Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 12
Factors Affecting Survival Rates Among Adult TB/HIV Co-Infected Patients in Mizan Tepi University Teaching Hospital, South West Ethiopia
Authors Wondimu W , Dube L, Kabeta T
Received 17 December 2019
Accepted for publication 7 April 2020
Published 23 April 2020 Volume 2020:12 Pages 157—164
DOI https://doi.org/10.2147/HIV.S242756
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Bassel Sawaya
Wondimagegn Wondimu,1 Lamessa Dube,2 Teshome Kabeta2
1Mizan Tepi University, College of Health Science, Department of Public Health, Mizan Aman, Ethiopia; 2Jimma University, Faculty of Public Health, Department of Epidemiology, Jimma, Ethiopia
Correspondence: Wondimagegn Wondimu
Mizan Tepi University, College of Health Science, Department of Public Health, Mizan Aman, Ethiopia
Tel +251 917255007
Email [email protected]
Background: Tuberculosis (TB) and human immunodeficiency virus (HIV) co-infection was responsible for approximately 300,000 deaths worldwide in 2017. Despite this burden of death, factors associated with the survival of TB-HIV co-infected patients were not adequately studied; and some of the existing evidences are inconsistent. This study was aimed to identify factors associated with survival rates of TB/HIV co-infected patients.
Methods: The current study was a retrospective analysis of data extracted from 364 TB/HIV co-infected patients treated at Mizan Tepi University Teaching Hospital, Ethiopia, during the years 2007– 2017. Time to event was measured from the date of TB treatment initiation till death, loss to follow-up or completion of treatment. Since the event was death, patients lost from follow-up and those on follow-up were considered as censored. Using Cox-regression, the 95% CI of hazard ratio (HR) and P-value < 0.05 were used to identify the significant variables in multivariable analysis.
Results: All the 364 patients were followed up for 1654 person-months. There were 83 (22.8%) deaths and the majority, 38 (45.8%), were occurring within the first two months of anti-TB treatment initiation. The overall incidence rate and median survival time were 5.02 per 100 person-months (95% CI: 4.05, 6.22) and 10 months, respectively. Not using CPT (adjusted hazard ratio [AHR] =1.72; P=0.023), bedridden functional status (AHR=2.55; P=0.007), not disclosing HIV status (AHR=4.03; P< 0.001) and CD4 < 200 cells/mm3 (AHR=6.05; P< 0.001) were factors associated with survival rates of TB/HIV co-infected patients.
Conclusion: Our finding signals that care and attention should be given to the victims of these synergistic diseases. There is room to improve the survival of the patients if those with low CD4 count and bedridden functional status are closely monitored; and if CPT is promptly initiated with encouraging HIV status disclosure.
Keywords: TB/HIV, co-infection, survival rate, MTUTH
Introduction
TB/HIV co-infection synergistically increases the adverse effects of both diseases and complicates a number of factors such as epidemiology, pathology, clinical presentation, treatment, and prevention.1
It has also social, economic and political crises as consequences.2,3 The most common consequences are stigma, high susceptibility to other comorbidities like mental disorders, cost of care, increased number of orphans and decreased productivity due to long time illness and absence from the workplace.2,3 In most of the situations, this co-infection affects the productive segment of the population and increases the crisis by many folds, especially for low-income countries like Ethiopia.4
For people living with HIV/AIDS (PLWHA), the risk of developing TB is estimated to be between 17–23 times greater than those without HIV infection in 2017. Globally, the proportion of HIV-positive patients died during the TB treatment was 11%, in the same year.5,6
TB was the leading cause of death among people with HIV and there were about 300,000 people died from HIV-associated TB in 2017, in which the masses of deaths (84%) occurred in Africa.6
Ethiopia is among the 30 high TB/HIV burden countries and out of the TB patients with known HIV status, about 11% were HIV co-infected. It was estimated that there were about 3600 deaths due to TB-HIV co-infection in 2017.5,7 On the top of the knowledge gap, the problems of lay beliefs, misconceptions, and prejudices regarding TB/HIV co-infection are other devastating issues in Ethiopia; since these all might contribute to delay from care and in turn for poor survival among co-infected patients.8–10
Currently, there is no published study in the study area on factors associated with survival rate of TB/HIV co-infected patients; although it is nearest to Gambella region, which is among the regions with higher TB/HIV co-infection in Ethiopia and from where also a significant number of TB/HIV co-infected patients come to Mizan-Tepi University Teaching Hospital (MTUTH).
