Back to Journals » International Journal of Nanomedicine » Volume 14
Fabrication and evaluation of novel quercetin-conjugated Fe3O4–β-cyclodextrin nanoparticles for potential use in epilepsy disorder
Authors Hashemian M, Ghasemi-Kasman M , Ghasemi S, Akbari A, Moalem-Banhangi M, Zare L, Ahmadian SR
Received 4 June 2019
Accepted for publication 19 July 2019
Published 13 August 2019 Volume 2019:14 Pages 6481—6495
DOI https://doi.org/10.2147/IJN.S218317
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Thomas Webster
Mona Hashemian,1 Maryam Ghasemi-Kasman,2,3 Shahram Ghasemi,4 Atefeh Akbari,5 Monire Moalem-Banhangi,1 Leila Zare,3 Seyed Raheleh Ahmadian1
1Student Research Committee, Babol University of Medical Sciences, Babol, Iran; 2Cellular and Molecular Biology Research Center, Health Research Institute, Babol University of Medical Sciences, Babol, Iran; 3Neuroscience Research Center, Health Research Institute, Babol University of Medical Sciences, Babol, Iran; 4Faculty of Chemistry, University of Mazandaran, Babolsar, Iran; 5Infertility and Reproductive Health Research Center, Health Research Institute, Babol University of Medical Sciences, Babol, Iran
Background: Despite the numerous pharmacological activities of quercetin, its biomedical application has been hampered, because of poor water solubility and low oral bioavailability. In the present study, we fabricated a novel form of quercetin-conjugated Fe3O4–β-cyclodextrin (βCD) nanoparticles (NPs), and the effect of these prepared NPs was evaluated in a chronic model of epilepsy.
Methods: Quercetin-loaded NPs were prepared using an iron oxide core coated with βCD and pluronic F68 polymer. The chronic model of epilepsy was developed by intraperitoneal injection of pentylenetetrazole (PTZ) at dose of 36.5 mg/kg every second day. Quercetin or its nanoformulation at doses of 25 or 50 mg/kg were administered intraperitoneally 10 days before PTZ injections and their applications continued 1 hour before each PTZ injection. Immunostaining was performed to evaluate the neuronal density and astrocyte activation of hippocampi.
Results: Our data showed successful fabrication of quercetin onto Fe3O4–βCD NPs. In comparison to free quercetin, quercetin NPs markedly reduced seizure behavior, neuronal loss, and astrocyte activation in a PTZ-induced kindling model.
Conclusion: Overall, quercetin–Fe3O4–βCD NPs might be regarded as an ideal therapeutic approach in epilepsy disorder.
Keywords: quercetin, Fe3O4 nanoparticles, anticonvulsant, neuroprotection, astrocyte activation
Introduction
Epilepsy is considered one of the most common neurological disorders, and is characterized by recurrent and unpredicted epileptic seizures.1 Although numerous antiepileptic drugs have been designed in recent years, available therapies are inefficient for control of seizure attacks in around 30% of patients. Therefore, several approaches have emerged to design novel drugs for treatment of epileptic patients.2 Most recently, in order to overcome the side effects of existing chemical drugs,3 natural products have been introduced as effective supplementary agents in the treatment of several neurological disorders by modulating reactive oxygen species (ROS) generation and inflammation.4
Among natural compounds, quercetin is regarded as the most widely existing flavonoid in fruits and vegetables. It possesses a wide range of pharmacological activities, including anti-ischemic effects,5 neuroprotective activity, antioxidant properties,6 and antiapoptotic applications7 in different kinds of neurological disorders.8–10 There are some controversies about the beneficial anticonvulsant activity of quercetin.2,11,12 It has been demonstrated that application of quercetin reduces seizure severity in a pentylenetetrazole (PTZ)-induced kindling model.13 In an interesting study, Sefil et al showed that administration of quercetin at low doses, especially 10 mg/kg, prolonged the onset of seizures and decreased seizure duration, while its application at a higher dose (40 mg/kg) could not prevent PTZ-induced seizures.12 Conversely, Nieoczym et al suggested that quercetin and rutin possess only weak and short-term anticonvulsant potential in some acute seizure models in mice.2Despite the numerous biological effects of quercetin, its action in the central nervous system is limited, due to its low water solubility and bioavailability, poor absorption, and difficulty in passage from the blood–brain barrier (BBB).14–16 Therefore, several strategies have been developed to overcome these drawbacks in the usage of quercetin and providing an elevated pool of this bioflavonoid in the brain.17
Nanotechnology-based drug-delivery systems have emerged as a novel and promising strategy for enhancing the bioavailability of drugs.18,19 Furthermore, nanotechnology can establish a suitable system for targeting of drugs to desirable tissue, as well as sustained drug release.20 It has been shown that a nanoencapsulated form of quercetin remarkably reduces oxidative stress in a cerebral ischemia–induced injury model and improves neuronal preservation in the hippocampus.17 Furthermore, Ahmad et al demonstrated that intranasal delivery of a quercetin-loaded mucoadhesive nanoemulsion enhances quercetin bioavailability and its passage through the BBB.15
