Back to Journals » Clinical Ophthalmology » Volume 13
Eye drop emulsion containing 0.1% cyclosporin (1 mg/mL) for the treatment of severe vernal keratoconjunctivitis: an evidence-based review and place in therapy
Authors Nebbioso M , Alisi L , Giovannetti F , Armentano M, Lambiase A
Received 17 April 2019
Accepted for publication 11 June 2019
Published 5 July 2019 Volume 2019:13 Pages 1147—1155
DOI https://doi.org/10.2147/OPTH.S181811
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Marcella Nebbioso, Ludovico Alisi, Francesca Giovannetti, Marta Armentano, Alessandro Lambiase
Department of Sense Organs, Faculty of Medicine and Odontology, Umberto I Policlinic, Sapienza University of Rome, Rome 00185, Italy
Abstract: Vernal keratoconjunctivitis (VKC) is a rare, recurrent and multifactorial ocular disease, which typically flares up during spring and affects especially male children and adolescents. This condition does not usually respond to common treatments with antihistamines or mast cells stabilizers, whereas corticosteroids have effective results. Corticosteroids need to be carefully administered, to avoid adverse effects, mainly the secondary development of glaucoma, cataracts, or infections. Immunosuppressive agents, such as cyclosporin (CyA) or tacrolimus are, therefore, frequently employed in VKC patients. Only the 0.1% CyA (1 mg/mL) concentration has an approved and specific clinical indication for the treatment of VKC and this drug was given the denomination of orphan drug by the European Commission (EU/3/06/360) in 2006. So far, few studies have been conducted to evaluate the efficacy and the side effects of topical 0.1% CyA. Different topical CyA concentrations, ranging from 0.05% to 2%, and various types of formulation are available at the moment. In the future, 0.1% CyA will presumably take an important part in the management of VKC. The present review focuses on eye drops containing 0.1% CyA; however, more studies will be needed to define its long-term efficacy in the natural course of this severe ocular disease.
Keywords: atopy, conjunctivitis, cyclosporin, keratitis, ocular inflammation, vernal keratoconjunctivitis
Introduction
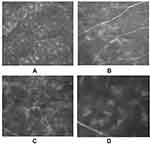
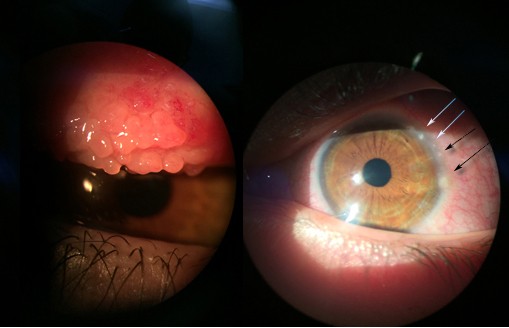
Vernal keratoconjunctivitis (VKC) is a chronic and bilateral inflammation of the ocular surface mostly observed in the pediatric age group. The average age of onset of symptoms is 6–7 years and the male to female ratio is 4:1. The disease tends to resolve during puberty in most cases.1 The prevalence of VKC in Western Europe is estimated to be around 3.2/10,000 inhabitants. The disease is more common in dry and temperate areas, such as the Mediterranean area and central Africa; the prevalence in Italy is 27.8/10,000 inhabitants.2 VKC is usually seasonally exacerbated, in spring or early autumn, but it may also occur as a circannual disease. The most characteristic symptoms are itching, photophobia, watery eyes, foreign body sensation, and mucous secretions. The literature describes four types of VKC: the tarsal form, that affects mostly the tarsal conjunctiva with papillae sometimes with a cobblestone-like appearance; the limbal form, characterized by Horner-Trantas dots and multiple inflammatory nodules (Figure 1); the corneal form, with intense photophobia and alterations highlighted only with confocal microscopy (Figure 2), and the mixed type which has features of the other three types.3,4 Corneal involvement is not common, includes shield ulcers, punctate epithelial keratitis, corneal neovascularization, and can lead to the development of serious complications such as keratoconus or corneal perforation.4
 |
Figure 1 Vernal keratoconjunctivitis (VKC): the tarsal form with giant papillae (left panel) and limbal form with Horner-Trantas dots (right panel, black and white arrows). |
