Back to Journals » Patient Preference and Adherence » Volume 15
Factors associated with COVID-19 Vaccine Hesitancy in Thai Seniors
Authors Thanapluetiwong S , Chansirikarnjana S , Sriwannopas O, Assavapokee T, Ittasakul P
Received 8 September 2021
Accepted for publication 19 October 2021
Published 31 October 2021 Volume 2021:15 Pages 2389—2403
DOI https://doi.org/10.2147/PPA.S334757
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Saran Thanapluetiwong,1 Sirintorn Chansirikarnjana,1 Orapitchaya Sriwannopas,1 Taweevat Assavapokee,1 Pichai Ittasakul2
1Division of Geriatric Medicine, Department of Medicine, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand; 2Department of Psychiatry, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
Correspondence: Pichai Ittasakul
Department of Psychiatry, Faculty of Medicine Ramathibodi Hospital, Mahidol University, 270 Rama VI Road, Ratchathewi, Bangkok, 10400, Thailand
Tel +66 2-201-1235
Fax +66 2-354-7299
Email [email protected]
Objective: Older people are the most vulnerable group for developing SARS-CoV-2 infection. Although vaccination against coronavirus disease 2019 (COVID-19) reduces infection, hospitalization, and mortality rates, some older people have refused to get vaccinated. Our study aimed to evaluate factors associated with COVID-19 vaccine hesitancy among Thai seniors.
Methods: We conducted a cross-sectional telephone survey on vaccine hesitancy in a geriatric clinic at Ramathibodi Hospital in Bangkok, Thailand. Patients aged ≥ 60 years were contacted and interviewed by trained interviewers between June 20 and July 25, 2021.
Results: In total, we interviewed 282 participants aged 60– 93 years (mean age 73.0± 7.5 years). We found that 44.3% of participants were hesitant to get a COVID-19 vaccination. Factors associated with high vaccine hesitancy were low education, lack of confidence in the healthcare system’s ability to treat patients with COVID-19, vaccine manufacturers, being offered a vaccine from an unexpected manufacturer, and a low number of new COVID-19 cases per day.
Conclusion: The prevalence of COVID-19 vaccine hesitancy among Thai seniors is relatively high, and is associated with specific factors. These findings will help in promoting COVID-19 vaccination among Thailand’s senior citizens.
Keywords: vaccine acceptance, vaccine refusal, elderly, older adult, SARS-CoV-2
Introduction
As of the end of June 2021, over 180 million people worldwide have suffered from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, with 3.9 million deaths (2.17% mortality rate).1 COVID-19 caused disruptions in many aspects of the health system,2 and systematic reviews suggested the high prevalence of COVID-19 is potentially associated with long-term burden.3,4 In addition, co-infection of SARS-CoV-2 and endemic infections in Asia is a major concern.5,6 As of June 30, 2021, 259,301 people in Thailand had been infected with COVID-19, and there were 2,023 deaths.7 The most vulnerable group in the infected population was older people. SARS-CoV-2 infection in older people, particularly males, has been associated with more severe symptoms.8–11 In the United States, people aged 65 years and over accounted for approximately 80% of all COVID-19 deaths. Individuals over age 85 years had 630 times the mortality rate of those aged 18–29 years.12 The Ministry of Public Health in Thailand reported death from SARS-CoV-2 among people over age 70 years was up to 12.1%.13
Many studies showed that COVID-19 vaccination decreased infection, hospitalization, and mortality rates. An observational study in Israel using national surveillance data found that the Pfizer-BioNTech mRNA vaccine BNT162b2 was 96.5%, 98.0%, and 98.1% effective in preventing SARS-CoV-2 infection, hospitalization, and death, respectively.14 An interim analysis of the Oxford-AstraZeneca ChAdOx1 nCoV-19 vaccine showed 70.4% efficacy in preventing SARS-CoV-2 infection.15 A study in Chile using an inactivated SARS-CoV-2 vaccine (CoronaVac) showed its effectiveness in preventing infection, hospitalization, and death was 65.9%, 90.3%, and 86.3%, respectively.16 Therefore, to successfully manage SARS-CoV-2 infection, countrywide COVID-19 vaccination is needed to achieve herd immunity.