In addition, studies conducted in the North West and the southwest part of Ethiopia identified inconsistent predictors.11,12 This study was aimed to identify the factors associated with the survival rate of TB-HIV co-infected adult patients in MTUTH.
Methods
Study Design and Setting
A retrospective analysis of data was done for TB/HIV co-infected patients enrolled from July 2, 2007, to July 1, 2017, at Mizan Tepi University Teaching Hospital. Each patient was followed up retrospectively from the date of TB treatment initiation till death, loss to follow-up or completion of treatment. Since the event was death, patients lost from follow-up and those on follow-up were considered as censored. The hospital is found in Mizan-Aman town, which is 593 km to South West of Addis Ababa, the capital of Ethiopia. The hospital started offering free ART services in 2006. About 5946 PLWHA were enrolled till January 2019 and among these 1830 were active. Assessment for TB was being done routinely for every HIV patient. Similarly, if their test results become positive, all TB patients were supported to know their HIV status and linked to the ART clinic.
Study Population and Sampling Techniques
All adult TB/HIV co-infected patients, who received care from MTUTH and being on anti-TB from July 2, 2007, to July 1, 2017, were included. Patients who started ART earlier than six months before the diagnosis of TB and later than two months after the diagnosis of TB were excluded. Stopping ART for any reason during TB treatment is one of the exclusion criteria since it is the known risk factor that increases death by many folds. Additionally, incomplete registries, transfer-out, and death on the same day of anti-TB treatment initiation were excluded.
The sample size was determined using the online calculator for test time-to-event data for the Cox proportional hazard.13 The CD4 count ≤50 is a known predictor of mortality and used to classify the groups into two (ie subjects with CD4 count ≤50 cells/mm3 and subjects with CD4 count >50 cells/mm3).4,14 Using the parameters (power=80%, type one error rate=5%, hazard ratio=2.31, overall probability of event=13% and proportion of sample in group of subjects with CD4 count ≤50 cells/mm3=38.7%) from previous study,15 the sample size became 364. The samples were selected by simple random sampling technique after listing the medical registration number of all 670 eligible records as a sampling frame.
Data Extraction Technique and Data Quality Control
Data were extracted using a data extraction tool adapted from national ART and anti TB treatment standard registries. Data sources were standard registries of ART and anti-TB treatments, electronic format, patient medical record (card) and intake forms. Two trained nurses each from the ART and TB clinics of the hospital extracted the data. The trained supervisor supervises all the extraction processes. Both the supervisor and principal investigator daily checked the completeness and consistency of checklists during data extraction. Socio-demographic, clinical, behavioral and health service-related baseline data were extracted from the sources.
Data Entry, Processing and Analysis
Data were entered into Epi data 3.1 and exported to SPSS version 21 for analysis. Exploration of the data was done to check the missing values, outliers, and proportionality of hazards over time. Continuous variables were described using means and medians with respective 95% CI; while categorical variables were described using counts, percentages and tables. The median survival time, survival probability and the incidence rate were determined. The association between the independent variables and outcome variable was assessed by the Cox-proportional hazard model, and hazard ratio (HR) was used as a measure of association. The survival status of TB/HIV co-infected patients was presented by the life table.
All variables with p-value ≤0.25 and/or clinical importance were candidates for the final model. By using a backward stepwise likely hood ratio technique and p-value <0.05 the final significant factors were identified.
Ethical Consideration
The study proposal was approved by an institutional review board (IRB) of Jimma University. A support letter was obtained from the IRB of Jimma University to MTUTH administrator’s office. Confidentiality was assured strictly. In addition, patient identifiers (medical registration number and ART unique number) were replaced by a new identification number during data extraction and the name of the patients was not used.
