Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 8
Facial primer provides immediate and long-term improvements in mild-to-moderate facial hyperpigmentation and fine lines associated with photoaging
Authors Roberts WE, Jiang LI, Herndon Jr J
Received 12 May 2015
Accepted for publication 28 July 2015
Published 2 September 2015 Volume 2015:8 Pages 471—477
DOI https://doi.org/10.2147/CCID.S88443
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Wendy E Roberts,1 Lily I Jiang,2 James H Herndon Jr3
1Generational and Cosmetic Dermatology, Rancho Mirage, CA, 2Thomas J Stephens and Associates, Richardson, 3Dermatology Center of Dallas, Dallas, TX, USA
Background: Photoaged skin results from various environmental factors, most importantly chronic sun exposure. Dyschromia and fine lines/wrinkles are common clinical manifestations of photodamaged skin.
Purpose: This single-center clinical trial was conducted to assess the efficacy and tolerability of a new multifunctional facial primer (camouflage, broad-spectrum SPF 50, and a treatment for hyperpigmentation) when used by females with mild-to-moderate facial hyperpigmentation and fine lines due to photoaging over a course of 12 weeks.
Patients and methods: Subjects were provided test material (Even Up-Clinical Pigment Perfector) and supporting products to use on their face and neck. Products were used according to specific application instructions. Clinical grading for efficacy and tolerability assessments were performed by an expert grader at baseline, baseline (post-application primer), week 4, week 8, week 12, and week 12 (post-application primer). Standardized digital photographs were taken, and self-assessment questionnaires were conducted.
Results: Twenty-eight female subjects completed the 12-week trial. The facial primer improved scores for the appearance of hyperpigmentation and other photoaging parameters immediately after the first application. The treatment also showed a progressive improvement in the clinical assessment of hyperpigmentation and other photoaging parameters over the 12-week trial. These long-term benefits can be attributed to an improvement in the underlying skin condition. The facial primer was well tolerated. Subject questionnaires showed that the product was highly rated at all visits.
Conclusion: The facial primer was shown to be effective and well tolerated for immediate and long-term improvement in the appearance of mild-to-moderate hyperpigmentation and fine lines associated with photodamage when used over a 12-week period.
Keywords: photodamaged facial skin, medical makeup, dermocosmetic, cosmetic, SPF
Introduction
Photoaged skin is largely a result of chronic exposure to ultraviolet (UV) radiation.1 Photoaging, which causes premature aging in the appearance and function of the skin, is similar to chronological aging in that it is cumulative over time. The amount of photodamage depends on the duration and intensity of exposure to the sun along with inherent pigmentation of the skin.2,3 Photodamaged skin is characterized by various undesirable esthetic manifestations including dyspigmentation, wrinkling, roughness, laxity, dullness, lentigines, atrophy, and purpura on areas of the body chronically exposed to the sun. Clinical dyschromia is often a prominent feature in sun-exposed skin, especially the face.4–6 While benign in nature, dyschromias can have an adverse effect on social functioning, lower work productivity and self-esteem, and produce a negative impact on a patient’s quality of life.7,8 Women frequently seek effective treatment for their irregular pigmentation as well as other clinical manifestations of photodamaged skin. Dermatological procedures, including chemical peels,9,10 along with lasers and pulsed light,11,12 are often utilized alone or in combination with various topical treatments for hyperpigmentation.12–16
Broad-spectrum sunscreens (blockers of both UVA and UVB) are considered the cornerstone of effective management of hyperpigmentary disorders. Evidence now strongly suggests that augmenting sunscreens with active ingredients that provide antioxidant and anti-inflammatory benefits can help increase the photo-protective qualities of sunscreens.17 In addition to UVA and UVB radiation, sunlight contains a large amount of infrared (IR) radiation. Protection against IR is equally important as it penetrates deeper into the skin and can produce significant damage in the dermis.18 One of the early and visible manifestations of both UV and IR exposure to skin is hyperpigmentation. In addition to providing an effective protection against sunlight, inclusion of actives in sunscreen that can decrease melanin synthesis and distribution can provide significant benefit in improving overall appearance of skin after prolonged sun exposure.
A major limitation with topical treatments for hyperpigmentation is that the beneficial effects generally are not seen for several weeks or even months after initiation of treatment. Patients typically do not want to wait so long for visual improvement in their condition, particularly if the uneven pigmentation is on their face. A colored medical makeup was developed to provide immediate cosmetic correction including photo-protection and long-term pigmentation reduction benefits.