The ChAdOx1 nCoV-19 and CoronaVac vaccinations have been the most commonly available vaccines in Thailand. The ChAdOx1 nCoV-19 vaccination is mostly given to people over age 60 years. However, some older people refuse to be vaccinated against COVID-19. The Strategic Advisory Group of Experts on Immunization from the World Health Organization (WHO) defined vaccine hesitancy as the delay in acceptance or refusal of vaccination despite availability of vaccination services.17,18 Factors that can influence vaccine hesitancy can be grouped into three categories: contextual, individual and group, and vaccine/vaccination-specific influences.17,18
A systematic review and meta-analysis of intended uptake and refusal of COVID-19 vaccines from 13 countries showed 60% of participants intended to get vaccinated, and 20% intended to refuse.19 Factors associated with vaccine refusal were being female, younger, having a lower income or education level, and being in an ethnic minority group.19 Despite older people benefiting the most from COVID-19 vaccination, few studies have investigated COVID-19 vaccine hesitancy in this group. To date, there has been no study on COVID-19 vaccine hesitancy in Thailand. Different social contexts, cultures, and politics may affect a person’s intention to get vaccinated. Our study aimed to evaluate factors associated with COVID-19 vaccine hesitancy in Thai seniors. The findings will offer important insights for promoting vaccination among older people in Thailand.
Materials and Methods
Setting and Study Design
This study protocol was approved by the Human Research Ethics Committee of the Faculty of Medicine, Ramathibodi Hospital, Mahidol University (COA. MURA2021/441). We conducted a cross-sectional telephone survey about vaccine hesitancy in an outpatient geriatric clinic at Ramathibodi hospital. Over the last 2 years, this hospital had approximately 2,600 senior patients and 16,000 clinic visits. Patients aged 60 years and over who visited the geriatric clinic in the past 2 years were retrieved from the hospital database. These patients were contacted and invited to participate in this survey. Because we performed a telephone-based survey and because it was inconvenient for participants to sign written informed consent forms and manage documents during the pandemic, we did not obtain written informed consent. However, all participants gave verbal informed consent, which was recorded in accordance with the verbal informed consent protocol approved by the the Human Research Ethics Committee of the Faculty of Medicine, Ramathibodi Hospital, Mahidol University. Patients who agreed to participate were interviewed via telephone by a trained interviewer between June 20 and July 25, 2021. The survey was conducted in the Thai language. This study was conducted according to the Declaration of Helsinki and Good Clinical Practice guidelines.20,21
Sample Size and Sampling
The sample size was calculated with based on a 95% confidence level, 0.05 margin of error, assuming an observed proportion of respondents selecting a specific response option of 50%, and finite correction for the study population of 2,600 patients. Using this method, the calculated sample size was 335 patients. The list of patients from the database was reordered using computerized randomization, and patients were contacted according to their order in the new randomized list.
Questionnaire
The questionnaire used in this study was self-developed following a review of the literature22–34 and agreement among experts. The questionnaire was structured into four sections covering sociodemographic data, medical history, COVID-19 pandemic-related information, and COVID-19 vaccine-related information. A pilot sample (N=10) was used to improve the language and clarity of expression of the survey items. The data from the pilot sample were not used in subsequent analyses. The final version of the questionnaire required around 30–45 minutes to complete. The questionnaire was originally developed in the Thai language.
