Back to Journals » Clinical Ophthalmology » Volume 14
Factors Affecting Compliance to Anti-Vascular Endothelial Growth Factor Treatment of Diabetic Macular Edema in a Cohort of Jordanian Patients
Authors Abu-Yaghi NE , Abed AM , Khlaifat DF, Nawaiseh MB , Emoush LO, AlHajjaj HZ, Abojaradeh AM, Hattar MN, Abusaleem SK, Sabbagh HM, Abu Gharbieh YA , Quaqazeh SA
Received 6 February 2020
Accepted for publication 4 March 2020
Published 24 March 2020 Volume 2020:14 Pages 921—929
DOI https://doi.org/10.2147/OPTH.S248661
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Nakhleh E Abu-Yaghi,1 Alaa M Abed,1 Dana F Khlaifat,2 Mohammed B Nawaiseh,2 Laith O Emoush,2 Heba Z AlHajjaj,2 Ala M Abojaradeh,2 Mariana N Hattar,2 Sura K Abusaleem,2 Hashem M Sabbagh,1 Yazan A Abu Gharbieh,1 Sura A Quaqazeh2
1Department of Special Surgery, Ophthalmology Division, The University of Jordan, Amman, Jordan; 2School of Medicine, The University of Jordan, Amman, Jordan
Correspondence: Nakhleh E Abu-Yaghi
The University of Jordan, P.O. Box 7599, Amman 11118, Jordan
Tel +962 798504937
Email [email protected]
Purpose: To determine compliance rates and characteristics and to investigate factors affecting patients’ adherence to treatment with anti-vascular endothelial growth factors (anti-VEGFs) for diabetic macular edema (DME) in a cohort of Jordanian patients.
Methods: A retrospective case series wherein the files of DME patients treated with anti-VEGFs were reviewed and analyzed for factors affecting treatment compliance was undertaken. Demographic, clinical and ocular characteristics were recorded. All patients were also interviewed by phone using a structured questionnaire. Univariate and multivariate analyses were performed to determine factors associated with compliance.
Results: A total of 117 patients (65 males 52 females) were included in this study with a mean age of 62.93 years (± 9.75). Approximately, 85% of patients were compliant to their treatment and follow-up plan during the first year of management. Subjective perception of visual improvement after receiving three loading doses was the only independent variable with a unique statistically significant contribution to compliance. All other studied factors in this group of patients were not significantly associated with patient compliance.
Conclusion: VEGF suppression via the intravitreal route to treat DME is a long-term process that requires caregiver dedication but also proper patient compliance. Addressing real-life barriers in those patients may help guide future strategies to improve the treatment experience, lower the financial burden and contribute to better outcomes. Patients’ perceptions of possible treatment outcomes at the short term may influence their long-term commitment to therapy.
Keywords: diabetic macular edema, anti-vascular endothelial growth factor, retinopathy, compliance
Introduction
According to the World Health Organization, more than 422 million people live with diabetes.1 Diabetic macular edema (DME) is one of the many complications of the disease,2 with an estimated 21 million people suffering from this complication.3 DME is the leading cause of decreased vision in diabetic patients and has become one of the leading causes of blindness worldwide.3–8 The management of DME has changed over the years. Laser photocoagulation therapy was the reference treatment and the most cost-effective option, but improvement in vision was not sustained over the long term.9–12 Intravitreal injections of corticosteroids were the next choice,13–18 but their side effects limit their benefits.19,20
Currently, first-line therapy for DME is intravitreal anti-vascular endothelial growth factor (anti-VEGF) medications which are proven to be effective and safe.21–25 They were also proven to be efficient in the treatment of wet age-related macular degeneration (AMD) and macular edema secondary to central and branch retinal vein occlusions.26–28 Despite these injections becoming the standard treatment for DME, adverse effects are still present. Intraocular pressure (IOP) elevations, subretinal hemorrhage, retinal detachment, uveitis and endophthalmitis are among the reported adverse effects.29,30 Systemic adverse effects such as strokes, acute myocardial infarction, and thromboembolic events have also been reported.31 Bilateral involvement in DME is very high due to the systemic nature of the disease.32 For the convenience of the patients and to reduce the number of clinic visits, simultaneous bilateral intravitreal injections are being done more often.33 Same-day injections for DME are safe and well-tolerated by patients.34–36 No significant difference in the occurrence rate of adverse effects has been found between simultaneous bilateral injections and single injections.37–39
