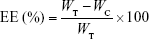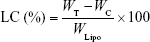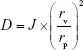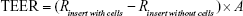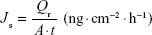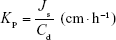Back to Journals » International Journal of Nanomedicine » Volume 13
Facilitated permeation of insulin across TR146 cells by cholic acid derivatives-modified elastic bilosomes
Authors Bashyal S , Seo JE, Keum T, Noh G, Choi YW , Lee S
Received 15 March 2018
Accepted for publication 3 July 2018
Published 6 September 2018 Volume 2018:13 Pages 5173—5186
DOI https://doi.org/10.2147/IJN.S168310
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Webster
Santosh Bashyal,1 Jo-Eun Seo,1 Taekwang Keum,1 Gyubin Noh,1 Young Wook Choi,2 Sangkil Lee1
1College of Pharmacy, Keimyung University, Daegu, Republic of Korea; 2College of Pharmacy, Chung-Ang University, Seoul, Republic of Korea
Background: Buccal delivery of insulin is still a challenging issue for the researchers due to the presence of permeability barrier (buccal mucosa) in the buccal cavity. The main objective of this study was to investigate the safety, effectiveness, and potential of various liposomes containing different bile salts to improve the permeation of insulin across in vitro TR146 buccal cell layers.
Methods: Elastic bilosomes containing soy lecithin and bile salt edge activators (sodium cholate [SC], sodium taurocholate [STC], sodium glycocholate [SGC], sodium deoxyglycocholate [SDGC], or sodium deoxytaurocholate [SDTC]) were fabricated by thin-film hydration method. The prepared liposomes were characterized, and in vitro permeation studies were performed. The fluorescein isothiocyanate-insulin-loaded elastic bilosomes were used to evaluate the quantitative and qualitative cellular uptake studies.
Results: The prepared elastic bilosomes had a particle size and an entrapment efficiency of ~140–150 nm and 66%–78%, respectively. SDGC-lipo (SDGC-incorporated liposome) was observed to be the most superior with an enhancement ratio (ER) of 5.24 (P<0.001). The SC-incorporated liposome (SC-lipo) and SDTC-incorporated liposome (SDTC-lipo) also led to a significant enhancement with ERs of 3.20 and 3.10 (P<0.05), respectively, compared with insulin solution. These results were further supported by quantitative and qualitative cellular uptake studies performed employing fluorescence-activated cell sorting analysis and confocal microscopy, respectively. The relative median fluorescence intensity values of elastic bilosomes were counted in the order of SDGC-lipo > SC-lipo > SDTC-lipo > SGC-incorporated liposome > STC-incorporated liposome, and similarity in the permeability profile of the employed elastic bilosomes was noted.
Conclusion: This study presents the employment of various derivatives of cholic acid-loaded elastic bilosomes as a promising strategy to enhance the permeation of insulin through buccal route.
Keywords: insulin, peptide delivery, bile salts, elastic liposomes, buccal mucosa
Introduction
As per the statistics released by the International Diabetes Federation, 425 million people were affected by diabetes worldwide in 2017. The prevalence is expected to increase to 629 million by 2045, rendering diabetes as a serious health concern in the world.1 It is reported that diabetes is a major cause for several chronic diseases such as heart attacks, kidney failure, blindness, stroke, and lower limb amputation.2 Insulin is the promising and most common medication used for the treatment of diabetes. It is administered to patients through a subcutaneous route with multiple daily injections, creating physical and mental burden on patients, and it reduces patient compliances and desired therapeutic outcome.3–5 Despite the advancements in innovative therapeutic approaches in pharmaceutical field, the noninvasive buccal delivery of biopharmaceuticals, particularly insulin, is still a major challenge.6 However, the presence of well-vascularized tissues, blood vessels draining directly into the jugular vein, and less proteolytic enzymes and bypassing the first-pass effects in the buccal mucosa as compared to gastrointestinal tract project the transbuccal delivery as a desired site for the delivery of biopharmaceuticals.7,8 Further, based on the ease of administration, short cellular turnover time of 4–14 days, and high patient compliances, transbuccal delivery has recently garnered an increasing attention worldwide.9
After multiple attempts, various pharmaceuticals have been registered for buccal drug delivery and commercialized in the market. For instances, Breakyl®, Setofilm®, Chloraseptic®, Donepezil Hexal®, Risperidone Hexal®, Triaminic®, and Theraflu® have been commercialized.10 In addition, several buccal products are on clinical trials such as buccal prochlorperazine (Phase III), buccal misoprostol (Phase IV), buccal midazolam (Phase IV), and Generex’s Oral-lyn™ insulin spray (Phase III).11 Oral-lyn™ insulin spray, a mixed micelles liquid formulation with sodium caprate and bile salts as an enhancer, has already been approved for marketing in Ecuador and Lebanon.12,13 The growing interest in the area of transbuccal delivery over the last two decades prompted us toward the initiation of study on buccal delivery of biopharmaceuticals. In our previous studies, we have demonstrated the successful delivery of salmon calcitonin along with chemical enhancers or in combination with iontophoresis across the buccal mucosa both in vitro and in vivo in rabbits.5,14