Nanoparticles (NPs) are well accepted as effective drug carriers, due to various technological advantages, including high carrier capacity, feasibility of various routes of administration, and sustained drug-release ability in the body.17 Moreover, NPs are generally considered atoxic, biodegradable, and nonimmunogenic.21 It has been shown that solid-lipid quercetin NPs effectively attenuate PTZ-induced neurocognitive impairment, along with amelioration of acetylcholinesterase activity, lipid peroxidation, and augmentation of glutathione levels in brains of zebrafish.22 Among NPs, magnetic NPs (MNPs) are widely utilized as nanocarriers for drugs, contrast imaging agents in magnetic resonance imaging, local hyperthermia, and magnetic targeting.23,24 However, because of high surface, volume ratios and van der Waals forces that trigger opsonization, extensive aggregation of MNPs commonly occurrs, which hampers their biomedical application.24 Interestingly, it has been shown that surface engineering of MNPs via various stabilizer coatings, including surfactants and synthetic and natural polymers, can solve the problem of MNP aggregation.25 Superparamagnetic iron oxide NPs (SPIONs) have recently attracted considerable attention, due to their biocompatibility, biodegradability, and safety.24,25 Sadhokha et al showed the beneficial effects of SPIONs in cancer stem cell therapy.27 Similarly, Kumar et al introduced SPIONs as promising anticancer agents.31 In addition, hyperthermia resulting from SPIONs can effectively destroy cancer stem cells.30 More recently, it has been shown that conjugation of quercetin with SPIONs significantly enhances its bioavailability in brain tissue.26 Further studies have also demonstrated that quercetin SPIONs markedly improve spatial learning and memory.27,28 The present study aimed to fabricate quercetin-loaded NPs using an iron oxide core coated with β-cyclodextrin (βCD) and pluronic F68 polymer. This formulation was evaluated for its anticonvulsant effect in a model of chronic epilepsy. Levels of neuronal loss and astrocyte activation were examined in PTZ receiving mice.
Methods
Chemicals
Quercetin powder (≥95% purity) and PTZ were purchased from Sigma-Aldrich. Fe(II) and Fe(III) ions, βCD, and pluronic F68 polymer (polyethylene oxide-co-polypropylene oxide-co-polyethylene oxide) were obtained from Merck. Quercetin dosages were selected based on previous study by Nassiri-Asl et al.13
Preparation of quercetin-loaded MNPs
MNPs were prepared by coprecipitating an aqueous solution of Fe(II) and Fe(III) ions in the presence of an aqueous ammonia solution under a nitrogen atmosphere, as previously reported.24,29 Briefly, 100 mg βCD and 45 mL deionized (DI) water containing 810 mg FeCl3 and 297 mg FeCl2 (molar ratio 2:1) were poured into a 100 mL beaker and stirred at 400 rpm under a nitrogen atmosphere for 20 minutes. After that, 3 mL ammonium hydroxide (28% ammonia in water) was added slowly at a stirring speed of 900 rpm. After 6 hours, 200 mg pluronic F68 in 5 mL water was added to the suspension under a nitrogen atmosphere, and the resulting solution was stirred overnight. The precipitate was washed five times with DI water and collected by magnet to obtain MNPs. In order to load quercetin in the MNPs, 1 mg quercetin in 400 μL acetone was added dropwise to an aqueous dispersion of MNPs and the mixture stirred overnight at 400 rpm on a magnetic stirrer to facilitate the penetration of quercetin molecules into the βCD–F68 polymer layers. The quercetin-loaded MNPs were washed three times by resuspension of MNPs in DI water and separated using a magnet.
Characterization of quercetin-loaded MNPs
The crystalline nature and phase analysis of prepared MNPs were investigated using X-ray diffraction (Rigaku Ultima IV) at 2θ=10°–80°. The morphology of samples was investigated by field-emission scanning electron microscopy (FE-SEM) (Mira3, Tescan). Elemental composition of quercetin-loaded MNPs was analyzed using energy-dispersive X-ray spectroscopy. Atomic force microscopy (AFM) (Ara 0101) was also used to obtain more information about the size and morphology of synthesized MNPs. Fourier-transform infrared (FTIR) spectroscopy (Vector 22; Bruker) was used to investigate the structural information of quercetin-loaded MNPs.
Animals
In the present study, male NMRI mice (6–8 weeks, 20–30 g) were obtained from the animal house of Babol University of Medical Sciences (Babol, Iran). Animals were kept in a 12-hour light–dark cycle with free access to food and water. All experimental procedures were approved by the local ethics committee of Babol University of Medical Sciences which was in accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals (approval number: MUBABOL.HRI.REC.1396.98).
Chemical kindling model
PTZ has been introduced as the most popular chemoconvulsant agent to establish experimental models of seizure. Although, the mechanisms by which PTZ elicits its action are not fully understood, a generally accepted mechanism is noncompetitive antagonism of the γ-aminobutyric acid type A–receptor complex.30 A PTZ-induced kindling model was developed based on our previous reports.31–33 In brief, PTZ was intraperitoneally (IP) adminisered at a dose of 36.5 mg/kg every second day, and mice were monitored for 20 minutes after each injection. Seizure stages were classified: stage 0, no response; stage 1, ear and facial twitching; stage 2, myoclonic jerks (MJs); stage 3, clonic forelimb convulsions; stage 4, generalized clonic seizures with turning to a side position; and stage 5, generalized tonic–clonic seizures (GCTSs) or death.32 Behavioral manifestations of seizure, including mean seizure stage, latency of MJs, latency of GTCSs, and duration of GTCSs, were analyzed for each experimental group.