Among the most widely used anti-VEGF agents for the treatment of DME are ranibizumab (Lucentis®, Genentech), bevacizumab (Avastin®, Genentech) and aflibercept (Eylea®, Regeneron Pharmaceuticals).22,39,40 Ranibizumab is FDA approved for ophthalmic use and is a recombinant humanized antibody (Fab) fragment that binds all active forms of VEGF-A.41 It was the first anti-VEGF agent to show benefit in terms of visual acuity (VA).42–44 Although improvement in VA varies, functional outcomes are similar.45–48 Bevacizumab is used in an off-label manner to inhibit VEGF in the eye and is a humanized full-length monoclonal antibody that binds to and inhibits VEGF; it has also shown good results.49–51 Aflibercept’s mode of action incorporates the second binding domain of the VEGFR-1 receptor and the third domain of the VEGFR-2 receptor, preventing VEGF from binding to its original receptors, thereby “trapping” the molecule and reducing its activity due to its very high VEGF affinity.52,53 When comparing the efficacy of the three types of injections, the relative effect depended on baseline VA. Mild initial visual loss showed no apparent differences, but at worse levels of initial VA, aflibercept was more effective at improving vision.24
Visual outcomes correlate with the number of injections given which makes compliance a key component to successful therapy.45 Since DME causes progressive loss of vision and occurs in younger patients, it is important to address factors that prevent proper patient compliance. Cost-effectiveness of various interventions for DME has been scrutinized, and lack of therapy compliance was shown to lead to a worse outcome and therefore represents a huge economic burden.54–57
The purpose of this study is to determine patients’ treatment compliance rates and investigate factors that may have a positive or negative effect on compliance with anti-VEGF treatment in a cohort of Jordanian patients with DME.
Materials and Methods
Study Design and Data Collection
After securing the Institutional Review Board approval to conduct this study and obtaining written informed consent from all participants, a retrospective analysis was performed on 117 consecutive patients diagnosed with DME and slated to receive anti-VEGF injections for treatment at Jordan University Hospital. Identified patients were subsequently contacted by phone and interviewed regarding their disease awareness, progression and adherence to their original treatment plan. All patients were prescribed three loading doses of an anti-VEFG agent to be given 4–6 weeks apart. This was followed by a clinical exam and an ocular coherence tomography (OCT) of the treated eye(s) with a PRN regimen of VEGF suppression according to clinical and tomographic findings. Patients were considered compliant if they received the three loading doses in a timely manner and maintained follow up for 12 months (including receiving all their prescribed injections during that period if indicated). Patients who failed those two conditions were considered noncompliant for the sake of this analysis.
Clinical records were extracted and reviewed to obtain data related to the dates of injections, clinical examinations (including VA and IOP) and OCT images from the day of diagnosis and up to 1 year follow-up for each patient.
The telephone questionnaire contained pre-determined questions divided into 2 sections; the first section included questions about age, marital status, educational level, employment status, place of residence, co-morbid systemic diseases, insurance, mode of transportation, number of companions (chaperones) and the duration of symptoms prior to diagnosis. The second section tackled the injection procedure itself: the average waiting time, attitude towards the injection process, complaints after the injection, whether the patient felt a subjective improvement in VA after receiving their injections and perceived challenges to adhere to the treatment plan and the follow-up visits.
Patients with incomplete medical records or who were unreachable by phone and patients who were deceased during the study period were excluded from the study.