Various nanovesicular systems have been greatly investigated to evade the absorption barriers as well as to enhance the bioavailability of protein/peptide drugs.15,16 Among these systems, liposomes have already demonstrated their efficiency in increasing the bioavailability of various drugs in several studies.17–23 Since bile salts stabilize the vesicles and increase the fluidity of biomembrane as well as internalization of vesicles, their uses in liposomes-based delivery are on a constant rise.24 The tissue damages caused by bile salts are reversible; thus, they have been used abundantly to enhance the permeation of various pharmaceuticals across the buccal mucosa.25 In general, it has been reported that the bile salts enhance the absorption of drugs by the extraction of the membrane protein or lipids, membrane fluidization, forming reverse micelles in the membrane, and inducing aqueous channels.26,27 Bilosomes are a novel colloidal delivery system formed by the incorporation of bile salts into liposomes.17 Bilosomes are more elastic, flexible, and ultra-deformable than conventional liposomes.28 Recently, several studies using bilosomes have shown promising potential in the delivery of insulin across different alternative routes such as oral, transdermal, and buccal.29 In different studies, Niu et al incorporated three different bile salts in liposomes and revealed that sodium glycocholate (SGC)-incorporated liposomes (SGC-lipo) exhibited high potency for the oral delivery of insulin than sodium taurocholate (STC), sodium deoxycholate (SDC), or conventional liposomes.30,31 It has also been reported that SGC-lipo was the most superior in protecting gastrointestinal tract against enzymatic degradation, and this property was the main mechanism in enhancing the oral bioavailability of insulin.32,33 Furthermore, the enhanced oral bioavailability of insulin,34,35 cyclosporine A,18 and itraconazole36 has been demonstrated by employing liposomes containing bile salts. Similarly, bilosomes incorporating various drugs have shown their efficient applicability toward a transdermal route such as insulin,37 methotrexate,28 and tenoxicam.38 Additionally tacrolimus-loaded bilosomes have been successfully designed to function as an ocular delivery system.39 Moreover Yang et al revealed that the SDC-incorporated liposomes (SDC-lipo) had a promising approach on the buccal delivery of insulin than conventional liposomes.29 To the best of our knowledge, this is the first report on the buccal delivery of insulin-loaded bilosomes. Accordingly, it is hypothesized that our study might be another milestone in demonstrating the delivery of insulin employing different bile salt-loaded liposomes through a buccal route.
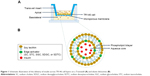
Due to variation in the thickness of individual’s buccal mucosae of porcine, cell model was selected.4 TR146 cell culture model was used as an in vitro model, as the TR146 cells form a multilayered squamous epithelium resembling human buccal epithelium.40,41 In the present study, different derivatives of cholic acids were used to prepare different elastic bilosomes. Table 1 summarizes the chemical structures and molecular weights of different derivatives of cholic acids used in this experiment. The main aim of this study was to investigate the safety, effectiveness, and potential of various liposomes containing different bile salts to improve the permeation of insulin across in vitro TR146 buccal cell layers.
Materials and methods
Materials
Human insulin was purchased from ProSpec-Tany TechnoGene Ltd. (Ness-Ziona, Israel). Soy lecithin (Solec 2F-UB) was purchased from Bunge North America, Inc. (Chesterfield, MO, USA). Sodium cholate (SC) and STC were purchased from Alfa Aesar (Heysham, UK). SGC and sodium deoxytaurocholate (SDTC) were purchased from Acros Organics (Morris Plains, NJ, USA). Fluorescein isothiocyanate-labeled insulin (FITC-insulin) and sodium deoxyglycocholate (SDGC) were purchased from Sigma-Aldrich (St Louis, MO, USA). TR146 cell line was purchased from Public Health England (London, UK). Hank’s balanced salt solution (HBSS), fetal bovine serum (FBS), Ham’s F-12 nutrient, and trypsin–ethylenediaminetetraacetic acid (EDTA; 0.25%) were purchased from WELGENE Inc. (Gyeongsan, Republic of Korea). All other chemicals were of reagent-grade and used without further purification.
Fabrication of elastic bilosomes
Elastic bilosomes were fabricated using a previously published thin-film hydration method with a slight modification.42 Briefly, soy lecithin and bile salt edge activators (SC, STC, SGC, SDGC, and SDTC; 85:15 w/w%) were dissolved in chloroform and methanol (3:1) in a round-bottom flask. The organic solvent was removed by rotary vacuum evaporation above the lipid transition temperature. The remaining traces of dry film were removed under nitrogen gas. Subsequently, the dry lipid film was hydrated with insulin solution (1.82 mg/mL) for 30–40 minutes under water bath at 35°C. Thus, prepared multilamellar liposome was extruded five times through a 200-nm polycarbonate membrane filter for homogeneous size distribution and efficient entrapment.
Particle characterization
The mean particle size, polydispersity index (PDI), and ζ potential were determined using dynamic light scattering (DLS) with a NanoBrook ZetaPALS (Version 5.69; Brookhaven Instruments Corp., Holtsville, NY, USA). Elastic bilosomes were diluted to 1:50 with distilled water, and the light scattering property was measured promptly. All measurements were performed under ambient conditions and in triplicate.
Entrapment efficiency (EE) and loading capacity (LC)
The efficiency of insulin entrapment in various elastic bilosomes was calculated by determining the free and total amount of insulin initially added in the formulations. Briefly, 1 mL of insulin-loaded liposome was placed in a centrifuge bottle (Beckman Coulter Inc., Carlsbad, CA, USA) and diluted with 9 mL of PBS of pH 7.4. Then, it was centrifuged at 200,000× g for 2 hours at 4°C (Beckman Optima™ LE-80K Ultracentrifuge). Insulin concentration in each sample was determined by Quantikine® enzyme-linked immunosorbent assay (ELISA; DINS00; R&D Systems Inc., Minneapolis, MN, USA). Finally, the drug EE and LC were calculated based on the following equations, respectively:
|
|
|
|
where WT, WC, and WLipo are the total amounts of drug initially added, the amount of drug detected in the supernatant, and the weight of liposome formulations, respectively.