VKC is included in the broad spectrum of allergic conjunctivitis. However, many different factors are involved in its pathogenesis, linked mainly to allergic and autoimmune diseases. Only 50% of VKC patients have a history of systemic atopic sensitization.5 The allergens involved differ geographically and not all of them can account for the seasonal exacerbations.6,7 Studies suggested that both IgE-dependent (type-I allergic) and IgE-independent (type-IV allergic) mechanisms may be involved in the immunopathogenesis of VKC.8 The role of type I allergic reactions has been highlighted by increased levels of IgE in serum and tears and increased number of mast cells in conjunctival tissue.9 An increased number of CD4+ Th2 cells and of their cytokines has been observed in tears and conjunctival biopsies, with the percentage of Th2 lymphocytes being associated with the severity of the disease.10,11
Extensive eosinophilia of the conjunctiva and high levels of IL-5 may contribute to tissue remodeling through the expression of high levels of TGF-β.12,13 A potential role of innate immune response has been highlighted by some authors. In fact, the increase of natural killer cells count and expression of toll-like receptors-4 in conjunctival biopsies have been recently demonstrated.14,15
The association of specific human leukocyte antigen (HLA) classes I and II has been investigated and has led to the finding of a characteristic overexpression of HLA-DRB1 and HLA-DQB1.16
Sex-hormone-related conditions such as gynecomastia have been reported in patients with VKC, suggesting the potential role of hormonal factors. This hypothesis is supported by positive staining for estrogen and progesterone receptors in conjunctiva and frequent resolution of symptoms after puberty.6,17 Moreover, male children with VKC have been shown to have decreased levels of dihydrotestosterone, a hormone able to alter the systemic immune response.18
The role of the immune system is also supported by the reduced levels of Vitamin D found in patients with active VKC and also by the fact that Vitamin D levels appear to be replenished after a few months treatment with 1% cyclosporin A (CyA).19 Several studies have suggested a strong relationship between Vitamin D levels and atopic and immuno-mediated diseases.20,21
Recent studies indicate that IL-17 may play an important role in the pathogenesis of VKC. VKC patients have higher levels of this cytokine compared to healthy subjects. IL-17 is involved in the development of autoimmune inflammatory diseases such as Sjӧgren’s syndrome, rheumatoid arthritis, systemic lupus erythematosus, and others.22,23
Lastly, antinuclear antibodies positivity, even though it is an unspecific parameter, was found in a relatively high proportion of VKC patients and could be linked to the severity of the disease.24–26
The lack of a globally accepted grading system to standardize and classify the severity of VKC determines that the choice of medications varies greatly from physician to physician.
The use of grading scores to rate the severity of VKC and of an algorithm to select the most appropriate medications is crucial for management and to allow more extensive use of steroid-sparing agents such as CyA and tacrolimus.27
Well-defined management guidelines can lead to using less potent medications in the presence of mild disease and to switch to more potent medications for the more severe forms of disease.28
Sacchetti et al provide a five-tier classification for VKC. VKC is defined as quiescent in the absence of symptoms, mild in the presence of symptoms with no corneal involvement moderate (mild plus photophobia), severe (moderate adding superficial punctate keratopathy) and very severe (moderate adding diffuse superficial punctate keratopathy or corneal ulcer).29
Gokhale’s grading system provides a different five-tier classification that does not consider quiescent VKC and divides moderate VKC in intermittent, if the inflammation-free intervals (during which the patient is off medications) last >2–3 months and chronic if they last <1 month. In both classifications, corneal findings are more important than conjunctival findings.27
Several other scores such as the VKC-CLEK have been specifically developed to evaluate epithelial damage in VKC patients.30
