, Elia M, Vellas B, Plauth M; Educational and Clinical Practice Committee. ESPEN guidelines for nutrition screening 2002. Clin Nutr. 2003;22(4):415–421. doi:10.1016/S0261-5614(03)00098-0
9. Zhisong W, Mao H, Nanlan M. Nutritional risk screening of pulmonary tuberculosis patients with intestinal tuberculosis and its evaluation of nutritional status before and after treatment. J Clin Pulm Med. 2019;24(1):126–129.
10. Fang X, Mao Y, Tang L, et al. Evidence summary for nutritional risk screening and assessment in patients with pulmonary tuberculosis. Chin J Nurs. 2019;54(9):1406–1409.
11. Xie W, Deng G, Shi W, Hui L, Jing W. Analysis of nutritional risk and nutritional index in patients with pulmonary. J New Med. 2018;49(10):740–744.
12. Li Y, Yang F, Zhou H, Shu L, Wang R, Zhao C. Clinical application of NRS-2002 in nutritional risk screening of tuberculosis inpatients. Ann Palliat Med. 2021;10(5):5322–5328. doi:10.21037/apm-21-610
13. Mao Y, Chen Y, Guihui W, Chen J. Correlation analysis of nutritional risk and clinical features in patients with pulmonary tuberculosis. Int J Tuberc Lung Dis. 2020;1(2):24–28.
14. Feleke BE, Feleke TE, Biadglegne F. Nutritional status of tuberculosis patients, a comparative cross-sectional study. BMC Pulm Med. 2019;19(1):182. doi:10.1186/s12890-019-0953-0
15. Rhee CK, Yoo KH, Lee JH, et al. Clinical characteristics of patients with tuberculosis-destroyed lung. Int J Tuberc Lung Dis. 2013;17(1):67–75. doi:10.5588/ijtld.12.0351
16. Zeng H, Liu H, Ye M, Zhang L, Tao S. Effect of nursing intervention on health education of patients with pulmonary tuberculosis who received surgery. Chin J Antitubere. 2013;35(08):601–605.
17. Wang X, Wang L, Xia F, Huang P, Wang Y. The relation between TB patients’ malnutrition and cellular immunity. J Clin Pulm Med. 2010;15(08):1132–1133.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