Deformability of elastic bilosomes
The comparative measurement of deformability of different elastic bilosomes was carried out against the standard liposomes (control) preparation using single syringe infusion pump (KDS 100 series; KD Scientific Inc., Holliston, MA, USA).43 One end of the syringe pump was fixed on the wall, and another end was adjusted by the home-built device so that it can withstand the pressure generated by the extruder. The vesicles were extruded through 50-nm polycarbonate membranes at a constant flow rate of 15.55 mL/h. The size of vesicles was measured by DLS with a NanoBrook ZetaPALS (Version 5.69). The deformability index (D) of elastic bilosomes was obtained as follows:
|
|
where D is the deformability of elastic bilosomes, J is the amount of suspension that was extruded during 2 minutes, rv is the particle size after extrusion, and rp is the pore diameter of the membrane.44
Cell lines and cell culture conditions
Human TR146 (passages #11–20) cells were used for in vitro cellular studies. The cells were cultured in Ham’s F-12 supplemented with 10% FBS, 2 mM glutamine, penicillin (10,000 units/mL), and streptomycin (10,000 μg/mL) and incubated at 37°C under 5% CO2 and 95% air. The media were replaced every 2–3 days. At 70%–80% confluency, cells were split using 0.25% trypsin–EDTA.
Cell viability studies
The in vitro cell cultures of TR146 cells were subjected to a 2,3-bis-(2-methoxy 4-nitro-5-sulfophenyl)–2H-tetrazolium-5-carboxanilide salt (XTT) containing N-methyl dibenzopyrazine methyl sulfate (PMS) Cell Proliferation Assay Kit (PanReac AppliChem Co., Barcelona, Spain) in order to quantify viability. The cells were seeded in 96-well plates at a density of 2×104 cells per well and incubated for 24 hours at 37°C. After 24 hours, the media were removed, and 150 μL of solution, containing various blank liposomes of different bile acids, with different concentrations was added to each well. Subsequently, the cells were incubated for 8 hours (the duration of permeability experiments). Then, 50 μL of reaction mixture (XTT and PMS reagent) was added to each well and incubated for further 4 hours at 37°C. Absorbance was read at 450 nm (reference absorbance at 690 nm) using a microplate reader.
Cell viability was calculated as follows:
|
|
where OD is the optical density.
Transepithelial electrical resistance (TEER)
In order to evaluate the integrity of the TR146 cell layers, before and after each transport during permeability studies, TEER was monitored. It was calculated by measuring resistance (R in Ω) using a Millicell® ERS-2 (Electrical Resistance System; EMD Millipore Corporation, Billerica, MA, USA) according to the manufacturer’s instruction. The TEER value was obtained as follows:
|
|
where R(insert with cells), R(insert without cells), and A refer to the resistance of cells with insert, the resistance of cell-free insert, and the surface area (cm2) of the filter, respectively.
In vitro cell permeation studies
The permeability studies were performed as described by Iyire et al with a slight modification (Figure 1).3 The studies were done across TR146 cell layers from apical (0.5 mL) to basolateral direction (1.5 mL) in HBSS–hydroxyethylpiperazine ethane sulfonic acid (HEPES) buffer (pH 7.4). The cells were seeded across 12-well Transwell® inserts (Corning Inc., Corning, NY, USA) at a density of 5×104 cells/cm2, and the medium was replaced every subsequent day until the formation of the monolayer (26–30 days). Typically, 500 μL of 1.25 mg/mL insulin-loaded liposomes (SC-incorporated liposomes [SC-lipo], STC-incorporated liposomes [STC-lipo], SGC-lipo, SDGC-incorporated liposomes [SDGC-lipo], or SDTC-incorporated liposomes [SDTC-lipo]) was added to the apical chamber of the Transwell and kept at 37°C. At different time points (0.5, 1, 2, 4, 6, and 8 hours), 500 μL of sample was withdrawn from the basolateral chamber and replaced by the same volume of HBSS–HEPES buffer (pH 7.4) to retain the constant volume of the medium. The amount of permeated insulin across TR146 cell layers was determined by a Quantikine® ELISA (DINS00, R&D systems).
The steady state flux (Js), permeability coefficient (Kp), and enhancement ratio (ER) were calculated from the linear part of the permeation curve as described by Oh et al.5 Js was obtained from Equation 6, where Qr is the total permeated insulin (ng), A is the cross-sectional diffusion area (cm2), and t is the time of exposure (hour).
|
|
Kp was calculated using Equation 7, where Js is the flux from the steady state (ng·cm−2·h−1), and Cd is the initial concentration in the donor chamber (ng·cm−3). Finally, ER was obtained by dividing the Kp value of each formulation with that of the control.
|
|
Cellular uptake studies
Fluorescence-activated cell sorting analysis (FACS) was used to study the cellular uptake characteristics of TR146 cells. The cells were seeded at a density of 3×105 cells per well in a 12-well plate and incubated for 24 hours in a humidified incubator under 5% CO2 atmosphere at 37°C. Then, the cells were treated with FITC-insulin-loaded liposomes and incubated for 8 hours. Subsequently, the cells were washed twice with HBSS–HEPES buffer (pH 7.4) to remove the traces of liposomal vesicles left in the wells, harvested, and suspended in 0.5 mL of ice-cold FACS buffer (10% FBS and 2% sodium azide in PBS, pH 7.4). The dispersed cells were introduced immediately to FACS analysis using BD FAC suit software (BD Biosciences, San Jose, CA, USA). For the quantification of median fluorescence intensity (MFI) values, 5×103 designated cells were collected per histogram.
