Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Facial Erythema Due to Lupus Vulgaris and Candida albicans Infections: A Case Report
Authors Xue T , Lu Z, Zhang W, Wang Z , Shi Y , Jiang H, Wang H
Received 26 April 2022
Accepted for publication 13 July 2022
Published 22 July 2022 Volume 2022:15 Pages 1397—1402
DOI https://doi.org/10.2147/CCID.S372359
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Tianping Xue,1 Zhenzhong Lu,1 Wenyue Zhang,2 Zhenzhen Wang,2 Ying Shi,2 Haiqin Jiang,2 Hongsheng Wang2,3
1Department of Dermatology, Suzhou Wuzhong People’s Hospital, Suzhou, Jiangsu, People’s Republic of China; 2Jiangsu Key Laboratory of Molecular Biology for Skin Diseases and STIs, Institute of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College, Nanjing, People’s Republic of China; 3Centre for Global Health, School of Public Health, Nanjing Medical University, Nanjing, People’s Republic of China
Correspondence: Hongsheng Wang, Jiangsu Key Laboratory of Molecular Biology for Skin Diseases and STIs, Institute of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College, 12 JangWangmiao Street Xuanwu District, Nanjing, Jiangsu Province, People’s Republic of China, Tel +86-13002571330 ; +86-25-85478953, Email [email protected]
Abstract: Co-infection of Mycobacterium tuberculosis (MTB) and Candida albicans with erythema on the face is rare. A familiar red spot on the face can easily lead to missed diagnosis and misdiagnosis. Untreated lupus vulgaris (LV) can form scar tissue. And the fungal infection that cannot be diagnosed and treated timely can also lead to failure of LV treatment, resulting in facial scarring, disfigurement, and psychological stress. In this study, we reported a case of a 58-year-old immunocompetent female co-infected with MTB and Candida albicans on her face. After anti-tuberculous and anti-fungal therapy, she recovered with no scar on her face.
Keywords: lupus vulgaris, Candida albicans, facial erythema
Introduction
It is uncommon for a facial erythema to present with co-infection of Mycobacterium tuberculosis (MTB) and Candida albicans in people without immunosuppression. Lupus vulgaris (LV) is a common type of skin tuberculosis and belongs to extrapulmonary tuberculosis, which often occurs on the face. LV can occur alone in people with normal immunity and is usually caused by adjacent infection sites or lymphatic or hematogenous spread. Moreover, it is chronic and progressive.1
Candida albicans is also a rare cause of primary cutaneous infection, with occasional cases reported in the literature. Under normal circumstances, Candida spp. are a commensal yeast of the human body. When the immune environment of the body changes, they can be transformed into pathogenic fungus. These patients are usually immunosuppressed, but immunocompetent hosts can also be affected.
Both MTB and Candida albicans can reside on human skin and mucous membrane for a long time without causing significant lesions if the immune system is intact. They maintain the local immune dynamic balance of the body and participate in normal immune regulation. Still, they can result in disease when innate immunity disorders cause changes in their abundance.
Case Reports
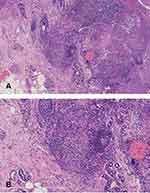
A 58-year-old woman admitted to an outpatient department with erythematosus and mild itching on her right cheek for six months was reported. She had type 2 diabetes and was being treated with metformin and glimepiride. Her fasting blood sugar remained slightly high, fluctuating around 8.0 mmol/L. She reported no personal or family history of tuberculosis. Further investigation revealed that she had stable, fingernail-sized erythema on her right cheek for 20 years. It did not change until six months ago, and her facial lesions expanded significantly. Physical examination of her right cheek revealed erythema, papules, and slight desquamation (Figure 1). The patient said there had been pustules and exudation when the disease worsened early. A T-cell spot test of MTB showed positive. Skin biopsy histopathology showed mild epidermal hyperplasia, with several epithelial cell masses of different sizes in the superficial and deep dermis. Lymphocytes were infiltrated around, and the boundary was clear. Caseous necrosis was not observed (Figure 2A and B). Direct immunofluorescence test showed that IgG basal membrane was suspiciously weak positive (±), and IgM, IgA, and C3 were negative (-). Findings on computed tomographic (CT) scan of her chest were normal. The positive colonies that grew on the Lowenstein-Jensen medium tissue sample inoculated after 35 days at 37°C were identified as MTB by PCR and DNA sequencing and acid-fast bacilli staining (Figure 3). And the tissue samples were also inoculated on the Sabouraud dextrose agar (SDA) plate at 28°C. The positive colonies that grew on the medium after ten days were identified as Candida albicans (Figure 4). Microscopic examination of the local erythema fungus on the face showed positive hypha. Although infection of the face is a rare extrapulmonary manifestation of tuberculosis, it is exceptional to have a concurrent infection with Candida albicans at the same site. It is an essential consideration for a patient with type 2 diabetes who has a history of poor glycemic control. Itraconazole 0.2 g bid was administered for seven days, and rifampicin 0.45 g qd, isoniazid tablet 0.3 g qd, and ethambutol hydrochloride 0.25 g tid were administered for five months (Figure 5).