Statistical Analysis
SPSS version 25.0 has been used in our analysis. Mean (± standard deviation) values have been used to describe continuous variables (i.e. age, symptoms, duration and VA). Count (frequency) has been used to describe other nominal variables (i.e. gender, laterality and others).
A p value of 0.05 has been adopted as a significant threshold.
Results
A total of 117 consecutive patients who met the inclusion criteria and visited the retina clinic at Jordan University Hospital between January 2017 and December 2017 were included in this study with a mean age of 62.93 years (±9.75). They were 65 (55.6%) men and 52 (44.4%) women. In terms of disease laterality, 3 (2.6%) had their right eyes treated, 5 (4.3%) their left eyes, and the remaining 109 (92.3%) had both eyes treated. The average number of injections per treated eye was 6 (range 3–10). Detailed patients’ characteristics are presented in (Table 1).
 |
Table 1 Characteristics of Patients Included in This Study |
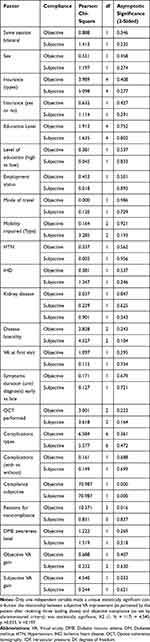 |
Table 2 Relation Between Compliance and Other Factors |
Discussion
VEGF pharmacological suppression using intravitreal injections has emerged as the first line of treatment for DME and other ophthalmic pathologies like wet AMD and macular edema secondary to retinal vascular disease. The treatment strategy often entails loading the patient with three or more monthly injections, then following up the patient with a scheduled clinical exam and ancillary tests. The treatment burden in DME is enormous with multiple injections in the first year, often bilateral, and adherence to the treatment plan can face multiple hurdles.
A study by Habib et al found that approximately 21% of DME patients were noncompliant to follow up and treatment with anti-VEGFs.58 Main factors for noncompliance included cost of the drug being injected, whether the patient is covered by medical insurance or not, as well as the psychological burden and the degree of patients’ satisfaction with having repeated intraocular injections. However, lack of formal education was not found to be a significant factor affecting compliance. Absence of funding/insurance, perceived susceptibility, perceived barriers, perceived benefits, and unilaterality of the injection are all factors that may affect patient compliance.58 A quarter of DME patients were noncompliant in a similar study by Best et al.45 Since diabetic patients must attend different medical consultations, sometimes with several specialists, this burden of repeated consultations may be a barrier to regular follow up. In another recent study by Weiss et al, only 35% of patients were compliant.59 The number of break-offs and change of visual acuity was found to be significantly correlated. In 60% of break-off cases, visual acuity was worse than before break-off. The most common reason for abstaining in that study was having other co-morbidities, and many patients were found to have little disease insight.59 The psychological burden including stress, discomfort, and fear from possible side effects has also been reported to affect patient compliance.23,58,60
In this study, we assessed compliance of DME patients to anti-VEGF treatment schedules over a period of 12 months. Approximately, 85% of patients were compliant with their treatment and follow-up plan during the first year of management, which mirrors results by previous investigators. In Egypt, Habib and coworkers followed patients for 1 year and noted the rate of dropped injections as a measure of compliance in 343 patients. They found that receiving bilateral injections at the same session correlated with the rate of adherence.58 Our center previously reported a trend towards more bilateral simultaneous injections but this factor was not found to affect compliance in this study.36
It is noteworthy that in our cohort of DME patients, compliance rate was positively correlated with the improvement of VA as perceived by the patient receiving the treatment. Subjects who reported a subjective improvement in vision adhered more to the treatment plan. Interestingly, a study by Polat et al on AMD patients receiving anti-VEGF treatment found an inverse relationship with the initial best-corrected VA. Patients who lost more vision at diagnosis were more compliant down the line; their perceived drop of vision probably evoking fear of progression and spurring aggressive adherence.61 Ehlken and colleagues found that higher age and poor baseline VA were associated with a higher risk for noncompliance in wet AMD but not in DME patients. They reported that DME subjects have the highest overall risk of patient-associated noncompliance, associated with a higher risk for significant visual loss. A possible explanation may be the presence of additional co-morbidities in patients with DME. This highlights that factors contributing to noncompliance may be different between diseases and communities.62