Results
Characteristics of the Cohort
Among eligible 670 patients, 364 were included in the study. The mean age (±SD) of the participants was 30.52 (±9.03). From the total participants, 211 (58%) were females and 64 (30.3%) of them died during anti TB treatment giving the sex ratio (female to male) of death as 3.4 (64/19).
Similarly, among 150 (41.2%) married participants, 15 (10%) experienced death. Regarding the educational status more than one third, 133 (36.5%) had attended secondary school and out of them more than a quarter, 35 (26.3%) were encountered death (Table 1).
 |
Table 1 Baseline Socio-Demographic Characteristics of Study Subjects in MTUTH, South West Ethiopia, 2007–2017 |
Participants with a baseline CD4 count of less than 200 cells/mm3 were 124 (34.1%) and more than half, 73 (58.9%) of them died during the follow-up period. The significant proportion, 232 (63.7%) of the study participants had one or two opportunistic infections other than TB; among these, 57 (24.6%) had the outcome of death. The proportion of death among cotrimoxazole preventive therapy user participants [283 (77.7%)] was 15.9% (Table 2).
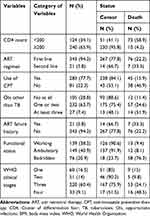 |
Table 2 Clinical Characteristics of Study Participants in MTUTH, South West Ethiopia, 2007–2017 |
From the total subjects included in the study, the disclosure status of two participants was not recorded. About three fourth, 270 (74.6%) disclosed their HIV status to family or any other and out of these 18 (6.7%) have died. On the other hand, among the 92 (25.4%) participants who did not disclose their status to anybody, 65 (70.7%) were experienced death.
Survival Status and Associated Factors
Three hundred sixty-four participants were followed for a total of 1654 person-months. There were 83 (22.8%) deaths and 41 (11.3%) loss to follow-up. From the total deaths, 38 (45.8%) occurred within the first two months of anti-TB treatment initiation. The overall incidence rate of death was 5.02 per 100 person-months (95% CI: 4.05, 6.22). The median survival time was around the 10th month. Survivals at 2, 6 and 12 months after initiating TB treatment were 82.02%, 73% and 45% respectively (Table 3).
 |
Table 3 Overall Life Table of TB/HIV Co-Infected Patients in MTUTH, South West Ethiopia, 2007–2017 |
The factors significantly associated with the survival rate of TB/HIV co-infected patients were; not using CPT (AHR=1.72; 95% CI: 1.08, 2.74), bedridden functional status (AHR=2.55; 95% CI: 1.29, 5.06), not disclosing HIV status (AHR=4.03; 95% CI: 2.21, 7.35) and CD4 count of less than 200 cells/mm3 (AHR=6.05; 95% CI: 2.83, 12.95) (Table 4).
 |
Table 4 Multivariable Cox-Regression Analysis of Factors Associated with Survival Rate of TB/HIV Co-Infected Patients in MTUTH, South West Ethiopia, 2007–2017 |
Discussion
This study revealed that there were 22.8% deaths among TB/HIV co-infected patients during TB treatment and this finding is comparable to the studies done in Ethiopian towns, Jimma11 and Bahirdar;12 but higher than the Guinean finding.16 The overall incidence rate was 5.02 per 100 person-months, which is comparable to a study done in Bahirdar.12 The comparability of death rates in Ethiopian studies and higher than Guinean finding are suggestive for Ethiopia’s rank of being among TB/HIV high burden countries in the world and the need of future high attention and priority particularly for the timely achievement of ending both epidemics.7,17
The majority of the deaths, 38 (45.8%) occurred within the two months of anti-TB treatment commencement. This is more than two times higher compared to finding from South Africa.16 The difference might be due to the dissimilarity of study designs and populations. The current finding is fairly comparable with a study conducted in Malaysia18 and Cameroon.16 This is suggestive for the need of appropriate management of drug interactions and overlapping toxicities of anti TB and ART drugs, immune reconstitution inflammatory syndrome (IRIS) and other OIs which were factors highly associated with early deaths in TB/HIV co-infected patients.19–21
The death among females was tripled compared to males in our study, and this might be the result of high social responsibility among females in the society due to which they may not take care of their health. This finding is higher than the Cameroonian finding22 and the inconsistency might be attributable to the difference in sample size which was smaller in the previous study.