Current treatment options for VKC include lubricants, antihistamines, and mast-cell stabilizers commonly used for mild disease.27 Severe to very severe VKC, especially with corneal involvement, is often treated with topical corticosteroids. Side effects of corticosteroid therapy such as glaucoma, cataracts and recurrent infections need to be strictly monitored.31 Lastly, CyA drops (0.05%, 0.1%, 0.5%, 1%, 2%) and tacrolimus (FK-506) 0.03% or 0.1% ointment are being developed for the treatment of VKC, but their role in each grade of VKC is still being evaluated.27,32
Cyclosporin A and currently available topical formulations
CyA is an 11-amino acid polypeptide, metabolite derived from the fungi Beauveria nevus and Tolypocladium inflatum, isolated in the early 1970s and initially used for its antifungal activity.33,34 It was not long before its more relevant immunosuppressive action was discovered. This cyclic hydrophobic neutral drug started to be employed as a treatment in organ transplantation35 and, later on, for inflammatory systemic diseases,36,37 due to the absence of significant bone marrow cytotoxicity.38 Its immunosuppressive role results from the inhibition of calcineurin 2,39,40 determining the block of transcription of nuclear factor of activated T-cells-dependent genes. This factor plays an important role in the synthesis of IL-2, fundamental for T-cell proliferation, IL-4 and CD40 ligand and essential for the activation of B-cell.41,42 CyA is also responsible for the inhibition of mast-cell degranulation and the transcription of IL-3, IL-5 and leukotriene.43
The introduction of CyA in ophthalmology dates back to the 1980s for the prevention of corneal graft rejection44,45 and subsequently for a large variety of inflammatory eye conditions, until it was approved by the United States Food and Drug Administration (FDA)46 in 2005 for the treatment of moderate to severe dry eye disease (DED), as confirmed by the latest DEWS report.77 The recent discovery of the anti-apoptotic effects of CyA has led to its use in ocular surface disorders such as VKC.47,48
From the pharmacokinetic point of view, it has been proved that local instillation of the drug does not require high concentrations of CyA in order to obtain an immunomodulating effect.49 Since there is low or no penetration in the humor aqueous, vitreous and ultimately in plasma, systemic side effects, such as nephrotoxicity and hypertension are practically absent.50,51 The hydrophobic structure of CyA has always represented a challenge, due to its poor aqueous solubility.52 In the form of eye drops, CyA was initially emulsionated using several vegetal oils, causing multiple side effects, ranging from uncomfortable symptoms, as conjunctival chemosis, itching or burning, to epithelial keratitis.53 New formulations, such as oil-in-water emulsion and micelle-based solution, are continuously being developed to avoid the aforementioned side effects.54 Different concentrations ranging from 0.05 to 2 mg/mL are available at the moment in different countries with different clinical indications (Table 1).55 Currently available products, main indications and country of distribution are summarized in Table 1. Finally, galenic formulations are available usually containing CyA in 1% or 2% concentrations in artificial tears.56
 |
Table 1 Most common concentration of CyA eye drops formulations and countries of distribution |
The lack of standardization related to both industrially produced and galenic formulations57 leads to several issues related to the development of reliable studies on CyA efficacy. To this day, the minimum effective concentration of CyA in VKC remains unknown.58
0.1% CyA in the treatment of VKC
VKC is a chronic, multifactorial, and remittent disease in which the activation of the immune system plays an active role. Symptoms can be controlled with steroid therapy, but treatment may be burdened by complications. In order to avoid as much as possible the use of cortisone, immunomodulators such as CyA may be used.59–61
CyA at a concentration of 1 mg/mL (0.1%) has been recently developed and commercialized for the treatment of VKC (Tables 1 and 2).
 |
Table 2 Summary of 0.1% (1 mg/mL) concentration CyA studies in VKC patients |
Currently, only Papilock mini® and Verkazia® both produced by Santen (Osaka, Japan) are specifically indicated for the treatment of VKC (Table 1).