Sociodemographic Characteristics and Medical History
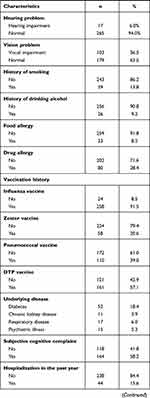
Participants’ sociodemographic characteristics were obtained, including age, gender, ethnicity, marital status, education, employment status, current residence, monthly income, and income loss due to COVID-19. In addition, participants were asked to report their medical history, including their body mass index (BMI), ambulation, hearing problems, vision problems, history of smoking and drinking alcohol, food and drug allergies, history of previous vaccinations (including influenza, pneumococcal, zoster and diphtheria-tetanus-pertussis vaccines), underlying diseases (including diabetes, chronic kidney disease, respiratory disease, and psychiatric illness), subjective cognitive complaints, hospitalization in the past year, and overall health perception.
COVID-19 Pandemic-Related Information
Participants were asked about their knowledge about COVID-19, primary source of COVID-19 information, confidence in COVID-19 information from government and public health agencies, confidence in the Thailand healthcare system’s ability to treat patients with COVID-19, government measurements for controlling COVID-19 infection, risk for COVID-19 infection, self-perception about having severe COVID-19 infection, and attitude toward social distancing. Moreover, participants were asked if there was a patient with COVID in their neighborhood, if they knew anyone who had been infected with COVID-19, and if they knew anyone who had died from COVID-19.
COVID-19 Vaccine-Related Information
Participants were asked if they were hesitant to get a COVID-19 vaccine, which was defined as delay in acceptance or refusal of vaccination despite availability of vaccination services. They were asked if they knew anyone who had had a serious reaction to the COVID-19 vaccine, wanted to be vaccinated for COVID-19, thought the manufacturer of the COVID-19 vaccine influenced their decision to get vaccinated, and who they thought was the most desirable COVID-19 vaccine manufacturer. They were also asked whether they still wanted to get vaccinated if they were offered vaccines from a manufacturer they did not expect. Furthermore, those who were hesitant to receive a COVID-19 vaccine were asked why they were hesitant, and all participants were asked if they were willing to have the vaccine.
Association Between Daily New COVID-19 Cases and Vaccine Hesitancy
Thailand was experiencing a new surge of COVID-19 infections at the time of our study, which was caused by a highly contagious Delta variant.35 We retrieved daily new COVID-19 patient data for Thailand from the WHO (COVID-19) official website36 to explore the association between the number of daily new cases and COVID-19 vaccine hesitancy.
Statistical Analysis
Nominal data (eg, presence of underlying diseases) were summarized as numbers and percentages. Continuous data (eg, age) were summarized as mean ± standard deviation (SD), or median and interquartile range based on the normality of the distribution. Group comparisons were performed using chi-square or Fisher’s tests for categorical variables and independent t-tests or Mann–Whitney U-tests for continuous parameters of continuous variables. Factors influencing vaccine hesitancy were investigated using binary logistic regression. Only variables that were statistically significant in the univariate logistic regression model were investigated in the multivariate logistic regression model. All statistical analyses were performed using SPSS version 26.0 for Windows (IBM Corp., Armonk, NY, USA). A p-value <0.05 was considered statistically significant.
Results
Of the 1,095 patients contacted, 282 (25.8%) patients were able to participate in the interview and were enrolled in this study. The remaining 813 (74.2%) patients were excluded from our study (Figure 1). In total, 125 (44.3%) of the 282 participants had vaccine hesitancy, and 157 (55.7%) indicated willingness to receive a vaccine.
 |
Figure 1 Study flow diagram. |
Participants’ Sociodemographic Characteristics and Medical History
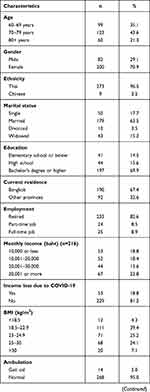
Sociodemographic data are shown in Tables 1 and 2. Participants were older adults aged 60–93 years (mean ± SD age: 73.0 ± 7.5 years). Most participants were female (70.9%), of Thai ethnicity (96.5%), married (63.5%), and lived in Bangkok (67.4%). A comparison of characteristics between the vaccine hesitancy and vaccine acceptance groups showed that those who had an education level of elementary school or below had higher vaccine hesitancy than those who had graduated with a bachelor’s degree or higher (odds ratio [OR] 2.56, 95% confidence interval [CI]: 1.06–6.16, p=0.037) (Table 3).