Experimental procedures
A total of 36 mice were randomly assigned to six experimental groups (n=6 in each group): group 1, saline + PTZ — mice received the IP injections of saline as PTZ vehicle and then PTZ was administrated every second day; group 2, blank MNPs + PTZ, mice were treated by blank MNP injections IP and then PTZ was administered every second day; groups 3 and 4, quercetin + PTZ — mice received free quercetin at doses of 25 or 50 mg/kg; and groups 5 and 6, mice were treated with quercetin-loaded MNPs at doses of 25 or 50 mg/kg. All interventions were injected IP, and applications were started 10 days before PTZ injection and continued for 1 hour before each PTZ injection.
Immunostaining
Immunostaining was carried out as mentioned previously.21 Briefly, hippocampal sections were washed with (PBS), then non-specific binding of antibodies to antigens was blocked by incubation of tissue sections with blocking solution (10% normal goat serum, 0.3% Triton X-100 in PBS) for 1 hour. Then, sections were incubated with primary antibodies, including rabbit anti-GFAP (1:400, Z0334; Dako) or rabbit anti-NeuN (1:500, ab177487; Abcam) overnight at 4°C. After washing with PBS, appropriate secondary antibodies, including anti-rabbit IgG conjugated with Alexa 488 (1:1,000, ab150077; Abcam) and anti-rabbit IgG conjugated with Alexa 594 (1:1,000, ab150080; Abcam) were added to the slides for 1 hour at room temperature. In the final step, after washing with PBS, nuclear staining was done using DAPI and sections were evaluated under fluorescence microscopy (Olympus IX71). Quantification of immunostaining results was carried out using Image J software as we reported previously.21,32
Statistical analysis
Two-way repeated measure ANOVA followed by Tukey's post hoc test was used for analyzing MJ latency, GTCS latency, and duration of GTCS. Maximum seizure stage was measured by Kruskal–Wallis nonparametric analysis followed by Dunn’s post hoc test. Analysis of immunostaining results was performed using one-way ANOVA followed by Tukey post hoc test. All data are expressed as means ± SEM, and P<0.05 was considered statistically significant.
Results
Characterization of quercetin-loaded MNPs
In X-ray diffraction patterns, characteristic peaks were observed at 2θ=30.5°, 35.7°, 43.4°, 53.4°, 57.3°, 63.2°, and 74.7°, corresponding to (220), (311), (400), (422), (511), (440), and (533), respectively, which were in accordance with the standard sample of Fe3O4 (Joint Committee of Powder Diffraction Standards file 19–0629) with inverse cubic spinel structure without any impurities (Figure 1A). Figure 1B shows field-emission scanning electron–microscopy of quercetin-loaded MNPs at two magnifications. Nearly spherical MNPs (<50 nm in size) and some degree of aggregation were observed (Figure 1B).
Energy-dispersive X-ray spectroscopy was used to analyze the elemental composition of quercetin-loaded MNPs. Appearances of characteristic Kα lines of C, O, and Fe in the context of samples confirmed the presence of Fe3O4, as well as quercetin, which contained only C and O elements (Figure 2A). In addition, Figure 2B shows elemental analysis of samples.
 |
Figure 2 (A) Energy-dispersive X-ray spectra for quercetin-loaded MNPs; (B) Elemental analysis of synthesized nanoparticles. Abbreviation: MNPs, magnetic nanoparticles. |
Elemental mapping analysis was also performed for selected areas of Fe3O4–quercetin samples (Figure 3A). X-ray maps showed that Fe, O, and C elements were distributed homogeneously on the surface of quercetin-loaded MNPs (Figure 3, B–D).
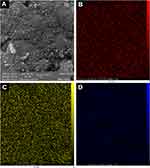 |
Figure 3 (A) Selected area for mapping analysis of quercetin-loaded mMNPs, with elemental distribution for (B) Fe, (C) O, and (D) C. Abbreviation: MNPs, magnetic nanoparticles. |
Figure 4, A and B shows two dimensional (2D) and three dimensional (3D) atomic force microscopy of quercetin-loaded MNPs. However, some larger particles can be observed, which could be due to formation of aggregated particles during loading of quercetin onto Fe3O4 NPs.
 |
Figure 4 (A) 2D and (B) 3D atomic force microscopy of quercetin-loaded magnetic nanoparticles. Abbreviations: 2D, two dimensional; 3D, three dimensional. |
FTIR spectra of quercetin, CD–Fe3O4, and quercetin-loaded MNPs are shown in Figure 5. The broad peaks around 3,300–3,500 cm−1 in spectra of three samples show the OH-stretching vibration of hydroxyl groups in CD–Fe3O4, quercetin-loaded MNPs, and quercetin. Quercetin FTIR spectroscopy, characteristic bands related to C=O stretching at 1,664 cm−1 and region corresponding to C-O stretching at 1150–1070 cm−1 are observed. Also, C–H bending vibration of aromatic groups for quercetin appeared at 932 cm−1. In quercetin-loaded MNPs, the same bands with lower intensity are observed, which confirmed the loading of quercetin on the MNPs.