This clinical trial is designed to assess the effects of a multifunctional facial primer (cosmetic correction, UV/IR protection, and pigmentation treatment) when used by patients with both mild-to-moderate hyperpigmentation and fine lines associated with photodamage.
Patients and methods
Thirty-one women, aged 40–64 years with Fitzpatrick skin types II–IV, who were regular users of foundation and in good general health, were recruited for the study. Inclusion criteria were the presence of mild-to-moderate facial hyperpigmentation and fine lines associated with photodamage. Subjects were required to have a baseline score of 3–6 on both the hyperpigmentation scale (0–9) and the fine lines scale (0–9). Primary exclusion criteria included use of topical skin-lightening and antiaging products within 4 weeks prior to the study as well as facial dermatologic procedures within 12 months prior to the study.
Subjects were provided a facial regimen of four products (Even Up-Clinical Pigment Protector, Pigment Perfecting Facial Primer, Sunforgettable Setting Mist, Pressed Mineral Foundation and Sunforgettable SPF 30; Colorescience, Inc., Carlsbad, CA, USA) along with usage instructions. All the products were applied in the morning with Sunforgettable SPF 30 reapplied as needed throughout the day. Subjects were advised to avoid extended periods of sun exposure and all use of tanning beds during the study and to use protective clothing (including sunglasses) and avoid sun exposure from 10 am to 2 pm. Subjects were allowed to continue the use of their regular cosmetics (except foundation) for the duration of the study but were not allowed to use any antiaging or skin-lightening products. Daily diary recordings of test material applications and comments by the subjects were made during the course of the study. Diaries were reviewed, and test materials were visually inspected at each post-baseline visit.
Table 1 lists the facial primer’s key ingredients and functions. The product contains zinc oxide and titanium dioxide to provide broad-spectrum SPF 50 sun protection. Protection against IR exposure is provided by Thermus thermophilus ferment. It also provides protection against UV damage and improves epidermal and dermal structural integrity. Improvement in skin pigmentation is provided by a combination of three actives that prevent stimulation of melanocytes by anti-inflammatory mechanisms (tocopheryl phosphate), suppress tyrosinase activity and transfer of melanin to keratinocytes (acetylated Rheum rhaponticum root extract), and increase skin turnover to remove existing melanin with retinoid-like activity (Bidens pilosa extract).
  | Table 1 Key ingredients in facial primer |
The conduct of the study followed all applicable guidelines for the protection of human subjects as outlined in 21 CFR50.25, in accordance with the accepted standards for good clinical practice, International Conference of Harmonisation (ICH), and the standard practices of Thomas J Stephens & Associates. Prior to treatment, subjects provided informed consent. The study was conducted in Dallas, TX, USA, from January to April 2014.
Subjects were treated over a 12-week period and arrived at the clinic for each visit having removed all makeup at least 30 minutes prior to the appointment. Clinical efficacy and tolerability evaluations were made by an expert grader at baseline, week 4, week 8, and week 12 on bare skin (without primer application). Additionally, following the application of facial primer by a certified makeup artist, clinical assessments were performed by the same expert grader after the baseline application of the facial primer and after the week 12 application.
Clinical grading of efficacy parameters
- Hyperpigmentation (0= faint spots or not visible, 9= discrete dark spots)
- Fine lines (0= none, 9= numerous)
- Overall appearance (0= excellent, 9= poor)
- Pore size (0= small or not noticeable pores, 9= pores large and prominent)
- Radiance (0= radiant, luminous appearance, 9= dull/matte or sallow appearance)
- Skin smoothness (0= smooth, even-looking texture, 9= rough, uneven-looking texture)
- Skin tone (0= even, healthy skin color, 9= uneven, discolored appearance)
The expert grader also performed global assessments of each subject’s face using a modified Griffith’s ten-point scale19 according to the following numerical definitions:
0= none (best possible)
1–3= mild
4–6= moderate
7–9= severe (worst possible)
Tolerability
At each assessment, the subjects were evaluated by an expert grader for objective evidence of erythema, edema, and dryness and peeling using a four-point scale (0= none, 3= severe). Subjects also assessed themselves for subjective irritation parameters including itching, stinging, tingling, and burning on a four-point scale (0= none, 3= severe).