 |
 |
 |
Table 1 Baseline Characteristics of All Participants (N=282). |
 |
 |
 |
Table 2 Baseline Characteristics Associated with COVID-19 Vaccine Hesitancy. |
 |
Table 3 Logistic Regression Results for COVID-19 Vaccine Hesitancy. |
COVID-19 Pandemic Related Information
Table 4 presents the results for the items covering COVID-19 pandemic-related information. Most participants (62.0%) thought they knew quite a lot or a lot about COVID-19, and the majority (55.3%) said their COVID-19 information came from television or radio. Comparison of the vaccination hesitancy and vaccine acceptance groups showed that those who were not confident in the ability of the Thailand healthcare system to treat patients with COVID-19 were more likely to have vaccine hesitancy than those who were confident (OR 6.41, 95% CI: 1.28–32.10, p=0.024) (Table 3).
 |
Table 4 COVID-19 Pandemic- and Vaccine-Related Factors Associated with COVID-19 Vaccine Hesitancy. |
COVID-19 Vaccine-Related Information
COVID-19 vaccine related information outcomes are depicted in Table 4. Most participants (89.3%) did not know anyone who was severely allergic to the COVID-19 vaccine, but 44.3% expressed vaccine hesitancy. Approximately one-third of participants (33.3%) desired the Oxford-AstraZeneca ChAdOx1 nCoV-19 vaccine, followed by Moderna mRNA-1273 SARS-CoV-2 vaccine (16.7%), and the Pfizer-BioNTech mRNA vaccine BNT162b2 (9.6%) (Figure 2). The most common reasons for COVID-19 vaccine hesitancy were fear of COVID-19 vaccine-related adverse effects (35.2%), possible complications caused by an underlying disease (26.4%), and lack of confidence in COVID-19 vaccine efficacy or quality (16.8%) (Figure 3). The most important reasons for vaccination were that the COVID-19 vaccine could prevent severe COVID-19 infection or death (42.9%) and protect them from COVID-19 infection (21.3%), and that they were vulnerable group for COVID-19 infection (13.1%) (Figure 4). The comparison of the vaccine hesitancy and vaccine acceptance groups showed that people who believed COVID-19 vaccine manufacturers influenced their decision to receive vaccination had higher vaccine hesitancy (OR 5.16, 95% CI: 2.65–10.04, p<0.001). People who said they would reject vaccination if they were offered a vaccine from a manufacturer they had not heard of before had more vaccine hesitancy compared with those who accepted receiving different vaccines (OR 3.16, 95% CI: 1.26–7.95, p=0.014) (Table 3).
 |
Figure 2 Most desirable vaccine manufacturer as perceived by participants. |
 |
Figure 3 Most important reason for COVID-19 vaccine hesitancy. |
 |
Figure 4 Most important reasons to get vaccinated against COVID-19. |
Association Between Daily New COVID-19 Cases and Vaccine Hesitancy
On the first day of our survey (June 20, 2021), there were 3,682 new COVID-19 cases. The number of new cases per day had substantially increased by the end of this study (on July 22, 2021) to 15,355 patients per day. We categorized the number of daily new cases into four groups: <5,000, 5,000–7,499, 7,500–9,999, and ≥10,000 cases per day (Table 4). We discovered that when there were <5,000 new cases per day, people were more hesitant to get vaccinated than when there were ≥10,000 new cases per day (OR 6.66, 95% CI: 2.48–17.68, p<0.001) (Table 3).