 |
Figure 5 Fourier-transform infrared spectra of quercetin, CD–Fe3O4, and quercetin-loaded MNPs. Abbreviations: CD, cyclodextrin; MNPs, magnetic nanoparticles. |
Quercetin-loaded MNPs attenuated seizure behavior in PTZ-induced kindling model
In order to evaluate the possible antiepileptogenic effects of quercetin-loaded MNPs on behavioral manifestations of seizure, maximum seizure stage, MJ and GTCS latency and GTCS duration were measured in mice following repeated administration of PTZ. Analysis of mean seizures stage showed that there was a significant difference between quercetin-loaded MNPs and blank MNPs (50 mg/kg) at injection 11 (P<0.05) of PTZ. Our data also indicated that application of quercetin or quercetin-loaded MNPs did not significantly reduce the seizure stage compared to the saline or blank-MNPs experimental groups (Figure 6A). Furthermore, analysis of MJ latency indicated that application of quercetin at a dose of 25 mg/kg significantly increased MJ latency compared to saline + PTZ at PTZ injections 7 (P<0.01), 9 (P<0.05), and 10 (P<0.001). Our data showed that application of free quercetin 50 mg/kg increased MJ latency compared to saline + PTZ only at PTZ injection 11 (P<0.05). There was also a significant difference in MJ latency between quercetin 50 mg/kg + PTZ and blank MNPs + PTZ at PTZ injection 5 PTZ (P<0.01). Interestingly, administration of quercetin-loaded MNPs (50 mg/kg) significantly increased the MJ latency compared to saline + PTZ at PTZ injections 7 (P<0.05), 9 (P<0.001), 10 (P<0.001), and 11 (P<0.01). There was also a significant increase in MJ latency following administration of quercetin-loaded MNPs (50 mg/kg) compared to blank MNPs + PTZ at PTZ injections 10 (P<0.001) and 11 (P<0.01). Quercetin-loaded MNPs (50 mg/kg) also significantly augmented MJ latency compared to free quercetin (50 mg/kg) at PTZ injections 9 and 10 (P<0.001). In comparison to quercetin-loaded MNPs (25 mg/kg), application of quercetin MNPs at 50 mg/kg increased MJ latency at PTZ injection 9 (P<0.05, Figure 6B).
In addition, analysis of GTCS latency indicated that application of free quercetin (25 mg/kg) significantly increased GTCS latency compared to saline + PTZ at PTZ injections 7 (P<0.01), 9 (P<0.05), and 10 (P<0.001). In comparison to saline + PTZ, a significant increase in GTCS latency was also found in quercetin (50 mg/kg)-treated mice at PTZ injections 5 (P<0.001), 10 (P<0.05), and 11 (P<0.05). A significant difference in GTCS latency was observed between quercetin (50 mg/kg) + PTZ and blank MNPs + PTZ at PTZ injection 5 (P<0.01). Application of quercetin-loaded MNPs (50 mg/kg) significantly increased GTCS latency compared to saline + PTZ at PTZ injections 7 (P<0.05), 9 (P<0.001), 10 (P<0.01), and 11 (P<0.01). Additionally, in comparison to blank MNPs and quercetin MNPs (25 mg/kg), quercetin-loaded MNPs (50 mg/kg) significantly increased GTCS latency (injection 9 for quercetin MNPs [25 mg/kg], P<0.05); injection 10 for blank MNPs, P<0.001). There was also a significant difference in GTCS latency between free quercetin and quercetin-loaded MNPs (50 mg/kg) at PTZ injections 9 and 10 (P<0.001) (Figure 7A). Our results also demonstrated that application of free quercetin at both doses (P<0.001) and quercetin MNPs (25 mg/kg, P<0.01) significantly decreased GTCS duration compared to blank MNPs at PTZ injection 10. A significant reduction in GTCS duration was also found in quercetin-loaded MNPs (50 mg/kg) compared to saline (injection 9, P<0.05) and blank MNPs (injections 10 [P<0.001] and 11 [ P<0.01, Figure 7B).
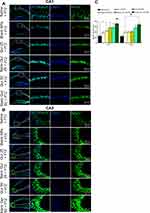
Quercetin-loaded MNPs reduced hippocampal neuronal loss following PTZ administration
To determine the protective effect of quercetin or its nanoformulation on the neuronal density of hippocampi, immunostaining against NeuN as a mature neuronal marker was done on hippocampal sections (Figure 8, A and B). The intensity of NeuN fluorescent signals was significantly increased in mice treated with free quercetin 25 or 50 mg/kg compared to saline + PTZ (CA1, quercetin 25 mg/kg + PTZ, P<0.05, and quercetin 50 mg/kg, P<0.001; CA3, quercetin 50 mg/kg + PTZ, P<0.01). In comparison with blank NPs + PTZ, application of free quercetin at 50 mg/kg increased the intensity of NeuN-positive cells in the CA1 region of hippocampi (P<0.05). There was also a significant difference in the intensity of NeuN-positive cells among quercetin-loaded NP–treated mice and the blank MNPs + PTZ groups (CA1, quercetin MNPs 25 mg/kg, P<0.05, and quercetin MNPs 50 mg/kg, P<0.001; CA3, quercetin MNPs 50 mg/kg, P<0.01). However, in comparison with free quercetin at 50 mg/kg, application of quercetin-loaded MNPs significantly attenuated levels of hippocampal neuronal loss following PTZ administration, no statistical significant difference was found between free quercetin (50 mg/kg) and its nanoformulation (Figure 8C).