We looked into several patient factors for noncompliance including clinical parameters (e.g. co-morbidities, mobility) demographic and socioeconomic factors (e.g. education level, means of transport). However, no relevant correlation could be identified in this study. On the other hand, a French study by Boulanger-Scemama et al found that impaired mobility and lack of a chaperone were major causes for noncompliance for anti-VEGF regimens in wet AMD patients.63
Our study did not find a correlation between the patients’ knowledge and the level of education with compliance. Psychosocial and socioeconomic factors we looked into did not reveal any meaningful correlation as well. Patients’ profession and measured quality of life have been shown to affect patient adherence to treatment in previous studies nevertheless,64–67 with vision loss due to DME producing a significant socioeconomic strain on communities.68
Stress, discomfort, and fear from possible side effects have been shown to have an effect on compliance.23,58,60 In the study by Weiss et al, patients were followed for at least 1 year (up to 30 months) and compliance was measured by missed appointments (lateness >14 days) or therapy beak-offs (lateness >100days).59 The investigators showed that disease insight had a significant effect on compliance, but our study did not mirror a similar effect. Furthermore, our results demonstrated that the lack of formal education was not a significant factor affecting compliance. The cost of treatment had no significant impact on compliance in this cohort of Jordanian patients. Most patients are insured under the umbrella of the Ministry of Health and pay around 20% of the total cost of the injection procedure, which varies according to the cost of the medication injected. Still the financial burden of getting treatment was not a factor affecting compliance in this study.
The limitations of this study lie in its retrospective nature and the number of patients analyzed and the rather short follow-up period. We did not test the effect of the type of anti-VEGF medication on patients’ compliance as many factors may confound the relationship.39,69 In Jordan and many other developing countries, anti-VEGFs are used interchangeably and subject to availability, and the cost of treatment many times dictates the choice of the pharmacological agent. For example, patients who secure governmental medical exemption would often choose to be treated with either ranibizumab or aflibercept. Uninsured patients or those with medical insurance who have to co-pay will more likely choose bevacizumab due to its availability at a much lower cost. Moreover, we could not reliably investigate whether compliant patients were also stricter in their diabetic control, as concurrent HbA1c levels were not always readily available in patients’ records with many subjects choosing to follow with an endocrinologist or a primary care practitioner elsewhere.
Conclusion
In this study on a group of Jordanian diabetics, DME patients receiving anti-VEGF intravitreal injections who appreciated a subjective improvement in their vision after three loading doses appeared to adhere better to their treatment plan. As the burden of DME treatment grows for both patients and health systems, compliance with therapy will continue to pose a challenge for treating doctors who strive to accomplish better visual outcomes. Patients’ barriers and perceptions remain at the core of this ever-expanding approach to treat this common sight-threatening condition.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics and Consent Statement
Institutional ethical approval was obtained from the IRB committee at Jordan University Hospital. All participants provided written informed consent. This research complies with the tenants of the Declaration of Helsinki.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Global report on diabetes WHO Library Cataloguing-in-Publication Data Global report on diabetes, 2016.