Interaction of various elastic bilosomes with TR146 cells
The cell–particle interactions were analyzed using FITC-insulin with confocal laser scanning microscopy (CLSM; Leica Microsystems, Wetzlar, Germany). For CLSM, TR146 cells were seeded at a density of 5×104 cells per well in an 8-chamber cell culture slide (SPL Life Sciences Co., Ltd., Pocheon, South Korea) and incubated for 24 hours. Then, the cells were treated with FITC-insulin-loaded liposomes and further incubated for 2 hours. Following incubation in a humidified incubator under 50% CO2 atmosphere at 37°C for an appropriate time, the cells were washed thrice with HBSS–HEPES buffer (pH 7.4), and plasma membrane was stained by adding 150 μL of Cell Mask™ Deep Red for 20 minutes. Subsequently, the cells were rinsed four times with HBSS–HEPES buffer (pH 7.4), and 150 μL of 4′,6-diamidino-2-phenylindole (DAPI) was added in order to stain the nuclei of cells and further incubated for 10 minutes at 37°C. Next, the cells were washed thrice with HBSS–HEPES buffer (pH 7.4) to remove the excess staining solution and fixed with 4% paraformaldehyde solution for 10 minutes in the dark as well as fixed by coverslips using the mounting medium (Dako North America, Inc., Carpinteria, CA, USA) and observed by CLSM.
Statistical analysis
The results are expressed as the mean ± SD. The one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test was used to determine the level of statistical significance between the groups. For all data, a single, double, or triple asterisk was used if the P-value was <0.05, <0.01, or <0.001 level of significance, respectively.
Result and discussion
Preparation and characterization of elastic bilosomes
Various amphiphilic molecules are used as edge activators to provide deformability to vesicles. These vesicles are capable of squeezing through the intercellular regions of biomembrane.45,46 Hydrophilic surfactants such as bile salts and TWEEN® 80 possess more deformability than lipophilic surfactant due to the formation of transient holes in lipid bilayers by enhancing the biomembrane fluidity.43,47 Lipid vesicles fabricated using phospholipid and edge activators at a ratio of 85:15 (w/w%) induced highest deformability.28,43,44,46 Different derivatives of cholic acid were chosen as edge activators for comparison and study their effects on the deformability and permeation enhancement. Elastic bilosomes containing bile salts such as SC, STC, SGC, SDGC, or SDTC at a ratio of 85:15 w/w% were successfully prepared by thin-film hydration method. These liposomal formulations were evaluated with respect to vesicular size, PDI, ζ potential, EE, and LC as shown in Table 2. On average, the vesicular size was ~140–150 nm that is considered as an ideal size to achieve permeation across biomembrane.48 It is well known that PDI parameter is used to determine the level of homogeneity. PDI values <0.1 are considered as monodispersion of liposomal vesicles and >0.1 is considered as polydispersion of liposomal vesicles.49 All the prepared liposomes belonged to monodispersion category, ie, particles were supposed to be of same size, more narrow, and homogeneously distributed. All elastic bilosomes were negatively charged, and the ζ values were much higher, leading to the formation of a physically stable system, due to the presence of negatively charged bile salts. Our results were in accordance with many published types of research, and a similar trend was observed with regard to higher ζ potential values of liposomes prepared using STC and SGC.17,18,36,39 The EE and LC were ~66%–78% and ~0.11%–0.14%, respectively. These results indicate that the prepared elastic bilosomes successfully encapsulated higher amount of insulin.
Deformability of different elastic bilosomes
Deformability is the crucial parameter of elastic bilosomes which facilitates the permeation across biomembrane pores smaller than their diameter.48 It has been reported that stress-dependent adaptability and shape modification in an elastic bilosome can be expressed by deformability index (D).42 In the present study, we have calculated the relative deformability as compared with the control. Since 100% soy lecithin or soy lecithin/cholesterol (85:15 w/w%) were not successfully fabricated, we preferred the minimum amount of STC (5%) to fabricate control liposome (soy lecithin/STC =95:5 w/w%). Figure 2 presents the relative deformability indices of several elastic bilosomes. The relative deformability indices were in the order of SDGC-lipo > SDTC-lipo > SC-lipo > SGC-lipo > STC-lipo. The significant differences in relative deformability indices were observed in SDGC-lipo (P<0.001) and SDTC-lipo (P<0.001) with respect to control. However, there were no significant differences in the relative deformability indices between each group except in the cases of SDGC-lipo and SDTC-lipo.
In vitro cell viability studies
The in vitro cell viability of blank liposomes (SC-lipo, STC-lipo, SGC-lipo, SDGC-lipo, and SDTC-lipo) at different concentrations (10.00, 5.00, 2.50, and 1.25 mg/mL) was tested across TR146 cells, and Figure 3 presents the results. Trihydroxy bile salt-loaded liposomes (SC-lipo, SGC-lipo, and STC-lipo) at concentrations <5 mg/mL did not induce cytotoxicity. However, SC-lipo and STC-lipo induced significant cytotoxicity at a concentration of 5 mg/mL (P<0.001 and P<0.01, respectively), whereas all these three liposomes induced significant cytotoxicity at a concentration >5 mg/mL (P<0.001). Similarly, dihydroxy bile salt-loaded liposomes (SDGC-lipo and SDTC-lipo) at a concentration >1.25 mg/mL induced cytotoxicity at a concentration-dependent manner.39,50 These results were in accordance with the observations of some published researches. Lee and Yamamoto revealed that trihydroxy bile salts showed less toxicity than dihydroxy bile salts.51 In another study by Duchateau et al, taurocholate was demonstrated as relatively safe, less toxic with no effect on ciliary arrest for 30 minutes at 30 mM concentration; whereas deoxycholate induced serious ciliotoxicity at a concentration of 5 mM for intranasal delivery of gentamicin.52 Furthermore, Morimoto et al reported that SGC can be a safe and useful enhancer, even at 20 mM, for intratracheal drug delivery in rabbit tracheas.53 In addition, Gordon et al demonstrated that glycine and taurine conjugates of bile salts are relatively less irritating for insulin delivery across nasal mucosa.54
Overall, toxicity and surface activity of bile salts vary according to their structures; thus, all the formulations used in this experiment showed a concentration-dependent increase in cytotoxicity. As no significant toxic effects were observed, liposomes (SC-lipo, STC-lipo, SGC-lipo, SDGC-lipo, or SDTC-lipo) at a concentration of 1.25 mg/mL were used for permeability studies across TR146 cells.