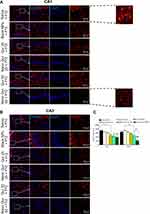
Quercetin-loaded MNPs ameliorated hippocampal astrocyte activation in PTZ-induced kindling model
In order to evaluate the effect of quercetin or quercetin nanoformulation on glial activation, the level of astrocyte activation was assessed using immunostaining against GFAP as astrocyte marker (Figure 9, A and B). The inset of Figure 9A depicts the change in morphology of hippocampal astrocytes following application of PTZ. The activated astrocytes displayed enlarged cell bodies and thick projections in the saline and blank-MNPs experimental groups, while the smaller cell bodies and thin processes of astrocytes were observed in hippocampi of mice that had been treated with high-dose quercetin–loaded MNPs. Analysis of immunostaining data indicated that application of quercetin-loaded MNPs at 25 mg/kg significantly reduced levels of astrocyte activation in the CA1 region of hippocampi compared to saline + PTZ (P<0.01). A significant reduction in the number of GFAP-expressing cells was also observed in free quercetin (50 mg/kg)-treated mice compared to the saline (CA1, P<0.01) and blank MNPs (CA1 and CA3, P<0.05) groups. However, in comparison to blank MNPs + PTZ, administration of quercetin MNPs at 25 mg/kg significantly ameliorated levels of astrocyte activation in the CA1 region of hippocampi (P<0.05), but application of quercetin-loaded MNPs at 50 mg/kg exerted a stronger inhibitory effect on astrocyte activation than saline (CA1, P<0.001; CA3, P<0.001) and blank MNPs (CA1, P<0.01; CA3, P<0.001). There was also a significant difference in number of GFAP-positive cells between free quercetin and quercetin-loaded MNPs (50 mg/kg) in the CA3 region of hippocampi (P<0.05, Figure 9C).
Discussion
In recent years, encapsulation of drugs in various types of NPs has emerged as a promising strategy for enhancing the water solubility and bioavailability of hydrophobic compounds. In the present study, a novel approach for fabrication of quercetin was developed using an iron oxide core coated with βCD and pluronic F68 polymer. Quercetin-loaded MNPs markedly inhibited the behavioral manifestations of seizure and reduced levels of hippocampal neuronal loss. Furthermore, astrocyte activation was ameliorated in quercetin-loaded MNP–treated mice.
Sefil et al demonstrated that application of quercetin, especially at 10 mg/kg, prolonged onset of seizures and decreased seizure stage and duration in PTZ-induced seizures.12 Furthermore, it has been shown that quercetin possesses a narrow therapeutic dose range for its anticonvulsant activity and exhibits different effects on the seizure threshold in acute and chronic seizure models.34 In contrast to this evidence, we did not observe a remarkable effect of free quercetin (25 or 50 mg/kg) on seizure behavior in a PTZ-induced kindling model. In line with our results, it has also been shown that quercetin and its glycosidic form as rutin display only weak and short-term anticonvulsant activity in a model of psychomotor seizures induced by 6 Hz stimulation.2 Several lines of evidence have suggested that the beneficial effects of quercetin are restricted because of its poor aqueous solubility and difficulty to pass across the BBB.16,27 To overcome these limitations, several approaches, such as complexation of quercetin with CD or liposomes or its encapsulation into NPs, have been developed.27 Most recently, quercetin conjugated with SPIONs has been introduced as a promising strategy for enhancing the half-life of quercetin in the blood and its bioavailability in brain tissue.27 The results of the present study showed that quercetin-loaded MNPs displayed stronger anticonvulsant effects than free quercetin. Interestingly, consistently with our data, a previous report also indicated that solid-lipid quercetin NPs reduce PTZ-induced neurocognitive impairments in zebrafish.22 Additionally, it has been shown that quercetin SPIONs can improve cognitive performance better than free quercetin via increasing quercetin’s bioavailability in the brain.27,28 In another study, Najafabadi et al showed that conjugation of quercetin with SPIONs markedly increased the bioavailability of quercetin in the brain about tenfold higher than free quercetin.26 Therefore, the better anticonvulsant effect of quercetin-loaded MNPs in the present study was presumably mediated through the smallness of the prepared MNPs and the enhancement of quercetin bioavailability in brain tissue.