The survivals at 2, 6 and 12 months after initiating TB treatment were 82.02%, 73% and 45% respectively. This is lower than the Malaysian finding.18 This might be due to the pioneering efforts in Malaysia to combat TB/HIV co-infection; which was evidenced by its early achievement of the first and third 90s of UNAIDS’ 90-90-90 target.23
Patients who did not use CPT were nearly two times (AHR=1.72) at higher risk of death and in agreement with this, a study conducted in Bahirdar revealed that not taking CPT was significantly associated with mortality among TB/HIV co-infected patients during TB treatment.12 Severe bacterial infections and malaria, which can be successfully prevented by CPT, might be attributable to the higher risk of death among CPT nonusers.14,24,25
The majority, 70 (92.1%) of the bedridden patients were on first-line ART and the remaining 6 (7.9%) were on second-line ART. Though they were on ART, their risk of death was two and a half times (AHR=2.55) higher compared to those who had working functional status. This is similar to the findings of the studies conducted in Jimma and Ambo.11,26,27 This might be due to the delayed presentation of bedridden patients to care after developing numerous infections and complications which were in turn associated with poor survival.10,18
The bidirectional and synergistic effect of TB and HIV infection has a profound impact on the survival of TB/HIV co-infected patients. Latent or active TB activates CD4 cells to be captured by HIV and HIV depletes CD4 cells, which in turn accelerates the progression of latent TB to active TB disease.1,28 CD4 count of less than two hundred cells per cubic millimeter was significantly associated with poor survival of TB/HIV co-infected patients in this study (AHR=6.05). This is supported by previous findings from different parts of the world including Ethiopia. Although the current estimation is consistent with the findings from Bahirdar and Malaysia, it is higher compared to that of a study conducted in Brazil.12,15,18 This might be due to the categorization CD4 count into small intervals in a previous study.
Failure to disclose status puts TB/HIV co-infected patients and their families at a higher risk of death. It prevents other at risk family members from being tested; and patients who do not disclose their status are highly likely to be lost to follow-up, as they cannot explain their ongoing need to visit health institutions.29 In the current study, patients who did not disclose their HIV status to anybody were about four times (AHR= 4.03) at higher risk of death than those who disclosed their status. This is in line with the finding of the study done in Jinka, Ethiopia.30 This might be due to patients who did not disclose their status would not be comfortable to take their medications appropriately, could not get important family supports and would not visit health institutions in the opposite of recommendation; which might contribute to the unwanted outcome like ART failure and poor survival.31 One of the main reasons for non-disclosure of the status is fear of stigmatization, and this needs a sustainable awareness creation in the community to bring positive attitudes toward TB/HIV co-infected patients and alleviate lay beliefs and misconceptions.8,9
Strength of the Study
Although they were insignificant in the final model, we have assessed the effect of new variables (ie which were not assessed previously for this particular study group), TB registration group and number of OIs other than TB on the survival of TB/HIV co-infected patients. In addition, we have also computed the overall life table of TB/HIV co-infected patients.
Limitation of the Study
Selection bias might be introduced; during the exclusion of incomplete registers and subjects with lost cards.
Conclusions
This study demonstrated a high proportion of deaths, particularly within the first two months of anti-TB treatment initiation; and it also identified the risk factors that can be addressed during the routine care. Our finding signals that attention should be given to the victims of these synergistic diseases. As a result, to improve the survival of TB/HIV co-infected patients, close monitoring of bedridden and low CD4 count patients, and prompt CPT initiation with encouraging HIV status disclosure are recommended to all concerned bodies.
List of Abbreviations
AHR, Adjusted Hazard Ratio; AIDS, Acquired Immuno Deficiency Syndrome; ART, Anti-Retro Viral Therapy; BMI, Body Mass Index; CD4, Cluster of Differentiation 4; CI, Confidence Interval; CPT, Cotrimoxazole Preventive Therapy; HIV, human immunodeficiency virus; HR, Hazard Ratio; IRB, Institutional Review Board; OIs, Opportunistic Infections; PLWHA, Peoples Living With HIV/IDS; TB, Tuberculosis; UNAIDS, The joint United Nations Programme on HIV/AIDS; WHO, World Health Organization.