In vitro cell permeation profiles of insulin
The TR146 cell line is a continuous cell line that is derived from human neck node metastasis originating from buccal carcinoma. It is capable of forming stratified nonkeratinized epithelium with four to seven cell layers of flattened cells on the surface after being cultured for 3–4 weeks.4,55 This cellular model has been used to simulate human buccal epithelium to predict the permeations of drug across the epithelium. The TR146 cell culture model closely mimics in vivo conditions; thus, it is used as in vitro model in our experiment.40 TEER is a decisive parameter to evaluate the integrity of the cell layers. In our study, the maximum TEER of confluent TR146 cell layers has reached at Day 30, and the value was 71.68±2.96 Ω·cm2 (97.39% recovery). All of these formulation-treated cells have revealed a recovery of above 96%. There were no any significant changes in the TEER before and after the permeability experiment, and Table 3 presents the results. This maximum TEER value of confluent TR146 cell layer was consistent with the previous reports.56 Jacobsen et al demonstrated that the maximum integrity of the TR146 cell layers has reached at around Day 30 in culture, and the maximum TEER value was 68.2±2.3 Ω·cm2.56 The in vitro cell culture models that form tight junctions such as Caco-2 cells (260 or 480 Ω·cm2)57,58 and MDCK stain I (1,500 Ω·cm2)59 have a higher magnitude of TEER compared with lacking tight junctions that could be attributed to a lower magnitude of TEER in TR146 cell layers.56 Thus, this proves that the TR146 cell layers maintain the cellular morphology as well as cell monolayer integrity during the experiment.
The cumulative permeation profiles of insulin-loaded various elastic bilosomes across the TR146 cell layers at each point were plotted as a function of time (Figure 4). The distinct differences in the cumulative amount of insulin between SDGC-lipo and SC-lipo or SDTC-lipo were observed after 2 hours. The potential of various elastic bilosomes in transporting the insulin across the TR146 cell layers over the period of 8 hours was evaluated by calculating the steady-state flux (Js), a permeability coefficient (Kp), and ER, and Table 4 presents the results. Improvement in permeation parameters was observed in the order of SDGC-lipo > SC-lipo > SDTC-lipo > SGC-lipo > STC-lipo > insulin solution. The Js, Kp, and ER values of insulin were significantly increased after incorporation into the elastic bilosomes with the most prominent effect found with the SDGC-lipo with an ER of 5.24 (P<0.001). The SC-lipo and SDTC-lipo also led to a significant enhancement with ERs of 3.20 and 3.10 (P<0.05), respectively, compared with insulin solution. This result can be explained as per Lipinski’s Rule of Five, ie, the permeability will increase across the biomembrane, if the number of H-bond donors and the number of H-bond acceptors are <5 and <10, respectively.60–62 Apparently, it means that the lower the value of H-bond donors and H-bond acceptors, the more will be the permeability. The SDGC has lower H-bond donors and acceptors than all the cholic acid derivatives as mentioned in Figure 5. Thus, the ER was improved in the order of SDGC-lipo > SC-lipo > SDTC-lipo > SGC-lipo > STC-lipo.
These findings were quite close with the results of relative deformability study (Figure 2). The higher permeation of SDGC-lipo in comparison with insulin solution could be explained based on their ability to squeeze themselves through the cell pores. The tiny space detention by buccal pore induces a modification in the shape of elastic vesicles and enables them to deliver the entrapped drugs across the buccal mucosa.28 Yang et al have already proposed alternative mechanisms for facilitating insulin transport across the buccal mucosa using SDC-lipo. One mechanism signifies the consequence of transbuccal hydration force across the buccal mucosa induced by the difference in water concentration between its surface and interior as dominant in the transdermal delivery of elastic bilosomes, thus facilitating the penetration of elastic bilosomes into the interstices across the buccal membrane.29 Another mechanism involves fusion of vesicles with the buccal membrane in a manner similar to the process involved in transdermal delivery. Accordingly, vesicles can act as penetration enhancers and penetrate the membrane, modify its intercellular lipids, and increase the membrane’s fluidity and weakness. In addition, the mixing of intercellular lipid layers of membrane and liposome–phospholipid bilayers can play an important role in enhancing the permeation of elastic bilosomes.43,63 It is hypothesized that these factors may be the plausible reasons for the observed enhanced permeation of insulin across TR146 cell layers. In addition, in one study, it has been demonstrated that dihydroxy bile salts (SDGC and SDTC) enhanced the permeation of decitabine than trihydroxy bile salts (SGC and STC) across the porcine buccal mucosa. The enhancement flux was 38-fold higher with 10 mM of SDGC compared with control. It is hypothesized that the enhancement may be due to a complex process including solubilization and micellar entrapment of intercellular lipids, extraction and denaturation of proteins, enzyme inactivation, and swelling of tissues.64 Similarly, our results also showed that permeation enhancement of dihydroxy bile salt-encapsulated liposome (SDGC-lipo, SDTC-lipo) was better compared with trihydroxy bile salt-encapsulated liposomes (SGC-lipo or STC-lipo).