Digital photography
At each assessment, digital photographs were taken using the VISIA CR2 (Canfield Imaging Systems, Fairfield, NJ, USA) with a Canon Mark II 5D digital SLR camera (Canon, Inc., Tokyo, Japan). Subjects had three sets of full-face images taken (right side, left side, and center view) using all lighting modes.
Self-assessment questionnaires
At each evaluation visit, subjects completed a self-assessment questionnaire regarding their experience with the test material, efficacy, esthetics, and other attributes using a 0–9 scale (0= best condition and 9= worst condition).
Statistical analysis
The per-protocol (PP) population was the primary population for all statistical analyses testing. The PP population included all subjects who were deemed eligible for participation and completed the study according to protocol. The average percentage change from baseline was calculated at each post-baseline evaluation visit. Wilcoxon signed-rank test was used for clinical grading of the efficacy parameters, tolerability evaluations, and skin condition-related self-assessment questionnaires. All differences were considered to be statistically significant at P<0.05 level.
Results
Twenty-eight female subjects completed the 12-week study. Three subjects discontinued the study (one was lost to follow-up, one was noncompliant, and one for a product-unrelated adverse event). The demographic data of the PP study population are presented in Table 2. The subjects were predominantly Caucasian with both mild-to-moderate hyperpigmentation and fine lines associated with photodamage.
  | Table 2 Demographic information of PP study population (n=28) |
Application of the facial primer produced a statistically significant 38.4% improvement in clinical grading scores for hyperpigmentation after first application when compared to bare skin condition at baseline. This immediate improvement is a result of covering up the imperfections and dark spots on the skin with micronized pigments. After 12 weeks of product use, a 43.9% improvement was seen compared to baseline (Figure 1). This additional improvement in clinical grading scores for hyperpigmentation at 12 weeks over the first application is the apparent result of the cumulative effect of key ingredients on the overall skin condition.
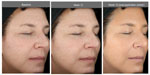
  | Figure 1 Facial primer provides immediate improvement in the hyperpigmentation score at first application of primer with greater improvement at week 12 compared to baseline. |
Similarly, statistically significant improvements were seen in other parameters of photoaging including skin tone, radiance, fine lines, skin smoothness, pore size, and overall appearance of the skin immediately following the first application which further improved after 12 weeks of product use (Figure 2). A statistical progressive improvement in hyperpigmentation scores upon assessment of bare skin (no primer applied) was seen at week 4, week 8, and week 12 indicating an improvement in skin quality with regular use of the products (Figure 3).
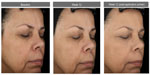
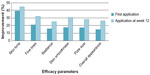  | Figure 2 Facial primer provides significant improvement in additional parameters of photodamage at first application with greater improvement at week 12 compared to baseline. |
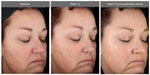
  | Figure 3 Significant progressive improvements in the hyperpigmentation score with use of the facial primer over 12 weeks (no facial primer applied for these assessments). |
The facial primer was well tolerated by the subjects with no statistically significant increases in scores for tolerability/safety parameters at any study visit compared to baseline scores.
The facial primer was well received by the subjects as indicated in the self-assessment questionnaires. 96.4% of subjects stated that the appearance of age spots/dark spots/sunspots/discolorations improved at 12 weeks, whereas 89.3% considered that the appearance of fine lines/wrinkles improved over this same time period with use of the facial primer. Figures 4–6 illustrate the beneficial effects of treatment for 12 weeks. Each figure shows baseline photos (before use of the primer) and 12-week photos (without primer application and following primer application).
Discussion
Color cosmetics may be useful for the cosmetic correction of pigment abnormalities and can provide emotional benefits to patients with such esthetic skin conditions, particularly for those conditions that are readily visible.20,21 Studies have shown that use of corrective makeup in cosmetically disfiguring dermatoses improves both appearance and quality of life.22 The appropriate use of corrective makeup may also complement the treatment of such disorders by improving adherence to therapy.22 Use of a concealer or foundation to mask hyperpigmented areas, however, may fail to uniformly cover imperfections, and spots can take on a dull gray coloration.23 While there are many different approaches to the treatment of disorders of hyperpigmentation, topical therapies are typically employed. Unfortunately, benefits from use of depigmenting agents are uncertain and often delayed for several weeks or longer.