Discussion
The present study is one of the few available studies on COVID-19 vaccination hesitancy in older adults, and the first study on COVID-19 vaccine hesitancy in Thailand. We conducted a telephone survey of patients from the geriatric clinic at Ramathibodi Hospital, using information acquired from the hospital’s database. Our survey was completed by 282 patients, of which 44.3% were hesitant to get vaccinated. Our study found that factors associated with high COVID-19 vaccine hesitancy were low education, lack of confidence in the healthcare system’s ability to treat patients with COVID-19, vaccine manufacturers, being offered vaccines from an unexpected manufacturer, and a lower number of new COVID-19 cases per day. However, factors that influenced vaccine hesitancy in earlier studies19,22,23,28,33,37–42 such as gender, income, marital status, and influenza vaccine history in the previous year, did not demonstrate associations in our study.
We found that people with an education level of elementary school or below had more vaccine hesitancy than those who had graduated with a bachelor’s degree or higher. This finding was consistent with previous studies22,25,40,41 from the United States, the United Kingdom, and Portugal. When compared with individuals who had confidence in the healthcare system’s competence to manage patients with COVID-19, those who lacked confidence had 6.4 times higher vaccine hesitancy. A study from Portugal25 reported comparable results, and a Saudi Arabian study found that people who trusted their healthcare system were three times more likely to receive vaccines.38
Vaccine manufacturers are one of Thailand’s most controversial issues. Currently, only the ChAdOx1 nCoV-19 and CoronaVac vaccinations are available. Although the Delta variant was the COVID-19 variant most commonly of concern in Thailand,35 research is only available on the efficacy of ChAdOx1 nCoV-19 against Delta (effectiveness: 67%, 95% CI: 6.3–71.8).43 This could explain why the COVID-19 vaccine manufacturer influenced people’s vaccine hesitancy. This was further supported by the finding from our study that people wanted to get vaccinated for two primary reasons: to prevent serious infection or death (42.9%) and to prevent infection (21.3%). To promote vaccination acceptability, the government and public health authorities should make high-potency COVID vaccine accessible to everyone. In addition, those who expected a certain vaccine but were instead given a different vaccine showed a higher rate of vaccine hesitancy. This could be a unique problem in the Thai situation, caused by the uncertain policies of the responsible agency. People’s vaccine hesitancy could be reduced by open communication and a clear vaccine delivery strategy.
We observed that when the number of new COVID-19 infections grew, people’s vaccine hesitancy decreased. Fear of COVID-19 was linked to risk perception in a previous investigation.44 Research suggested that those who perceived a greater risk of infection were more likely to receive vaccines.25,33 We hypothesized that as the number of new COVID-19 infections rose, so did the risk of becoming infected, which resulted in less vaccination hesitancy.
Some previous studies that suggested females had higher COVID-19 vaccine hesitancy;22,37,39,42 however, we were unable to replicate this finding. In addition, we found no link between income and COVID-19 vaccine hesitancy, although several previous studies demonstrated people with higher income had lower vaccine hesitancy.28,40,42 Our study found no relationship between marital status and COVID-19 vaccine hesitancy. However, in some previous research, married people exhibited less COVID-19 vaccine hesitancy.33,38 This may be related to the fact that our participants were older individuals with different marital status than younger groups. Furthermore, unlike some previous research,25,28,33,41 we were unable to show that those who had had an influenza vaccine injection in the previous year had less COVID-19 vaccine hesitancy. This could be because all older patients in our geriatric clinic were encouraged to get an annual influenza vaccine, with vaccination rates reaching 91.5%.
Our study was one of only a few studies on vaccine hesitancy that employed direct data gathering rather than an online volunteer survey. As a result, we were able to enroll diverse people in our study, including older adults who were unable to use technological devices. We began our research at a time when the number of COVID-19 patients was growing exponentially because of the Delta variant, so we could compare the incidence of COVID-19 vaccination hesitancy based on daily new cases. Furthermore, the majority of COVID-19 vaccine hesitancy studies were conducted while vaccinations were not generally available, whereas our study examined people’s attitudes after vaccines became widely accessible.