FITC-insulin uptake study in TR146 cell layers
FITC-insulin-loaded liposomes were prepared in order to evaluate the quantitative cellular uptake studies across TR146 cells. FACS analysis was performed for studying the cellular uptake of FITC-insulin-loaded liposomes. Figure 6A presents the representative fluorescence intensity of FITC-insulin-loaded various elastic bilosomes. The graph clearly distinguishes the greater shift in the fluorescence intensity of FITC-insulin after incorporation into various elastic bilosomes. However, there was a little shift in fluorescence intensity after treatment with FITC-insulin alone. In order to compare the obtained fluorescence intensity with the fluorescence intensity of FITC-insulin, the relative MFI values in different treatments were calculated and shown in Figure 6B. The results revealed that SDGC-lipo exhibited a 4.25-fold increase in the cellular uptake of FITC-insulin compared with FITC-insulin alone. A possible explanation for this could be the presence of less number of H-bond donors and acceptors and the nature of dihydroxy bile salt, which contributed toward higher lipophilicity of the system. Similarly, SC-lipo and SDTC-lipo revealed 3.30- and 2.56-fold increases in the cellular uptake of FITC-insulin, respectively. The relative MFI values of elastic bilosomes were counted in the order of SDGC-lipo > SC-lipo > SDTC-lipo > SGC-lipo > STC-lipo, which is in accordance with the results obtained from the drug permeability studies.
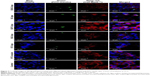
Interaction of various elastic bilosomes with TR146 cells
Qualitative cellular uptake was assessed employing confocal microscopy as presented in Figure 7. The blue fluorescence, green fluorescence, and red fluorescence represent the DAPI that labels the nuclei of cells, the FITC-insulin that indicates FITC-insulin-loaded liposomes, and the Cell Mask™ that labels the cell membranes, respectively. These results demonstrate minimal or no interactions between the cells and various FITC-insulin-loaded elastic bilosomes. In agreement with the FACS results, the cellular uptake of FITC-insulin-loaded SDGC-lipo was potentiated in TR146 cell layers, as presented by the enhancement of green fluorescence emitted by FITC-insulin. The results also showed that FITC-insulin was within or close to the nucleus. Thus, these findings reveal that some amount of insulin was permeated through the transcellular route.3
Conclusion
Liposomes containing bile salt edge activator (SC, STC, SGC, SDGC, or SDTC) were successfully fabricated using thin-film hydration method and revealed similar particle size and EE. The present study demonstrates that various derivatives of cholic acid-loaded elastic bilosomes can be advantageous for the buccal delivery of insulin. SDGC-lipo was found to be the most superior for the transport of insulin across TR146 cell layers. SC-lipo, SDTC-lipo, SGC-lipo, and STC-lipo were also good candidates for enhancing the insulin permeability across TR146 cells. However, further detailed studies are necessitated to explore the mechanism for the transport of insulin across TR146 cell layers. To conclude, the presented findings present elastic bilosomes as a potential approach for facilitating the buccal delivery of protein/peptide drugs. Another study on in vitro and in vivo application of these elastic bilosomes to porcine buccal tissues and diabetic rabbit models, respectively, is currently under investigation.
Acknowledgment
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Sciences, ICT and Future Planning (NRF-2016R1D1A1B01015369).
Disclosure
The authors report no conflicts of interest in this work.
References
Idf.org [homepage on the Internet]. International Diabetes Federation. Available from: https://www.idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html. Accessed January 11, 2018. | ||
Who.int [homepage on the Internet]. World Health Organization. Available from: http://www.who.int/news-room/fact-sheets/detail/diabetes. Accessed January 11, 2018. | ||
Iyire A, Alaayedi M, Mohammed AR. Pre-formulation and systematic evaluation of amino acid assisted permeability of insulin across in vitro buccal cell layers. Sci Rep. 2016;6:32498. | ||
Xue XY, Zhou Y, Chen YY, et al. Promoting effects of chemical permeation enhancers on insulin permeation across TR146 cell model of buccal epithelium in vitro. Drug Chem Toxicol. 2012;35(2):199–207. | ||
Oh DH, Chun KH, Jeon SO, Kang JW, Lee S. Enhanced transbuccal salmon calcitonin (sCT) delivery: effect of chemical enhancers and electrical assistance on in vitro sCT buccal permeation. Eur J Pharm Biopharm. 2011;79(2):357–363. | ||
Shrestha N, Shahbazi MA, Araújo F, et al. Chitosan-modified porous silicon microparticles for enhanced permeability of insulin across intestinal cell monolayers. Biomaterials. 2014;35(25):7172–7179. | ||
Bashyal S, Noh G, Keum T, Choi YW, Lee S. Cell penetrating peptides as an innovative approach for drug delivery; then, present and the future. J Pharm Investig. 2016;46(3):205–220. | ||
Caon T, Jin L, Simões CM, Norton RS, Nicolazzo JA. Enhancing the buccal mucosal delivery of peptide and protein therapeutics. Pharm Res. 2015;32(1):1–21. | ||