2. Delcourt C, Massin P, Rosilio M. Epidemiology of diabetic retinopathy: expected vs reported prevalence of cases in the French population. Diabetes Metab. 2009;35(6):431–438. doi:10.1016/j.diabet.2009.06.002
3. Yau JWY, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556–564. doi:10.2337/dc11-1909
4. Rabinowitz R, Yagev R, Shoham A, Lifshitz T. Comparison between clinical and ultrasound findings in patients with vitreous hemorrhage. Eye. 2004;18(3):253–256. doi:10.1038/sj.eye.6700632
5. Keenan TDL, Johnston RL, Donachie PHJ, Sparrow JM, Stratton IM, Scanlon P. United Kingdom National Ophthalmology Database Study: diabetic Retinopathy; Report 1: prevalence of centre-involving diabetic macular oedema and other grades of maculopathy and retinopathy in hospital eye services. Eye (London, England). 2013;27(12):1397–1404. doi:10.1038/eye.2013.196
6. Kobrin Klein BE. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol. 2007;14(4):179–183. doi:10.1080/09286580701396720
7. Bourne RRA, Stevens GA, White RA, et al. Causes of vision loss worldwide, 1990–2010: a systematic analysis. Lancet Glob Heal. 2013;1(6):e339–49. doi:10.1016/S2214-109X(13)70113-X
8. Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis. 2015;2(1):17. doi:10.1186/s40662-015-0026-2
9. Photocoagulation for diabetic macular edema. Arch Ophthalmol. 1985;103(12):1796. doi:10.1001/archopht.1985.01050120030015
10. Laursen ML, Moeller F, Sander B, Sjoelie AK. Subthreshold micropulse diode laser treatment in diabetic macular oedema. Br J Ophthalmol. 2004;88(9):1173–1179. doi:10.1136/bjo.2003.040949
11. Lee CM, Olk RJ. Modified grid laser photocoagulation for diffuse diabetic macular edema. Long-Term Visual Results. Ophthalmology. 1991;98(10):1594–1602.
12. Doft BH, Blankenship GW. Single versus multiple treatment sessions of argon laser panretinal photocoagulation for proliferative diabetic retinopathy. Ophthalmology. 1982;89(7):772–779. doi:10.1016/S0161-6420(82)34734-X
13. Haller JA, Kuppermann BD, Blumenkranz MS, et al. Randomized controlled trial of an intravitreous dexamethasone drug delivery system in patients with diabetic macular edema. Arch Ophthalmol. 2010;128(3):289. doi:10.1001/archophthalmol.2010.21
14. Diabetic Retinopathy Clinical Research Network. A Randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology. 2008;115(9):1447–1459.e10.
15. Gillies MC, Sutter FKP, Simpson JM, Larsson J, Ali H, Zhu M. Intravitreal triamcinolone for refractory diabetic macular edema: two-year results of a double-masked, placebo-controlled, randomized clinical trial. Ophthalmology. 2006;113(9):1533–1538. doi:10.1016/j.ophtha.2006.02.065
16. Gillies MC, McAllister IL, Zhu M, et al. Intravitreal triamcinolone prior to laser treatment of diabetic macular edema: 24-month results of a randomized controlled trial. Ophthalmology. 2011;118(5):866–872. doi:10.1016/j.ophtha.2010.09.029
17. Boyer DS, Yoon YH, Belfort R, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121(10):1904–1914. doi:10.1016/j.ophtha.2014.04.024
18. Veritti D, Sarao V, Diplotti L, Samassa F, Lanzetta P. Fluocinolone acetonide for the treatment of diabetic macular edema. Expert Opin Pharmacother. 2017;18(14):1507–1516. doi:10.1080/14656566.2017.1363182
19. Gillies MC, Simpson JM, Gaston C, et al. Five-year results of a randomized trial with open-label extension of triamcinolone acetonide for refractory diabetic macular edema. Ophthalmology. 2009;116(11):2182–2187. doi:10.1016/j.ophtha.2009.04.049
20. Gillies MC, Lim LL, Campain A, et al. A randomized clinical trial of intravitreal bevacizumab versus intravitreal dexamethasone for diabetic macular edema: the BEVORDEX study. Ophthalmology. 2014;121(12):2473–2481. doi:10.1016/j.ophtha.2014.07.002
21. Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 Phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789–801. doi:10.1016/j.ophtha.2011.12.039
22. Korobelnik J-F, Do DV, Schmidt-erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121(11):2247–2254. doi:10.1016/j.ophtha.2014.05.006
23. Fong DS, Luong TQ, Contreras R, et al. Treatment patterns and 2-year vision outcomes with bevacizumab in diabetic macular edema: an analysis from a large U.S. Integrated Health Care System. Retina. 2018;38(9):1830–1838.
24. Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;123(6):1351–1359. doi:10.1016/j.ophtha.2016.02.022
25. Jampol LM, Glassman AR, Bressler NM, et al. Anti-vascular endothelial growth factor comparative effectiveness trial for diabetic macular edema: additional efficacy post hoc analyses of a randomized clinical trial. JAMA Ophthalmol. 2016;134(12):1429. doi:10.1001/jamaophthalmol.2016.3698
26. Epstein DL, Algvere PV, von Wendt G, et al. Benefit from bevacizumab for macular edema in central retinal vein occlusion: twelve- month results of a prospective, randomized study. Ophthalmology. 2012;119(12):2587–2591. doi:10.1016/j.ophtha.2012.06.037
27. Huang P, Niu W, Ni Z, Wang R, Sun X. A meta-analysis of anti-vascular endothelial growth factor remedy for macular edema secondary to central retinal vein occlusion Wedrich A, Editor. . PLoS One. 2013;8(12):e82454. doi:10.1371/journal.pone.0082454
28. Tan MH, Mcallister IL, Gillies ME, et al. Randomized controlled trial of intravitreal ranibizumab versus standard grid laser for macular edema following branch retinal vein occlusion. Am J Ophthalmol. 2014;157(1):237–247.e1. doi:10.1016/j.ajo.2013.08.013
29. Nuzzi R, Tridico F. Local and systemic complications after intravitreal administration of anti-vascular endothelial growth factor agents in the treatment of different ocular diseases: a five-year retrospective study. Semin Ophthalmol. 2015;30(2):129–135. doi:10.3109/08820538.2013.835833
30. Day S, Acquah K, Mruthyunjaya P, et al. Ocular complications after anti-vascular endothelial growth factor therapy in Medicare patients with age-related macular degeneration. Am J Ophthalmol. 2011;152(2):266–272. doi:10.1016/j.ajo.2011.01.053
31. Group TCR. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897–1908. doi:10.1056/NEJMoa1102673
32. Gonder JR, Walker VM, Barbeau M, et al. Costs and Quality of Life in Diabetic Macular Edema: canadian Burden of Diabetic Macular Edema Observational Study (C-REALITY). J Ophthalmol. 2014;2014:939315.
33. Giocanti-Auregan A, Tadayoni R, Grenet T, et al. Estimation of the need for bilateral intravitreal anti-VEGF injections in clinical practice. BMC Ophthalmol. 2016;16(1):142. doi:10.1186/s12886-016-0317-y
34. Bakri SJ, Risco M, Edwards AO, Pulido JS. Bilateral simultaneous intravitreal injections in the office setting. Am J Ophthalmol. 2009;148(1):66–69.e1. doi:10.1016/j.ajo.2009.02.013
35. Lima LH, Zweifel SA, Englrmbert M, et al. Evaluation of safety for bilateral same-day intravitreal injections of antivascular endothelial growth factor therapy. Retina. 2009;29(9):1213–1217. doi:10.1097/IAE.0b013e3181b32d27
36. Abu-yaghi NE, Shokry AN, Abu-sbeit RH. Bilateral same-session intravitreal injections of anti-vascular endothelial growth factors. Int J Ophthalmol. 2014;7(6):1017–1021. doi:10.3980/j.issn.2222-3959.2014.06.20
37. Davis RP, Schefler AC, Murray TG. Concomitant bilateral intravitreal anti-VEGF injections for the treatment of exudative age-related macular degeneration. Clin Ophthalmol. 2010;4:703–707. doi:10.2147/opth.s10008
38. Woo SJ, Han JM, Ahn J, et al. Bilateral same-day intravitreal injections using a single vial and molecular bacterial screening for safety surveillance. Retina. 2012;32(4):667–671. doi:10.1097/IAE.0b013e31822c296b