The traditional advantage of a cosmetic correction is that it offers the patient rapid and dramatic coverage, while the underlying condition is being treated with therapeutic modalities. A cosmetic primer is a cream or lotion applied before another cosmetic to improve coverage and lengthen the amount of time the cosmetic lasts on the face. Typically, a primer is used as a base cosmetic correction to even out the surface of the face prior to application of a foundation. The facial primer utilized in this study is unique in that it combines three functions, namely a cosmetic correction makeup to conceal facial imperfections, a broad-spectrum SPF 50 sunscreen, and multifunctional treatment for hyperpigmentation. The treatment component is based on ingredients having several activities: prevention of UV- and IR-mediated skin damage, prevention of melanocyte stimulation, tyrosinase inhibition (decreases production of melanin), decreased transfer of melanin to keratinocytes, keratolytic (increases cell exfoliation enhancing cell turnover and removal of epidermal melanin), and anti-inflammatory (reduces pro-inflammatory mediators). The facial primer studied here belongs to a new class of medical makeup that provides coverage to hide pigmentary skin imperfections while protecting skin and correcting underlying conditions.24
It is well known that sun exposure plays a major aggravating role in reinforcing existing dyschromias and causing new areas of pigmentation. As such, minimizing sun exposure and the regular use of broad-spectrum sunscreens are fundamentals in the management of hyperpigmentation. However, it is often difficult for physicians to convince their patients of the necessity to use sunscreen chronically, and in sufficient amounts, to prevent recurrence of dyschromias. The facial primer incorporates broad-spectrum SPF 50 sunscreen with IR protection as an integral component of the product.
The subjects in this study had both mild-to-moderate hyperpigmentation along with fine lines of the face. Not only was the facial primer effective in improving the appearance of hyperpigmentation after first application due to its cosmetic correction effects, but its continued use for the 12-week study period also improved dyschromias when assessed on bare skin. Daily application of the facial primer progressively decreased pigmentation from baseline by 4% at 4 weeks to 15% at 12 weeks. No plateau effect was reached suggesting that additional benefits may occur with continued use. This long-term effect on pigmentation can be attributed to the strong antioxidants that prevent activation of melanocytes by UV, IR, and oxidative stress, thereby reducing pigmentation over time. Additional actives also target various steps in the melanogenesis pathway by inhibiting tyrosinase, reducing melanin transfer to keratinocytes, and increasing epidermal turnover to remove existing melanin. Many preventive properties of this product may help reduce the occurrence of post-inflammatory hyperpigmentation. All of the other clinical efficacy assessments of the facial skin (overall appearance, pore size, radiance, smoothness, fine lines, and skin tone) showed a statistical improvement at their first use and at week 12 compared to baseline. These effects are likely a result of strong antioxidant properties of actives that help strengthen skin and improve its quality over time.
In addition to efficacy, a facial treatment must be safe and well tolerated. Tolerability assessments by both the investigator and subject confirmed that the facial primer was well tolerated.
Conclusion
This study demonstrates the efficacy and tolerability of a multifunctional facial primer for the treatment of mild-to-moderate facial hyperpigmentation and fine lines associated with photodamage.
Acknowledgments
Medical writing assistance was provided by Vincent Gotz, MS Pharm, MBA, of ProPharmaCon LLC (San Diego, CA, USA). Financial support for this study was provided by Colorescience, Inc.
Disclosure
The authors report no conflicts of interest in this work.