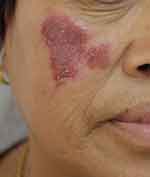 |
Figure 1 Red erythema and plaques on the right cheek before treatment. |
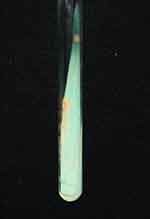 |
Figure 3 Round milky yellow colonies on Lowenstein-Jensen medium. |
 |
Figure 4 Positive white colonies grew on the SDA medium. |
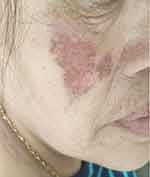 |
Figure 5 Dark red erythema on right cheek after treatment without scar. |
Discussion
LV is paucibacillary skin tuberculosis, accounting for 1–1.5% of the total number of skin tuberculosis cases.2 LV may occur mainly in five general forms, of which the plaque form is the most common.3 This may appear as an asymptomatic infiltrating papule or plaque that slowly spreads and develops into infiltrating erythema. LV can occur alone in people with normal immunity and is usually caused by adjacent infection sites or lymphatic or hematogenous spread. This condition can also develop at the site of direct injection. For example, trauma may be one of the causes of cutaneous tuberculosis.1
The thyroid gland and sex gland can cause endocrine maladjustment and bring about tuberculosis bacillus resurrection. This patient had no history of breast, uterus, or thyroid surgery, so that these factors could basically be ruled out. The erythema on her face remained stable for more than 20 years. Natural tuberculosis infection can coexist with the body for an extended period, ranging from a few weeks to decades, without any unpleasant symptoms. MTB protects it from being destroyed by the host through an avoidance strategy that enters macrophages and interferes with the microbicidal immune response.4 It has been proposed that the variable PE-PGRS proteins provide an “umbrella” for the tuberculosis bacilli and protect them from being detected and cleared by the immune system.5
Her facial lesions remained the same, despite occasional antibiotics for colds over the past two decades. The primary consideration is that antibiotics are usually active against growing bacteria but are ineffective against non-growing bacteria.5 In this case, the source of the infection could not be traced because her face had been infected for a long time. The BCG scar on her right arm proves that she had been vaccinated with the BCG vaccine. It is unknown whether the condition was caused by trauma or by the blood or lymphatic spread of the pre-existing MTB.
Unlike LV, Candida albicans most commonly infects immunosuppressive individuals. Candida albicans has evolved into a symbiotic fungus on mucous membranes and skin surfaces and is the most common opportunistic pathogen in humans. Half a year ago, the facial erythema of this patient experienced a sudden increase and exosmosis, which could be considered poor blood glucose control. Locally colonized Candida albicans has a crucial feature, switching its ability between yeast and mycelium growth.6 The yeast form predominantly exists as a commensal pathogen, and the mycelium form as an invasive pathogen.7,8 Candida albicans surface proteins have the function of superantigen and can activate T cells without antigen presentation and release proinflammatory cytokines.9 This process leads to the activation of epidermal cells and the proliferation of keratinocyte cytokines, thus resulting in disease.10
Both MTB and Candida albicans can colonize humans for long periods. An estimated >30% of the world population are colonized with Candida albicans and Mycobacteria spp., ~90% of whom show no clinical signs of disease.11 In this case, her prolonged elevated blood sugar may have led to changes in her defenses, and infection with one of these bacteria may have made the other susceptible. This may be the reason for the co-infection in this case.
Local lesion with anti-fungal and anti-tuberculosis drugs is fine and can be used as evidence of the co-infection. Local Mycobacterium tuberculosis (MTB) infection may cause systemic diseases including pulmonary tuberculosis. Nonetheless, the specific interaction between the various morphogenetic forms of fungi and the human immune system can still be understood insufficiently.