Acknowledgments
We would like to acknowledge the administrative bodies of Mizan Tepi University Teaching Hospital who allowed us to do this study on their institution and staffs of ART and TB clinics who participated in the extraction of data and supervision of its overall processes.
Disclosure
The authors have declared that no competing interests exist.
References
1. Bruchfeld J, Correia-neves M, Ka¨llenius G. Tuberculosis and HIV Coinfection. Cold Spring Harb Perspect Med. 2015;5(7):a017871. doi:10.1101/cshperspect.a017871
2. Deribew A, Tesfaye M, Hailmichael Y, et al. Common mental disorders in TB/HIV co-infected patients in Ethiopia. BMC Infect. Dis. 2010;10(1):1–8. doi:10.1186/1471-2334-10-201
3. Deribew A, HaileMichael Y, Tesfaye M, et al. The synergy between TB and HIV co-infection on perceived stigma in Ethiopia. BMC Res Notes. 2010;3:2–5. doi: 10.1186/1756-0500-3-249.
4. UNAIDS. Global AIDS Monitoring; 2016. Available from https://www.aidsdatahub.org/global-aids-monitoring-2017-indicators-monitoring-2016-united-nations-political-declaration-hiv-and. Accessed on January 22, 2018.
5. WHO. Global Tuberculosis Report; 2018. Available from https://apps.who.int/iris/handle/10665/274453. Accessed on October 10, 2019.
6. WHO. HIV-Associated Tuberculosis, Achievements in 2017; 2018. Available from http://www.who.int/tb/areas-of-work/tb-hiv/tbhiv_factsheet.pdf?ua=1. Accessed on January 5, 2019.
7. EPHI, WHO, FMOH of Ethiopia: Service Availability and Readiness Assessment 2016: Summary Report; 2016. Available from https://www.washinhcf.org/wp-content/uploads/2017/03/Final-SARA-Report-Jan-2017.pdf; Accessed on January 8, 2018
8. Gebremariam MK, Bjune GA, Frich JC. Lay beliefs of TB and TB/HIV co-infection in Addis Ababa, Ethiopia: a qualitative study. BMC Res Notes. 2011;4(1):2–6. doi:10.1186/1756-0500-4-277
9. Deribew A, Abebe G, Apers L, et al. Prejudice and misconceptions about tuberculosis and HIV in rural and urban communities in Ethiopia: a challenge for the TB/HIV control program. BMC Public Health. 2010;10(1):1–10. doi:10.1186/1471-2458-10-400
10. Gesesew H, Tsehaineh B, Massa D, Tesfay A, Kahsay H, Mwanri L. The prevalence and associated factors for delayed presentation for HIV care among tuberculosis/HIV co-infected patients in Southwest Ethiopia: a retrospective observational cohort. Infect Dis Poverty. 2016;5:1–10.
11. Gesesew H, Tsehayneh B, Massa D, Gebremedhin A, Kahsay H, Mwanri L. Predictors of mortality in a cohort of tuberculosis/HIV co-infected patients in Southwest Ethiopia. Infect Dis Poverty. 2016;5:1–9. doi:10.1186/s40249-016-0099-8
12. Sileshi B, Deyessa N, Girma B, Melese M, Suarez P. Predictors of mortality among TB-HIV Co-infected patients being treated for tuberculosis in Northwest Ethiopia: a retrospective cohort study. BMC Infect. Dis. 2013;13(1):1–10. doi:10.1186/1471-2334-13-297
13. Chow S, Shao JWH. Sample Size Calculations in Clinical Research; 2008. Available from http://powerandsamplesize.com/Calculators/Test-Time-To-Event-Data/Cox-PH-2-Sided-Equality. Accessed on May 1, 2018.
14. WHO. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommended for a Public Health Approach; 2016. Available from https://www.who.int/hiv/pub/arv/arv-2016/en/. Accessed on July 20, 2018.