References
Gilchrest BA. Biochemical and molecular changes in photodamaged skin. In: Gilchrest BA, editor. Photodamage. Cambridge: Blackwell Science; 1995:168–184. | |
Fisher GJ, Kang S, Varani J, et al. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;138:1462–1470. | |
Gilchrest BA. Skin aging and photoaging: an overview. J Am Acad Dermatol. 1989;21:610–613. | |
Taylor CR, Stern RS, Leyden JJ, Gilchrest BA. Photoaging/photodamage and photoprotection. J Am Acad Dermatol. 1990;22:1–15. | |
Ortonne J-P. Pigmentary changes of the ageing skin. Br J Dermatol. 1990;122:21–28. | |
Castanet J, Ortonne J-P. Pigmentary changes in aged and photoaged skin. Arch Dermatol. 1997;133:1296–1299. | |
Taylor A, Pawaskar M, Taylor SL, Balkrishnan R, Feldman SR. Prevalence of pigmentary disorders and their impact on quality of life: a prospective cohort study. J Cosmet Dermatol. 2008;7:164–168. | |
Grimes PE. Management of hyperpigmentation in darker racial ethnic groups. Semin Cutan Med Surg. 2009;28:77–85. | |
Berson DS, Cohen JL, Rendon MI, Roberts WE, Starker I, Wang B. Clinical role and application of superficial chemical peels in today’s practice. J Drugs Dermatol. 2009;8:803–811. | |
Fischer TC, Perosino E, Poli F, et al; Cosmetic Dermatology European Expert Group. Chemical peels in aesthetic dermatology: an update 2009. J Eur Acad Dermatol Venereol. 2010;24:281–292. | |
Bonan P, Bassi A, Troiano M, et al. Lasers for hyperpigmentation and melasma. Prime-J. 2013;1:32–41. | |
Rivas S, Pandya AG. Treatment of melasma with topical agents, peels and lasers: an evidence-based review. Am J Clin Dermatol. 2013;14:359–376. | |
Kligman AM, Grove GL, Hirose R, Leyden JJ. Topical tretinoin for photoaged skin. J Am Acad Dermatol. 1986;15:836–859. | |
Ortonne J-P. Retinoid therapy of pigmentary disorders. Dermatol Ther. 2006;19:280–288. | |
Rendon MI, Gaviria JI. Review of skin-lightening agents. Derm Surg. 2005;31:886–889. | |
Ke MS, Soriano T, Lask GP. Optimal treatments for hyperpigmentation. J Cosmet Laser Ther. 2006;8:7–13. | |
Matsui MS, Hsia A, Miller JD, et al. Non-sunscreen photoprotection: antioxidants add value to a sunscreen. J Invest Dermatol Symp Proc. 2009;14:56–59. | |
Holzer AM, Elmets CA. The other end of the rainbow: infrared and skin. J Invest Dermatol. 2010;130:1469–1499. | |
Griffiths CE, Wang TS, Hamilton TA, Voorhees JJ, Ellis CN. A photonumeric scale for the assessment of cutaneous photodamage. Arch Dermatol. 1992;128:347–351. | |
Draelos ZD. Colored facial cosmetics. Dermatol Clin. 2000;18:621–631. | |
Levy LL, Emer JJ. Emotional benefit of cosmetic correction in the treatment of facial skin conditions: personal experience and review. Clin Cosmet Invest Dermatol. 2012;5:173–182. | |
Seité S, Deshayes P, Dréno B, et al. Interest of corrective makeup in the management of patients in dermatology. Clin Cosmet Invest Dermatol. 2012;5:123–128. | |
Nonni J. Medical makeup: the correction of hyperpigmentation disorders. Ann Dermatol Venereol. 2012;139:s170–s176. | |
Guerrero D. Dermocosmetic management of hyperpigmentation. Ann Dermatol Venereol. 2012;139:s166–s169. | |
Lintner K, Mas-Chamberlin C, Mondon P, et al. Cosmeceuticals and active ingredients. Clin Dermatol. 2009;27:461–468. | |
Eberlin S, Costa A, Pereira A, et al. Effects of antioxidants in the protection of infrared radiation-induced matrix metalloproteinase-1 in human fibroblast. J Am Acad Dermatol. 2014;70:AB155. | |
Azzi A. Tocopheryl phosphate, a novel natural form of vitamin E: in vitro and in vivo studies. FASEB J. 2006;20:LB79–LB80. | |
Gianello R, Libinaki R, Azzi A, et al. Alpha-tocopheryl phosphate: a novel, natural form of vitamin E. Free Radic Biol Med. 2005;39:970–976. | |
Dell’Acqua G, Wagner C. Lightening and illuminating skin with acetylated hydroxystilbenes from Rheum rhaponticum. Cosmet Toilet. 2011;126:634. | |
Chiang YM, Chuang DY, Wang SY, Kuo YH, Tsai PW, Shyur LF. Metabolite profiling and chemopreventive bioactivity of plant extracts from Bidens pilosa. J Ethnopharmacol. 2004;95:409–419. | |
Dieamant G, Pereda Mdel C, Nogueira C, et al. Antiaging mechanisms of a standardized supercritical CO2 preparation of Black Jack (Bidens pilosa L.) in human fibroblasts and skin fragments. Evid Based Complement Alternat Med. 2015;2015:280529. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.