Our study had several limitations. We recruited participants from a geriatric clinic, most of whom lived in Bangkok, and the results should be interpreted with caution in other settings. Only one-fourth of those who were contacted were able to participate in this study and complete the questionnaires on their own; therefore the number of participants was fewer than planned. We were unaware that some participants would refuse to answer questions on sensitive subjects such as their incomes, and therefore were missing some information. Finally, although we demonstrated associations between higher COVID-19 vaccine hesitancy and low education level, lack of confidence in the healthcare system’s ability to treat patients with COVID-19, vaccine manufacturers, being offered vaccines from an unexpected manufacturer, and a lower number of new COVID-19 cases per day, we could not demonstrate causality.
Conclusion
The prevalence of COVID-19 vaccine hesitancy among Thai seniors is relatively high. Low education level, lack of confidence in the healthcare system’s ability to treat patients with COVID-19, vaccine manufacturers, being offered vaccines from an unexpected manufacturer, and a lower number of new daily COVID cases are linked to greater COVID-19 vaccine hesitancy. These findings will help in promoting COVID-19 vaccination among Thailand’s senior citizens.
Ethics Approval and Informed Consent
The study protocol was approved by the Human Research Ethics Committee of the Faculty of Medicine, Ramathibodi Hospital, Mahidol University (COA. MURA2021/441). This study was conducted according to the Declaration of Helsinki and Good Clinical Practice guidelines. Because we performed a telephone-based survey and it was inconvenient for participants to sign written informed consent and manage documents during the pandemic, we did not receive written informed consent. However, all participants provided verbal informed consent, which was recorded.
Acknowledgments
We would like to thank Piangporn Charernwat for assistance with data collection and project coordination, Krittika Saranburut for statistical analysis advice, and all interviewers and participants.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research project was supported by the Faculty of Medicine, Ramathibodi Hospital, Mahidol University.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. World Health Organization. WHO Coronavirus (COVID-19) DASHBOARD. Available from: https://covid19.who.int/.
2. Fahriani M, Anwar S, Yufika A, et al. Disruption of childhood vaccination during the COVID-19 pandemic in Indonesia. Narra J. 2021;1(1):e7. doi:10.52225/narraj.v1i1.7
3. Yusuf F, Fahriani M, Mamada SS, et al. Global prevalence of prolonged gastrointestinal symptoms in COVID-19 survivors and potential pathogenesis: a systematic review and meta-analysis. F1000Res. 2021;10:301. doi:10.12688/f1000research.52216.1
4. Fahriani M, Ilmawan M, Fajar JK, et al. Persistence of long COVID symptoms in COVID-19 survivors worldwide and its potential pathogenesis - a systematic review and meta-analysis. Narra J. 2021;1(2):e36. doi:10.52225/narraj.v1i2.36
5. Masyeni S, Santoso MS, Widyaningsih PD, et al. Serological cross-reaction and coinfection of dengue and COVID-19 in Asia: experience from Indonesia. Int J Infect Dis. 2021;102:152–154. doi:10.1016/j.ijid.2020.10.043
6. Bastola A, Sah R, Rajbhandari SK, et al. SARS-CoV-2 and orientia tsutsugamushi co-infection in a young teen, Nepal: significant burden in limited-resource countries in Asia? Narra J. 2021;1(2):e34. doi:10.52225/narraj.v1i2.34