In 2009, Ebihara et al conducted a multicentric study in Japan in which they examined a total number of 594 patients, of whom 320 (median age 16 years) were affected by VKC and 274 by allergic keratoconjunctivitis. Signs and symptoms of disease were evaluated in order to build a 4-point grading scale.62 Topical 0.1% CyA solution was instilled three times daily in 90% of patients, twice daily in 5%, and four times daily in 5%, in one or both eyes. Only the most affected eye was considered for the study. Follow-up lasted 6 months. All scores for symptoms and signs significantly decreased from month 1 to month 6 of treatment in all three groups. Corneal involvement decreased from 21.6% to 8.6% and one-third of the patients were able to discontinue topical steroids. No significant adverse reactions to CyA were reported. The study had some limitations: the study was not randomized, there was no control group, no designated treatment, and there were no inclusion/exclusion criteria. Therefore, the improvement observed in some cases could be associated with the natural course of these diseases, which is characterized by remissions and exacerbations.62
Subsequently in 2010, Baiza-Duran et al published a double-masked, comparative, prospective, multicenter clinical study to evaluate the safety and efficacy of a 0.1% and 0.05% CyA solution in moderate to severe steroid-dependent children with VKC. Overall, 112 patients (mean age 10.2 years) were enrolled in the study after a complete washout period of the steroid therapy. Patients were randomized to one of 2 groups (0.05% or 0.1% CyA) and both eyes were treated simultaneously with one drop in each eye every 12 hrs. Signs and symptoms significantly improved in all patients during the 6 months of the study. Better results were seen in the first 2 months of treatment in the group of patients that received the 0.1% solution, especially when evaluating conjunctival chemosis and foreign body sensation. However, improvement in symptomatology showed no significant difference between the 0.1% group and the 0.05% group after 60 days of treatment. No adverse reactions to CyA were observed during the follow-up period in both concentrations.54 The authors suggest that the comparable efficacy of low CyA concentration may be attributable to the aqueous solution. This allows an increased bioavailability of CyA in the cornea.63 The results were consistent with the results obtained by Ebihara in VKC patients treated with a 0.1% CyA formulation with an aqueous vehicle.62,63
Two years after commercialization of the 0.1% formulation in Japan, Takamura et al followed up for 6 months 2,597 patients with VKC treated with this formulation and confirmed that it was safe and effective. Around 30% of patients who also concomitantly used topical steroids were able to discontinue the steroid treatment. Only mild adverse drug reactions were observed. The main limitation of this study is its observational design and the lack of a placebo control group.64
Lastly, a randomized multicentric Phase III trial, called VEKTIS, published in 2018 compared the efficacy of CyA drops in a cationic emulsion, instilled 4 times daily or 2 times daily, against the vehicle alone, instilled 4 times daily. One-hundred sixty-nine patients (mean age 9.2 years) with active severe VKC and severe keratitis participated. Severe VKC was graded with the Bonini scale (grade 3 or 4),3 severe keratitis with a score of 4 or 5 corneal fluorescein staining (CFS) on the modified Oxford scale.65
Patients were enrolled early during the allergy season in order to extend the follow-up period to 4 months to cover the whole VKC season and were randomized into 3 groups: CyA 4 times daily, CyA 2 times daily, and vehicle 4 times daily. At 4 months follow-up, both the high and low dose groups reported an improvement in symptoms and CFS. Steroid rescue medication use was more frequent in the vehicle only group. CyA therapy also determined an improvement in the subjective perception of disease, evaluated through a quality of life questionnaire.66 The overall frequency of adverse reactions was low and the therapy was well tolerated for the duration of the study. The main limitations of this study are: the enrollment of patients with severe VKC only, the short-term follow-up, and the use of the Oxford grading system, which has been specifically developed to evaluate corneal involvement in DED but not in VKC.67
To the best of our knowledge, no other studies on 0.1% CyA concentration nor any efficacy comparison with other CyA concentrations have been published.