It has been well documented that oxidative stress, inflammatory factors, and excessive release of excitatory amino acids play an important role in the pathology of epilepsy disorder.21 In parallel with previous reports,21,35,36 our data also demonstrated that repetitive administration of PTZ led to hippocampal neuronal loss and astrocyte activation. Conversely, quercetin supplementation, especially its nanoformulation form, significantly attenuated neuronal loss and levels of astrocyte activation. Despite the beneficial effects of SPIONs, it has been demonstrated that these NPs may induce cell toxicity.37,38 The potential toxicity of SPIONs is highly dependent on the size of the NPs, exposure time, type of coating, and type of tissue.27 The present study does not provide data on the toxicity of quercetin-loaded SPIONs, but previous reports have shown that quercetin–Fe3O4 NPs have no remarkable toxic effects on liver, kidney, or heart samples of animals.28,39,40 Additionally, no cell toxicity was found in brain tissue of rats treated with Qur-SPIONs.27
In line with our findings, Ghosh et al revealed that oral treatment of nanoencapsulated quercetin downregulates the inducible nitric oxide synthase and caspase 3 activity and improves hippocampal neuronal density in combating ischemia reperfusion–induced neuronal damage.17 A previous report also suggested that quercetin supplementation reduces levels of apoptotic neurons in status epilepticus–induced neuronal injury. It has been postulated that the neuroprotective effects of quercetin may be associated with regulation of the X-linked inhibitor of apoptosis protein and caspase 3 protein.41 Additionally, antioxidant levels in different brain regions are increased in animals treated with quercetin NPs.17 Lee et al showed that quercetin-loaded silica NPs significantly reduced the production of proinflammatory cytokines by cultured macrophages.42 It has also been reported that triphenyl phosphonium–coated nanoquercetin attenuated histopathological severity in brain tissue by preserving structural and functional integrity of mitochondria in cerebral ischemia–reperfusion injury. Moreover, quercetin NPs ameliorated apoptotic cell numbers via reduction of ROS levels.43 In another study, Gosh et al illustrated that encapsulation of quercetin with arsenic-chelator nanocapsules reduced the generation of ROS and liver injury in a rat model of chronic arsenic toxicity.44 In parallel with our study, a previous report also indicated that quercetin-loaded zein NPs reduced memory impairment and ameliorated hippocampal astrocyte activation in a mouse model of Alzheimer’s disease.45
Conclusion
In the present study, quercetin-loaded MNPs were efficiently fabricated using an iron oxide core coated with βCD and pluronic F68 polymer. Quercetin-loaded MNPs reduced the behavioral signs of seizure, neuronal loss, and astrocyte activation in a PTZ-induced kindling model (Figure 10). It is postulated that the stronger anticonvulsant activity of quercetin-loaded MNPs might be resulted from enhancement of water solubility and bioavailability of quercetin. However, further studies are required to elucidate the stability, bioavailability of synthetized NPs, and the precise anticonvulsant mechanisms of quercetin-loaded MNPs in an experimental model of epilepsy.
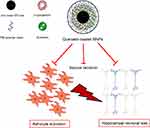 |
Figure 10 Schematic representation of quercetin-loaded magnetic nanoparticles (MNPs) and their effects on seizure behavior, neuronal loss and astrocyte activation. |
Abbreviations
WHO, World Health Organization; ROS, reactive oxygen species; PTZ, pentylenetetrazole; BBB, blood–brain barrier; NPs, nanoparticles; MNPs, magnetic NPs; SPIONs, superparamagnetic iron oxide NPs; CD, cyclodextrin; DI, deionized water; FTIR, Fourier-transform infrared spectroscopy; IP, intraperitoneally; MJ, myoclonic jerk; GTCS, generalized tonic–clonic seizure; iNOS, inducible nitric oxide synthase.
Acknowledgment
This work was supported by a grant from the Student Research Committee, Deputy of Research and Technology (9604029), Babol University of Medical Sciences, Babol, Iran.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol. 2011;7(1):31. doi:10.1038/nrneurol.2010.178
2. Nieoczym D, Socała K, Raszewski G, Wlaź P. Effect of quercetin and rutin in some acute seizure models in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54:50–58. doi:10.1016/j.pnpbp.2014.05.007
3. Garlich FM, Balakrishnan K, Shah SK, Howland MA, Fong J, Nelson LS. Prolonged altered mental status and bradycardia following pediatric donepezil ingestion. Clin Toxicol. 2014;52(4):291–294. doi:10.3109/15563650.2014.900182
4. Butler MS. Natural products to drugs: natural product-derived compounds in clinical trials. Nat Prod Rep. 2008;25(3):475–516. doi:10.1039/b514294f
5. Cho J-Y, Kim I-S, Jang Y-H, Kim A-R, Lee S-R. Protective effect of quercetin, a natural flavonoid against neuronal damage after transient global cerebral ischemia. Neurosci Lett. 2006;404(3):330–335. doi:10.1016/j.neulet.2006.06.010
6. Dajas F. Life or death: neuroprotective and anticancer effects of quercetin. J Ethnopharmacol. 2012;143(2):383–396. doi:10.1016/j.jep.2012.07.005
7. Baghel SS, Shrivastava N, Baghel RS, Agrawal P, Rajput S. A review of quercetin: antioxidant and anticancer properties. World J Pharm Pharmaceutical Sci. 2012;1(1):146–160.