7. World Health Organization. Thailand situation. Available from: https://covid19.who.int/region/searo/country/th.
8. Yanez ND, Weiss NS, Romand JA, Treggiari MM. COVID-19 mortality risk for older men and women. BMC Public Health. 2020;20(1):1742. doi:10.1186/s12889-020-09826-8
9. Ward H, Atchison C, Whitaker M, et al. SARS-CoV-2 antibody prevalence in England following the first peak of the pandemic. Nat Commun. 2021;12(1):905. doi:10.1038/s41467-021-21237-w
10. Pastor-Barriuso R, Perez-Gomez B, Hernan MA, et al. Infection fatality risk for SARS-CoV-2 in community dwelling population of Spain: nationwide seroepidemiological study. BMJ. 2020;371:m4509. doi:10.1136/bmj.m4509
11. O’Driscoll M, Ribeiro Dos Santos G, Wang L, et al. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature. 2021;590(7844):140–145. doi:10.1038/s41586-020-2918-0
12. Centers for Disease Control and Prevention (CDC). COVID-19 guidance for older adults. Available from: https://www.cdc.gov/aging/covid19-guidance.html.
13. World Health Organization. Coronavirus disease 2019 (COVID-19) WHO Thailand situation report – 17 April 2020. Available from: https://www.who.int/docs/default-source/searo/thailand/2020-04-17-tha-sitrep-55-covid19-final.pdf?sfvrsn=c59388b9_0.
14. Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819–1829. doi:10.1016/S0140-6736(21)00947-8
15. Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi:10.1016/S0140-6736(20)32661-1
16. Jara A, Undurraga EA, Gonzalez C, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021;385(10):875–884. doi:10.1056/NEJMoa2107715
17. Strategic Advisory Group of Experts (SAGE) on Immunization. Report of the SAGE working group on vaccine hesitancy. Available from: https://www.who.int/immunization/sage/meetings/2014/october/1_Report_WORKING_GROUP_vaccine_hesitancy_final.pdf.
18. MacDonald NE. Hesitancy SWGoV. Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015;33(34):4161–4164. doi:10.1016/j.vaccine.2015.04.036
19. Robinson E, Jones A, Lesser I, Daly M. International estimates of intended uptake and refusal of COVID-19 vaccines: a rapid systematic review and meta-analysis of large nationally representative samples. Vaccine. 2021;39(15):2024–2034. doi:10.1016/j.vaccine.2021.02.005
20. World Medical Association. World Medical Association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2494. doi:10.1001/jama.2013.281053
21. World Medical Association. WMA declaration of Helsinki - ethical principles for medical research involving human subjects. Available from: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/.
22. Robertson E, Reeve KS, Niedzwiedz CL, et al. Predictors of COVID-19 vaccine hesitancy in the UK household longitudinal study. Brain Behav Immun. 2021;94:41–50. doi:10.1016/j.bbi.2021.03.008
23. Lin C, Tu P, Beitsch LM. Confidence and receptivity for COVID-19 vaccines: a rapid systematic review. Vaccines. 2020;9(1):16. doi:10.3390/vaccines9010016
24. Okubo R, Yoshioka T, Ohfuji S, Matsuo T, Tabuchi T. COVID-19 vaccine hesitancy and its associated factors in Japan. Vaccines. 2021;9(6):662. doi:10.3390/vaccines9060662
25. Soares P, Rocha JV, Moniz M, et al. Factors associated with COVID-19 vaccine hesitancy. Vaccines. 2021;9(3):300. doi:10.3390/vaccines9030300
26. Fridman A, Gershon R, Gneezy A, Capraro V. COVID-19 and vaccine hesitancy: a longitudinal study. PLoS One. 2021;16(4):e0250123. doi:10.1371/journal.pone.0250123
27. Kreps S, Prasad S, Brownstein JS, et al. Factors associated with US adults’ likelihood of accepting COVID-19 vaccination. JAMA Netw Open. 2020;3(10):e2025594. doi:10.1001/jamanetworkopen.2020.25594