Discoid Lupus Erythematosus (DLE), Sarcoidosis, and Sporotrichosis are included in differential clinical diagnoses.
DLE may be demonstrated as symmetrical facial plaques accompanied by joint pain, elevated erythrocyte deposition (ESR), and leukopenia. Additionally, its pathological findings are specific.
Sarcoidosis can be accompanied by slight fever, night sweats, weakness, and the implicative involvement. The pathological findings were bare nodules, and PPD test was negative.
Sporotrichosis may be demonstrated as subcutaneous nodules, dark red infiltrating plaques, accompanied by lymphadenopathy. Cigar-shaped and stellate bodies can be seen pathologically.
Conclusion
Candida albicans can infect the mucous membranes and sometimes skin, especially the face. The necessary tests should be exerted when the patient demonstrates co-infection. For instance, when clinicians are diagnosed Mycobacterium tuberculosis, Candida albicans, acid-fast staining, or PCR pathological section, fungal microscopic examination, and colony culture should be taken into consideration. Facial damage may lead to disfigurement due to atrophic scar,12 and malignant changes may exist, or even cause systemic infection though symptoms of LV and fungal co-infection are mild; the early treatment is required. Therefore, clinicians are advised to widen the diagnostic horizon. Correct diagnosis is conclusively a critical, reasonable, and accurate drug-using process that may achieve positive clinical efficacy. Having exerted anti-fungal and anti-tuberculous therapies, the woman achieved good clinical results, with no facial scars or other uncomfortable symptoms.
Ethics and Consent Statement
The written informed consent was obtained from the patient for the publication of the case details and images. Institutional approval was not required to publish the case details.
Funding
This study did not receive any funding.
Disclosure
All authors have reviewed and agreed upon the manuscript content. The authors have no conflicts of interest to declare.
References
1. Brito AC, Oliveira CMM, Unger DA, Bittencourt MJS. Cutaneous tuberculosis: epidemiological, clinical, diagnostic and therapeutic update. An Bras Dermatol. 2022;97(2):129–144. doi:10.1016/j.abd.2021.07.004
2. Barbagallo J, Tager P, Ingleton R, Hirsch RJ, Weinberg JM. Cutaneous tuberculosis: diagnosis and treatment. Am J Clin Dermatol. 2002;3(5):319–328. doi:10.2165/00128071-200203050-00004
3. Chen Q, Chen W, Hao F. Cutaneous tuberculosis: a great imitator. Clin Dermatol. 2019;37(3):192–199. doi:10.1016/j.clindermatol.2019.01.008
4. Pirofski LA, Casadevall A. The state of latency in microbial pathogenesis. J Clin Invest. 2020;130(9):4525–4531. doi:10.1172/JCI136221
5. Zhang Y. Persistent and dormant tubercle bacilli and latent tuberculosis. Front Biosci. 2004;9:1136–1156. doi:10.2741/1291
6. Gow NA, Brown AJ, Odds FC. Fungal morphogenesis and host invasion. Curr Opin Microbiol. 2002;5(4):366–371. doi:10.1016/s1369-5274(02)00338-7
7. Netea MG, Joosten LA, van der Meer JW, Kullberg BJ, van de Veerdonk FL. Immune defence against Candida fungal infections. Nat Rev Immunol. 2015;15(10):630–642. doi:10.1038/nri3897
8. Gow NA, van de Veerdonk FL, Brown AJ, Netea MG. Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat Rev Microbiol. 2011;10(2):112–122. doi:10.1038/nrmicro2711
9. Kashem SW, Kaplan DH. Skin immunity to Candida albicans. Trends Immunol. 2016;37(7):440–450. doi:10.1016/j.it.2016.04.007
10. Wu Y, Zeng Z, Guo Y, et al. Candida albicans elicits protective allergic responses via platelet mediated T helper 2 and T helper 17 cell polarization. Immunity. 2021;54(11):2595–2610.e7. doi:10.1016/j.immuni.2021.08.009
11. Robinson RT, Huppler AR. The Goldilocks model of immune symbiosis with Mycobacteria and Candida colonizers. Cytokine. 2017;97:49–65. doi:10.1016/j.cyto.2017.05.015
12. Mei Y, Zhang W, Shi Y, et al. Cutaneous tuberculosis and nontuberculous mycobacterial infections at a National Specialized Hospital in China. Acta Derm Venereol. 2019;99(11):997–1003. doi:10.2340/00015555-3283
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.