8. Ansari MA, Abdul HM, Joshi G, Opii WO, Butterfield DA. Protective effect of quercetin in primary neurons against Aβ (1–42): relevance to Alzheimer’s disease. J Nutr Biochem. 2009;20(4):269–275. doi:10.1016/j.jnutbio.2008.03.002
9. Du G, Zhao Z, Chen Y, et al. Quercetin protects rat cortical neurons against traumatic brain injury. Mol Med Rep. 2018;17(6):7859–7865. doi:10.3892/mmr.2018.8801
10. Türedi S, Yuluğ E, Alver A, Bodur A, İnce İ. A morphological and biochemical evaluation of the effects of quercetin on experimental sciatic nerve damage in rats. Exp Ther Med. 2018;15(4):3215–3224. doi:10.3892/etm.2018.5824
11. Singh T, Kaur T, Goel RK. Adjuvant quercetin therapy for combined treatment of epilepsy and comorbid depression. Neurochem Int. 2017;104:27–33. doi:10.1016/j.neuint.2016.12.023
12. Sefil F, Kahraman I, Dokuyucu R, et al. Ameliorating effect of quercetin on acute pentylenetetrazole induced seizures in rats. Int J Clin Exp Med. 2014;7(9):2471.
13. Nassiri-Asl M, Moghbelinejad S, Abbasi E, et al. Effects of quercetin on oxidative stress and memory retrieval in kindled rats. Epilepsy Behav. 2013;28(2):151–155. doi:10.1016/j.yebeh.2013.04.019
14. Anwer MK, Al-Mansoor MA, Jamil S, Al-Shdefat R, Ansari MN, Shakeel F. Development and evaluation of PLGA polymer based nanoparticles of quercetin. Int J Biol Macromol. 2016;92:213–219. doi:10.1016/j.ijbiomac.2016.07.002
15. Ahmad N, Ahmad R, Naqvi AA, et al. Intranasal delivery of quercetin-loaded mucoadhesive nanoemulsion for treatment of cerebral ischaemia. Artif Cells Nanomed Biotechnol. 2018;46(4):717–729. doi:10.1080/21691401.2017.1337024
16. Nday CM, Halevas E, Jackson GE, Salifoglou A. Quercetin encapsulation in modified silica nanoparticles: potential use against Cu (II)-induced oxidative stress in neurodegeneration. J Inorg Biochem. 2015;145:51–64. doi:10.1016/j.jinorgbio.2015.01.001
17. Ghosh A, Sarkar S, Mandal AK, Das N. Neuroprotective role of nanoencapsulated quercetin in combating ischemia-reperfusion induced neuronal damage in young and aged rats. PLoS One. 2013;8(4):e57735. doi:10.1371/journal.pone.0057735
18. Zhang L, Gu F, Chan J, Wang A, Langer R, Farokhzad O. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther. 2008;83(5):761–769. doi:10.1038/sj.clpt.6100400
19. Gobbo OL, Sjaastad K, Radomski MW, Volkov Y, Prina-Mello A. Magnetic nanoparticles in cancer theranostics. Theranostics. 2015;5(11):1249. doi:10.7150/thno.11544
20. Lockman PR, Mumper RJ, Khan MA, Allen DD. Nanoparticle technology for drug delivery across the blood-brain barrier. Drug Dev Ind Pharm. 2002;28(1):1–13.
21. Hashemian M, Anissian D, Ghasemi-Kasman M, et al. Curcumin-loaded chitosan-alginate-STPP nanoparticles ameliorate memory deficits and reduce glial activation in pentylenetetrazol-induced kindling model of epilepsy. Prog Neuropsychopharmacol Biol Psychiatry. 2017;79:462–471. doi:10.1016/j.pnpbp.2017.07.025
22. Rishitha N, Muthuraman A. Therapeutic evaluation of solid lipid nanoparticle of quercetin in pentylenetetrazole induced cognitive impairment of zebrafish. Life Sci. 2018;199:80–87. doi:10.1016/j.lfs.2018.03.010
23. Laurent S, Bridot J-L, Elst LV, Muller RN. Magnetic iron oxide nanoparticles for biomedical applications. Future Med Chem. 2010;2(3):427–449. doi:10.4155/fmc.09.164
24. Yallapu MM, Othman SF, Curtis ET, et al. Curcumin-loaded magnetic nanoparticles for breast cancer therapeutics and imaging applications. Int J Nanomedicine. 2012;7:1761. doi:10.2147/IJN.S30631
25. Shubayev VI, Pisanic IITR, Jin S. Magnetic nanoparticles for theragnostics. Adv Drug Deliv Rev. 2009;61(6):467–477. doi:10.1016/j.addr.2009.03.007
26. Najafabadi RE, Kazemipour N, Esmaeili A, Beheshti S, Nazifi S. Using superparamagnetic iron oxide nanoparticles to enhance bioavailability of quercetin in the intact rat brain. BMC Pharmacol Toxicol. 2018;19(1):59. doi:10.1186/s40360-018-0242-1
27. Amanzadeh E, Esmaeili A, Abadi REN, Kazemipour N, Pahlevanneshan Z, Beheshti S. Quercetin conjugated with superparamagnetic iron oxide nanoparticles improves learning and memory better than free quercetin via interacting with proteins involved in LTP. Sci Rep. 2019;9(1):6876. doi:10.1038/s41598-019-43345-w
28. Ebrahimpour S, Esmaeili A, Beheshti S. Effect of quercetin-conjugated superparamagnetic iron oxide nanoparticles on diabetes-induced learning and memory impairment in rats. Int J Nanomed. 2018;13:6311. doi:10.2147/IJN.S177627
29. Yallapu MM, Othman SF, Curtis ET, Gupta BK, Jaggi M, Chauhan SC. Multi-functional magnetic nanoparticles for magnetic resonance imaging and cancer therapy. Biomaterials. 2011;32(7):1890–1905. doi:10.1016/j.biomaterials.2010.11.028
30. Hansen SL, Sperling BB, Sánchez C. Anticonvulsant and antiepileptogenic effects of GABAA receptor ligands in pentylenetetrazole-kindled mice. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(1):105–113. doi:10.1016/j.pnpbp.2003.09.026
31. Gol M, Ghorbanian D, Hassanzadeh S, Javan M, Mirnajafi-Zadeh J, Ghasemi-Kasman M. Fingolimod enhances myelin repair of hippocampus in pentylenetetrazol-induced kindling model. Eur J Pharm Sci. 2017;96:72–83. doi:10.1016/j.ejps.2016.09.016
32. Ahmadian SR, Ghasemi-Kasman M, Pouramir M, Sadeghi F. Arbutin attenuates cognitive impairment and inflammatory response in pentylenetetrazol-induced kindling model of epilepsy. Neuropharmacology. 2019;146:117–127. doi:10.1016/j.neuropharm.2018.11.038
33. Ghorbanian D, Ghasemi-Kasman M, Hashemian M, et al. Myristica fragrans Houtt extract attenuates neuronal loss and glial activation in pentylenetetrazol-induced kindling model. Iran J Pharm Res. 2019;18(2):812–825.