39. Brown DM, Nguyen QD, Marcus DM, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013;120(10):2013–2022. doi:10.1016/j.ophtha.2013.02.034
40. Rajendram R, Fraser-bell S, Kaines A, et al. A 2-year prospective randomized controlled trial of intravitreal Bevacizumab or Laser Therapy (BOLT) in the management of diabetic macular edema. Arch Ophthalmol. 2012;130(8):972–979. doi:10.1001/archophthalmol.2012.393
41. Krispel C, Rodrigues M, Xin X, Sodhi A. Ranibizumab in diabetic macular edema. World J Diabetes. 2013;4(6):310–318. doi:10.4239/wjd.v4.i6.310
42. Massin P, Bandello F, Garweg JG, et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter Phase II study. Diabetes Care. 2010;33(11):2399–2405. doi:10.2337/dc10-0493
43. Mitchell P, Massin P, Bressler S, et al. Three-year patient-reported visual function outcomes in diabetic macular edema managed with ranibizumab: the RESTORE extension study. Curr Med Res Opin. 2015;31(11):1967–1975. doi:10.1185/03007995.2015.1081880
44. Mj E, Ayala A, NM B, et al. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: 5-year randomized trial results. Ophthalmology. 2015;122(2):375–381. doi:10.1016/j.ophtha.2014.08.047
45. Best A-L, Fajnkuchen F, Nghiem-buffet S, et al. Treatment efficacy and compliance in patients with diabetic macular edema treated with ranibizumab in a real-life setting. J Ophthalmol. 2018;2018:1–7. doi:10.1155/2018/4610129
46. Dugel PU, Hillenkamp J, Sivaprasad S, et al. Baseline visual acuity strongly predicts visual acuity gain in patients with diabetic macular edema following anti-vascular endothelial growth factor treatment across trials. Clin Ophthalmol. 2016;10:1103–1110. doi:10.2147/OPTH
47. Hrarat L, Fajnkuchen F, Boubaya M, et al. Outcomes after a 1-year treatment with ranibizumab for diabetic macular edema in a clinical setting. Ophthalmologica. 2016;236(4):207–214. doi:10.1159/000453006
48. Granström T, Forsman H, Lindholm Olinder A, et al. Patient-reported outcomes and visual acuity after 12 months of anti-VEGF-treatment for sight-threatening diabetic macular edema in a real world setting. Diabetes Res Clin Pract. 2016;121:157–165. doi:10.1016/j.diabres.2016.09.015
49. Arevalo JF, Lasave AF, Wu L, et al. Intravitreal Bevacizumab plus Grid laser photocoagulation or intravitreal bevacizumab or grid laser photocoagulation for diffuse diabetic macular edema. Retina. 2013;33(2):403–413. doi:10.1097/IAE.0b013e3182695b83
50. Gunther JB, Altaweel MM. Bevacizumab (Avastin) for the treatment of ocular disease. Surv Ophthalmol. 2009;54(3):372–400. doi:10.1016/j.survophthal.2009.02.004
51. Carneiro AM, Mendonça LS, Falcão MS, Fonseca SL, Brandão EM, Falcão-reis FM. Comparative study of 1+PRN ranibizumab versus bevacizumab in the clinical setting. Clin Ophthalmol. 2012;6(1):1149–1157. doi:10.2147/OPTH.S33017
52. Holash J, Davis S, Papadopoulos N, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002;99(17):11393–11398. doi:10.1073/pnas.172398299
53. Saishin Y, Saishin Y, Takahashi K, et al. VEGF-TRAPR1R2 suppresses choroidal neovascularization and VEGF-induced breakdown of the blood-retinal barrier. J Cell Physiol. 2003;195(2):241–248.