15. Otavio R, Velasque L, Ribeiro SR, et al. Mortality in patients with HIV-1 and tuberculosis co-infection in Rio de Janeiro, Brazil - associated factors and causes of death. BMC Infect Dis. 2017;17:1–10. doi:10.1186/s12879-016-2122-x
16. Camara A, Sow MS, Toure A, et al. Treatment outcome, survival and their risk factors among new tuberculosis patients co-infected with HIV during the Ebola outbreak in Conakry. Epidemiol Public Heal. 2017:1–8. doi:10.1016/j.respe.2017.05.011.
17. WHO. Global Tuberculosis Report; 2017. Available from https://www.who.int/tb/publications/global_report/gtbr2017_main_text.pdf?ua=1. Accessed on January 8, 2018.
18. Ismail I, Bulgiba A. Predictors of death during tuberculosis treatment in TB/HIV co-infected patients in Malaysia. PLoS One. 2013;8(8):1–7. doi:10.1371/journal.pone.0073250
19. Aluoch JA. Tuberculosis/HIV Co-infection. East Afr Med J. 2013;89:68–74.
20. Swaminathan S, Padmapriyadarsini C, Narendran G. HIV-associated tuberculosis: clinical update. Clin. Infect. Dis. 2010;50(10):1377–1386. doi:10.1086/652147
21. Worodria W, Massinga-Loembe M, Mazakpwe D, et al. Incidence and predictors of mortality and the effect of tuberculosis immune reconstitution inflammatory syndrome in a cohort of TB/HIV patients commencing antiretroviral therapy. J Acquir Immune Defic Syndr. 2011;58:32–37. doi:10.1097/QAI.0b013e3182255dc2
22. Bigna JJR, Noubiap JJN, Agbor AA, et al. Early mortality during initial treatment of tuberculosis in patients co-infected with HIV at the yaoundé central hospital, cameroon: an 8-year retrospective cohort study (2006–2013). PLoS One. 2015:1–13. doi:10.1371/journal.pone.0132394.
23. UNAIDS. Global AIDS Update, Ending AIDS, Progress Towards the 90- 90-90 targets; 2017. Available from https://www.unaids.org/en/resources/documents/2017/20170720_Global_AIDS_update_2017. Acceessd on February 6, 2018.
24. Nunn AJ, Mwaba P, Chintu C, et al. Role of co-trimoxazole prophylaxis in reducing mortality randomised clinical trial. BMJ. 2008;337(jul10 1):2–8. doi:10.1136/bmj.a257
25. Hasse B, Walker AS, Fehr J, et al. Co-trimoxazole prophylaxis is associated with reduced risk of incident tuberculosis in participants in the swiss HIV cohort study. Antimicrob. Agents Chemother. 2014;58(4):2363–2368. doi:10.1128/AAC.01868-13
26. Hailu R, Eshetu W. Survival of HIV-TB co-infected adult patients under ART in ambo referral hospital, Ethiopia. Ethiop J Heal Dev. 2013;27:88–93.
27. Gesesew H, Tsehaineh B, Massa D, et al. The role of social determinants on tuberculosis/HIV co ‑ infection mortality in southwest Ethiopia: a retrospective cohort study. BMC Res Notes. 2016;9(1):4–11. doi:10.1186/s13104-016-1905-x
28. Sullivan ZA, Wong EB, Ndung T, Kasprowicz VO, Bishai WR. Latent and active tuberculosis infection increase immune activation in individuals co-infected with HIV. EBioMedicine. 2015;2(4):334–340. doi:10.1016/j.ebiom.2015.03.005
29. PEPFAR Ethiopia. Ethiopia Country/Regional Operational Plan (COP/ROP) 2017 Strategic Direction Summary; 2017. Available from https://copsdata.amfar.org/SDS/2017/Ethiopia.pdf. Accessed on April 21, 2020.
30. Erdaw T, Ameni G. Survival and predictors of mortality among human immunodeficiency virus patients on anti-retroviral treatment at Jinka Hospital, South Omo, Ethiopia: a six years retrospective cohort study. Epidemiol Health. 2016;38:1–10.
31. Yimer YT, Yalew AW. Magnitude and predictors of Anti-Retroviral Treatment (ART) failure in private health facilities in Addis Ababa, Ethiopia. PLoS One. 2015;10(5).
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