34. Nassiri-Asl M, Hajiali F, Taghiloo M, Abbasi E, Mohseni F, Yousefi F. Comparison between the effects of quercetin on seizure threshold in acute and chronic seizure models. Toxicol Ind Health. 2016;32(5):936–944. doi:10.1177/0748233713518603
35. Anissian D, Ghasemi-Kasman M, Khalili-Fomeshi M, et al. Piperine-loaded chitosan-STPP nanoparticles reduce neuronal loss and astrocytes activation in chemical kindling model of epilepsy. Int J Biol Macromol. 2018;107:973–983. doi:10.1016/j.ijbiomac.2017.09.073
36. Kaur H, Patro I, Tikoo K, Sandhir R. Curcumin attenuates inflammatory response and cognitive deficits in experimental model of chronic epilepsy. Neurochem Int. 2015;89:40–50. doi:10.1016/j.neuint.2015.07.009
37. Hsieh H-C, Chen C-M, Hsieh W-Y, Chen C-Y, Liu -C-C, Lin F-H. ROS-induced toxicity: exposure of 3T3, RAW264. 7, and MCF7 cells to superparamagnetic iron oxide nanoparticles results in cell death by mitochondria-dependent apoptosis. J Nanopart Res. 2015;17(2):71. doi:10.1007/s11051-015-2886-8
38. Singh N, Jenkins GJ, Asadi R, Doak SH. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev. 2010;1(1):5358. doi:10.3402/nano.v1i0.5358
39. Sabareeswaran A, Ansar EB, Varma PRVH, Mohanan PV, Kumary TV. Effect of surface-modified superparamagnetic iron oxide nanoparticles (SPIONS) on mast cell infiltration: an acute in vivo study. Nanomedicine. 2016;12(6):1523–1533. doi:10.1016/j.nano.2016.02.018
40. Chen J, Shi M, Liu P, et al. Reducible polyamidoamine-magnetic iron oxide self-assembled nanoparticles for doxorubicin delivery. Biomaterials. 2014;35(4):1240–1248. doi:10.1016/j.biomaterials.2013.10.057
41. Hu K, Li S-Y, Xiao B, Bi F-F, Lu X-Q, Wu X-M. Protective effects of quercetin against status epilepticus induced hippocampal neuronal injury in rats: involvement of X-linked inhibitor of apoptosis protein. Acta Neurol Belg. 2011;111(3):205.
42. Lee GH, Lee SJ, Jeong SW, et al. Antioxidative and antiinflammatory activities of quercetin-loaded silica nanoparticles. Colloids Surf B Biointerfaces. 2016;143:511–517. doi:10.1016/j.colsurfb.2016.03.060
43. Ghosh S, Sarkar S, Choudhury ST, Ghosh T, Das N. Triphenyl phosphonium coated nano-quercetin for oral delivery: neuroprotective effects in attenuating age related global moderate cerebral ischemia reperfusion injury in rats. Nanomedicine. 2017;13(8):2439–2450. doi:10.1016/j.nano.2017.08.002
44. Ghosh S, Dungdung SR, Chowdhury ST, et al. Encapsulation of the flavonoid quercetin with an arsenic chelator into nanocapsules enables the simultaneous delivery of hydrophobic and hydrophilic drugs with a synergistic effect against chronic arsenic accumulation and oxidative stress. Free Radical Bio Med. 2011;51(10):1893–1902. doi:10.1016/j.freeradbiomed.2011.08.019
45. Puerta E, Suárez-Santiago JE, Santos-Magalhães NS, Ramirez MJ, Irache JM. Effect of the oral administration of nanoencapsulated quercetin on a mouse model of Alzheimer’s disease. Int J Pharm. 2017;517(1–2):50–57. doi:10.1016/j.ijpharm.2016.11.061
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.