54. Ross EL, Hutton DW, Stein JD, Bressler NM, Jampol LM, Glassman AR. Cost-effectiveness of aflibercept, bevacizumab, and ranibizumab for diabetic macular edema treatment. JAMA Ophthalmol. 2016;134(8):888. doi:10.1001/jamaophthalmol.2016.1669
55. Brown GC, Brown MM, Turpcu A, Rajput Y. The cost-effectiveness of ranibizumab for the treatment of diabetic macular edema. Ophthalmology. 2015;122(7):1416–1425. doi:10.1016/j.ophtha.2015.03.032
56. Dewan V, Lambert D, Edler J, Kymes S, Apte RS. Cost-effectiveness analysis of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2012;119(8):1679–1684. doi:10.1016/j.ophtha.2012.01.049
57. Stein JD, Newman-casey PA, Kim DD, Nwanyanwu KH, Johnson MW, Hutton DW. Cost-effectiveness of various interventions for newly diagnosed diabetic macular edema. Ophthalmology. 2013;120(9):1835–1842. doi:10.1016/j.ophtha.2013.02.002
58. Habib AE, Abdel-kader AA, Eissa IM, Awadein A. Adherence to intravitreal Anti-Vascular Endothelial Growth Factor (Anti-VEGF) drugs in diabetic macular edema in an egyptian population: a health belief model. Curr Eye Res. 2019;44(3):303–310. doi:10.1080/02713683.2018.1543708
59. Weiss M, Sim DA, Herold T, et al. Compliance and adherence of patients with diabetic macular edema to intravitreal antivascular endothelial growth factors therapy in daily practice. Retina. 2018;38(12):2293–2300. doi:10.1097/IAE.0000000000001892
60. Senra H, Ali Z, Balaskas K, Aslam T. Psychological impact of anti-VEGF treatments for wet macular degeneration—a review. Graefe’s Arch Clin Exp Ophthalmol. 2016;254(10):1873–1880. doi:10.1007/s00417-016-3384-0
61. Polat O, Inan S, Özcan S, et al. Factors affecting compliance to intravitreal anti-vascular endothelial growth factor therapy in patients with age-related macular degeneration. Turkish J Ophthalmol. 2017;47(4):205–210. doi:10.4274/tjo
62. Ehlken C, Helms M, Böhringer D, Agostini HT, Stahl A. Association of treatment adherence with real-life VA outcomes in AMD, DME, and BRVO patients. Clin Ophthalmol. 2018;12:13–20. doi:10.2147/OPTH.S151611
63. Boulanger-scemama E, Querques G, About F, et al. Ranibizumab for exudative age-related macular degeneration: a five year study of adherence to follow-up in a real-life setting. J Fr Ophtalmol. 2015;38(7):620–627. doi:10.1016/j.jfo.2014.11.015
64. Brook RA, Kleinman NL, Patel S, Smeeding JE, Beren IA, Turpcu A. United States comparative costs and absenteeism of diabetic ophthalmic conditions. Postgrad Med. 2015;127(5):455–462. doi:10.1080/00325481.2014.994468
65. Chen E, Looman M, Laouri M, et al. Burden of illness of diabetic macular edema: literature review. Curr Med Res Opin. 2010;26(7):1587–1597. doi:10.1185/03007995.2010.482503
66. Gonder JR, Walker VM, Barbeau M, et al. Costs and Quality of Life in Diabetic Macular Edema: canadian burden of diabetic macular edema observational study (C-REALITY). J Ophthalmol. 2014;2014:1–9. doi:10.1155/2014/939315
67. Minassian DC, Owens DR, Reidy A. Prevalence of diabetic macular oedema and related health and social care resource use in England. Br J Ophthalmol. 2012;96(3):345–349. doi:10.1136/bjo.2011.204040
68. Gale R, Carneiro A, de Zaeytijd J, et al. The importance of ocular, systemic and psychosocial factors in the management of the diabetic macular oedema patient with antivascular endothelial growth factor therapy. Eur Ophthalmic Rev. 2016;10(02):117.
69. Giocanti-auregan A, Tadayoni R, Grenet T, et al. Estimation of the need for bilateral intravitreal anti-VEGF injections in clinical practice. BMC Ophthalmol. 2016;16(1):142. doi:10.1186/s12886-016-0317-y
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