Pather SI, Rathbone MJ, Şenel S. Current status and the future of buccal drug delivery systems. Expert Opin Drug Deliv. 2008;5(5):531–542. | ||
Montenegro-Nicolini M, Morales JO. Overview and future potential of buccal mucoadhesive films as drug delivery systems for biologics. AAPS PharmSciTech. 2017;18(1):3–14. | ||
Morales JO, Brayden DJ. Buccal delivery of small molecules and biologics: of mucoadhesive polymers, films, and nanoparticles. Curr Opin Pharmacol. 2017;36:22–28. | ||
Bernstein G. Delivery of insulin to the buccal mucosa utilizing the RapidMist system. Expert Opin Drug Deliv. 2008;5(9):1047–1055. | ||
Bernstein G. Buccal delivery of insulin: the time is now. Drug Dev Res. 2006;67(7):597–599. | ||
Oh DH, Kim MJ, Jeon SO, et al. Strategic approaches for enhancement of in vivo transbuccal peptide drug delivery in rabbits using iontophoresis and chemical enhancers. Pharm Res. 2015;32(3):929–940. | ||
Shrestha N, Araújo F, Shahbazi MA, et al. Thiolation and cell-penetrating peptide surface functionalization of porous silicon nanoparticles for oral delivery of insulin. Adv Funct Mater. 2016;26(20):3405–3416. | ||
Bashyal S, Lee S. Delivery of biopharmaceuticals using combination of liposome and iontophoresis: a review. J Pharm Investig. 2015;45(7):611–624. | ||
Cui M, Wu W, Hovgaard L, Lu Y, Chen D, Qi J. Liposomes containing cholesterol analogues of botanical origin as drug delivery systems to enhance the oral absorption of insulin. Int J Pharm. 2015;489(1–2):277–284. | ||
Guan P, Lu Y, Qi J, et al. Enhanced oral bioavailability of cyclosporine A by liposomes containing a bile salt. Int J Nanomedicine. 2011;6:965–974. | ||
Lankalapalli S, Tenneti VS. Formulation and evaluation of rifampicin liposomes for buccal drug delivery. Curr Drug Deliv. 2016;13(7):1084–1099. | ||
El-Samaligy MS, Afifi NN, Mahmoud EA. Increasing bioavailability of silymarin using a buccal liposomal delivery system: preparation and experimental design investigation. Int J Pharm. 2006;308(1–2):140–148. | ||
Abd El Azim H, Nafee N, Ramadan A, Khalafallah N. Liposomal buccal mucoadhesive film for improved delivery and permeation of water-soluble vitamins. Int J Pharm. 2015;488(1–2):78–85. | ||
Mašek J, Lubasová D, Lukáč R, et al. Multi-layered nanofibrous mucoadhesive films for buccal and sublingual administration of drug-delivery and vaccination nanoparticles – important step towards effective mucosal vaccines. J Control Release. 2017;249:183–195. | ||
Smistad G, Jacobsen J, Sande SA. Multivariate toxicity screening of liposomal formulations on a human buccal cell line. Int J Pharm. 2007;330(1–2):14–22. | ||
Moghimipour E, Ameri A, Handali S. Absorption-enhancing effects of bile salts. Molecules. 2015;20(8):14451–14473. | ||
Hassan N, Ahad A, Ali M, Ali J. Chemical permeation enhancers for transbuccal drug delivery. Expert Opin Drug Deliv. 2010;7(1):97–112. | ||
Sohi H, Ahuja A, Ahmad FJ, Khar RK. Critical evaluation of permeation enhancers for oral mucosal drug delivery. Drug Dev Ind Pharm. 2010;36(3):254–282. | ||
Nicolazzo JA, Reed BL, Finnin BC. Buccal penetration enhancers–how do they really work? J Control Release. 2005;105(1–2):1–15. | ||
Zeb A, Qureshi OS, Kim HS, Cha JH, Kim HS, Kim JK. Improved skin permeation of methotrexate via nanosized ultradeformable liposomes. Int J Nanomedicine. 2016;11:3813–3824. | ||
Yang TZ, Wang XT, Yan XY, Zhang Q. Phospholipid deformable vesicles for buccal delivery of insulin. Chem Pharm Bull. 2002;50(6):749–753. | ||
Niu M, Lu Y, Hovgaard L, et al. Hypoglycemic activity and oral bioavailability of insulin-loaded liposomes containing bile salts in rats: the effect of cholate type, particle size and administered dose. Eur J Pharm Biopharm. 2012;81(2):265–272. | ||
Niu M, Tan Y, Guan P, et al. Enhanced oral absorption of insulin-loaded liposomes containing bile salts: a mechanistic study. Int J Pharm. 2014;460(1–2):119–130. | ||
Niu M, Lu Y, Hovgaard L, Wu W. Liposomes containing glycocholate as potential oral insulin delivery systems: preparation, in vitro characterization, and improved protection against enzymatic degradation. Int J Nanomedicine. 2011;6:1155–1166. | ||
Hu S, Niu M, Hu F, et al. Integrity and stability of oral liposomes containing bile salts studied in simulated and ex vivo gastrointestinal media. Int J Pharm. 2013;441(1–2):693–700. | ||
Mahmud F, Jeon OC, Al-Hilal TA, et al. Absorption mechanism of a physical complex of monomeric insulin and deoxycholyl-l-lysyl-methylester in the small intestine. Mol Pharm. 2015;12(6):1911–1920. | ||
Kim SK, Lee S, Jin S, et al. Diabetes correction in pancreatectomized canines by orally absorbable insulin-deoxycholate complex. Mol Pharm. 2010;7(3):708–717. | ||
Li Z, Zhang M, Liu C, et al. Development of liposome containing sodium deoxycholate to enhance oral bioavailability of itraconazole. Asian J Pharm Sci. 2017;12(2):157–164. | ||
Malakar J, Sen SO, Nayak AK, Sen KK. Formulation, optimization and evaluation of transferosomal gel for transdermal insulin delivery. Saudi Pharm J. 2012;20(4):355–363. | ||
Al-Mahallawi AM, Abdelbary AA, Aburahma MH. Investigating the potential of employing bilosomes as a novel vesicular carrier for transdermal delivery of tenoxicam. Int J Pharm. 2015;485(1–2):329–340. | ||