28. Kelly BJ, Southwell BG, McCormack LA, et al. Predictors of willingness to get a COVID-19 vaccine in the U.S. BMC Infect Dis. 2021;21(1):338. doi:10.1186/s12879-021-06023-9
29. Syed Alwi SAR, Rafidah E, Zurraini A, Juslina O, Brohi IB, Lukas S. A survey on COVID-19 vaccine acceptance and concern among Malaysians. BMC Public Health. 2021;21(1):1129. doi:10.1186/s12889-021-11071-6
30. Daly M, Robinson E. Willingness to vaccinate against COVID-19 in the U.S.: representative longitudinal evidence from April to October 2020. Am J Prev Med. 2021;60(6):766–773. doi:10.1016/j.amepre.2021.01.008
31. Loomba S, de Figueiredo A, Piatek SJ, de Graaf K, Larson HJ. Measuring the impact of COVID-19 vaccine misinformation on vaccination intent in the UK and USA. Nat Hum Behav. 2021;5(3):337–348. doi:10.1038/s41562-021-01056-1
32. Callaghan T, Moghtaderi A, Lueck JA, et al. Correlates and disparities of intention to vaccinate against COVID-19. Soc Sci Med. 2021;272:113638. doi:10.1016/j.socscimed.2020.113638
33. Wang J, Jing R, Lai X, et al. Acceptance of COVID-19 vaccination during the COVID-19 pandemic in China. Vaccines. 2020;8(3):482. doi:10.3390/vaccines8030482
34. Ward JK, Alleaume C, Peretti-Watel P, Group C. The French public’s attitudes to a future COVID-19 vaccine: the politicization of a public health issue. Soc Sci Med. 2020;265:113414. doi:10.1016/j.socscimed.2020.113414
35. World Health Organization. Coronavirus disease 2019 (COVID-19) WHO Thailand situation report – 22 July 2021. Available from: https://cdn.who.int/media/docs/default-source/searo/thailand/2021_07_22_eng-sitrep-193-covid19.pdf?sfvrsn=a0fdd5a7_3.
36. World Health Organization. WHO Coronavirus (COVID-19) dashboard (Thailand). Available from: https://covid19.who.int/region/searo/country/th.
37. Neumann-Bohme S, Varghese NE, Sabat I, et al. Once we have it, will we use it? A European survey on willingness to be vaccinated against COVID-19. Eur J Health Econ. 2020;21(7):977–982. doi:10.1007/s10198-020-01208-6
38. Al-Mohaithef M, Padhi BK. Determinants of COVID-19 vaccine acceptance in Saudi Arabia: a web-based national survey. J Multidiscip Healthc. 2020;13:1657–1663. doi:10.2147/JMDH.S276771
39. Yoda T, Katsuyama H. Willingness to receive COVID-19 vaccination in Japan. Vaccines. 2021;9(1):48. doi:10.3390/vaccines9010048
40. Williams L, Flowers P, McLeod J, Young D, Rollins L; The Catalyst Project T. Social patterning and stability of intention to accept a COVID-19 vaccine in Scotland: will those most at risk accept a vaccine? Vaccines. 2021;9(1):48. doi:10.3390/vaccines9010017
41. Fisher KA, Bloomstone SJ, Walder J, Crawford S, Fouayzi H, Mazor KM. Attitudes toward a potential SARS-CoV-2 vaccine: a survey of U.S. adults. Ann Intern Med. 2020;173(12):964–973. doi:10.7326/M20-3569
42. Reiter PL, Pennell ML, Katz ML. Acceptability of a COVID-19 vaccine among adults in the United States: how many people would get vaccinated? Vaccine. 2020;38(42):6500–6507. doi:10.1016/j.vaccine.2020.08.043
43. Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 (delta) variant. N Engl J Med. 2021;385(7):585–594. doi:10.1056/NEJMoa2108891
44. Han MFY, Mahendran R, Yu J. Associations between fear of COVID-19, affective symptoms and risk perception among community-dwelling older adults during a COVID-19 lockdown. Front Psychol. 2021;12:638831. doi:10.3389/fpsyg.2021.638831
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