Dai Y, Zhou R, Liu L, Lu Y, Qi J, Wu W. Liposomes containing bile salts as novel ocular delivery systems for tacrolimus (FK506): in vitro characterization and improved corneal permeation. Int J Nanomedicine. 2013;8:1921–1933. | ||
Sander C, Nielsen HM, Jacobsen J. Buccal delivery of metformin: TR146 cell culture model evaluating the use of bioadhesive chitosan discs for drug permeability enhancement. Int J Pharm. 2013;458(2):254–261. | ||
Portero A, Remuñán-López C, Nielsen HM. The potential of chitosan in enhancing peptide and protein absorption across the TR146 cell culture model-an in vitro model of the buccal epithelium. Pharm Res. 2002;19(2):169–174. | ||
Kang MJ, Eum JY, Jeong MS, et al. Tat peptide-admixed elastic liposomal formulation of hirsutenone for the treatment of atopic dermatitis in NC/Nga mice. Int J Nanomedicine. 2011;6:2459–2467. | ||
El Zaafarany GM, Awad GA, Holayel SM, Mortada ND. Role of edge activators and surface charge in developing ultradeformable vesicles with enhanced skin delivery. Int J Pharm. 2010;397(1–2):164–172. | ||
Jain S, Jain P, Umamaheshwari RB, Jain NK. Transfersomes – a novel vesicular carrier for enhanced transdermal delivery: development, characterization, and performance evaluation. Drug Dev Ind Pharm. 2003;29(9):1013–1026. | ||
El Maghraby GM, Williams AC, Barry BW. Oestradiol skin delivery from ultradeformable liposomes: refinement of surfactant concentration. Int J Pharm. 2000;196(1):63–74. | ||
Lee EH, Kim A, Oh YK, Kim CK. Effect of edge activators on the formation and transfection efficiency of ultradeformable liposomes. Biomaterials. 2005;26(2):205–210. | ||
Cevc G, Gebauer D, Stieber J, Schätzlein A, Blume G. Ultraflexible vesicles, transfersomes, have an extremely low pore penetration resistance and transport therapeutic amounts of insulin across the intact mammalian skin. Biochim Biophys Acta. 1998;1368(2):201–215. | ||
Kang MJ, Eum JY, Jeong MS, et al. Facilitated skin permeation of oregonin by elastic liposomal formulations and suppression of atopic dermatitis in NC/Nga mice. Biol Pharm Bull. 2010;33(1):100–106. | ||
Karn PR, Jin SE, Lee BJ, et al. Preparation and evaluation of cyclosporin A-containing proliposomes: a comparison of the supercritical antisolvent process with the conventional film method. Int J Nanomedicine. 2014;9:5079–5091. | ||
Yang L, Zhang H, Fawcett JP, Mikov M, Tucker IG. Effect of bile salts on the transport of morphine-6-glucuronide in rat brain endothelial cells. J Pharm Sci. 2011;100(4):1516–1524. | ||
Lee VHL, Yamamoto A. Penetration and enzymatic barriers to peptide and protein absorption. Adv Drug Deliv Rev. 1989;4(2):171–207. | ||
Duchateau GSMJE, Zuidema J, Merkus FWHM. Bile salts and intranasal drug absorption. Int J Pharm. 1986;31(3):193–199. | ||
Morimoto K, Uehara Y, Iwanaga K, et al. Influence of absorption enhancers (bile salts) and the preservative (benzalkonium chloride) on mucociliary function and permeation barrier function in rabbit tracheas. Eur J Pharm Sci. 1998;6(3):225–230. | ||
Gordon GS, Moses AC, Silver RD, Flier JS, Carey MC. Nasal absorption of insulin: enhancement by hydrophobic bile salts. Proc Natl Acad Sci U S A. 1985;82(21):7419–7423. | ||
Shrestha N, Araújo F, Sarmento B, Hirvonen J, Santos HA. Cell-based in vitro models for buccal permeability studies. In: Bruno Sarmento, editor. Concepts and Models for Drug Permeability Studies. New York: Elsevier; 2015:31–40. | ||
Jacobsen J, van Deurs B, Pedersen M, Rassing MR. TR146 cells grown on filters as a model for human buccal epithelium: I. Morphology, growth, barrier properties, and permeability. Int J Pharm. 1995;125(2):165–184. | ||
Anderberg EK, Nyström C, Artursson P. Epithelial transport of drugs in cell culture. VII: Effects of pharmaceutical surfactant excipients and bile acids on transepithelial permeability in monolayers of human intestinal epithelial (Caco-2) cells. J Pharm Sci. 1992;81(9):879–887. | ||
Artursson P. Epithelial transport of drugs in cell culture. I: A model for studying the passive diffusion of drugs over intestinal absorptive (Caco-2) cells. J Pharm Sci. 1990;79(6):476–482. | ||
Cho MJ, Thompson DP, Cramer CT, Vidmar TJ, Scieszka JF. The Madin Darby canine kidney (MDCK) epithelial cell monolayer as a model cellular transport barrier. Pharm Res. 1989;6(1):71–77. | ||
Alex A, Millan DS, Perez M, Wakenhut F, Whitlock GA. Intramolecular hydrogen bonding to improve membrane permeability and absorption in beyond rule of five chemical space. Med Chem Comm. 2011;2(7):669–674. | ||
Choy YB, Prausnitz MR. The rule of five for non-oral routes of drug delivery: ophthalmic, inhalation and transdermal. Pharm Res. 2011;28(5):943–948. | ||
Bhal SK, Kassam K, Peirson IG, Pearl GM. The Rule of Five revisited: applying log D in place of log P in drug-likeness filters. Mol Pharm. 2007;4(4):556–560. | ||
Verma DD, Verma S, Blume G, Fahr A. Liposomes increase skin penetration of entrapped and non-entrapped hydrophilic substances into human skin: a skin penetration and confocal laser scanning microscopy study. Eur J Pharm Biopharm. 2003;55(3):271–277. | ||
Mahalingam R, Ravivarapu H, Redkar S, Li X, Jasti BR. Transbuccal delivery of 5-Aza-2′-deoxycytidine: effects of drug concentration, buffer solution, and bile salts on permeation. AAPS PharmSciTech. 2007;8(3):E28–E33. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.