Several studies have been conducted evaluating 2% CyA vs placebo. In all of them, CyA led to the improvement of VKC symptoms and was well tolerated.68,69 Moreover, 1% CyA has been proven effective for the treatment of VKC determining a decrease in the disease score severity after 2 weeks of therapy.70 Lastly, several trials on 0.05% CyA have found a consistent efficacy over placebo70,71 but not as a steroid-sparing therapy.72,73
In summary, 0.1% CyA has been proved to be effective in the treatment of VKC. Studies conducted have shown that patients treated with 0.1% CyA experience improvement of symptoms and signs, and in some cases are able to discontinue the steroid therapy. A consistent number of patients participated in the studies, allowing an adequate analysis of efficacy. However, only few studies have been published using this formulation and in three out of four studies reviewed, the enrolled patients belonged to a limited geographical area and ethnicity (Mexico and Japan). Only the VEKTIS study (2018) was a multicentric trial involving various European and extra-European countries. The follow-up in this study was 12 months, in which the authors analyzed disease severity scores, subjective symptoms and the need for steroid therapy. The follow-up period may be not long enough to allow an adequate evaluation over the course of the treatment because some patients show symptoms only during spring or summer while others present the symptomatology all the year.74,75
Conclusion
VKC is a recurrent disease, with exacerbations and remissions, therefore it is difficult to evaluate the persistence of efficacy over a long period of time. Also, the lack of diagnostic and prognostic biomarkers and of an internationally recognized grading score for VKC makes it difficult to define standardized clinical outcomes. The variability in clinical manifestations and severity of the disease even in the very short term can compromise evaluation of the therapy’s efficacy. Currently, the minimum CyA concentration to control symptoms of VKC is not known. In the future, it is auspicable that new studies be performed to compare 0.1% CyA concentration with higher doses, and using different formulations (such as oil-in-water or aqueous solution, that consistently affect the bioavailability of CyA), in order to establish the most effective and better-tolerated dosage and vehicle.76 Furthermore, a longer follow-up period is needed to evaluate the efficacy of CyA during the long natural course of the disease.
Ultimately, in the future, topical 0.1% CyA can be expected to play an increasingly important role in therapeutic strategies for severe VKC.
Disclosure
The authors have no proprietary interest in any materials or methods described in this article. The authors report no conflicts of interest in this work.
References
1. Nebbioso M, Iannaccone A, Duse M, Aventaggiato M, Bruscolini A, ZicariAM Vascular Endothelial Growth Factor (VEGF) serological and lacrimal signaling in patients affected by Vernal Keratoconjunctivitis (VKC). J Ophthalmol. 2018;2018:1–6. doi: 10.1155/2018/3850172.
2. Bremond-Gignac D, Donadieu J, Leonardi A Prevalence of vernal keratoconjunctivitis: a rare disease? Br J Ophthalmol. 2008;92(8):1097–1102. doi: 10.1136/bjo.2007.117812.
3. Bonini S, Sacchetti M, Mantelli F, Lambiase A Clinical grading of vernal keratoconjunctivitis. Curr Opin Allergy Clin Immunol. 2007;7(5):436–441. doi: 10.1097/ACI.0b013e3282efb726.
4. Nebbioso M, Zicari AM, Lollobrigida V, Marenco M, Duse M Assessment of corneal alterations by confocal microscopy in vernal keratoconjunctivitis. Semin Ophthalmol. 2015;30(1):40–43. doi: 10.3109/08820538.2013.821508.
5. Leonardi A, Busca F, Motterle L, et al. Case series of 406 vernal keratoconjunctivitis patients: a demographic and epidemiological study. Acta Ophthalmol Scand. 2006;84(3):406–410. doi: 10.1111/j.1600-0420.2005.00622.x.
6. Bonini S, Bonini S, Lambiase A, et al. Vernal keratoconjunctivitis revisited: a case series of 195 patients with long-term followup. Ophthalmology. 2000;107(6):1157–1163. doi: 10.1016/S0161-6420(00)00092-0.
7. Kosrirukvongs P, Vichyanond P, Wongsawad W Vernal keratoconjunctivitis in Thailand. Asian Pac J Allergy Immunol. 2003;21(1):25–30.
8. Zicari AM, Zicari A, Nebbioso M et al. High-mobility group box-1 (HMGB-1) and serum soluble receptor for advanced glycation end products (sRAGE) in children affected by vernal keratoconjunctivitis. Pediatr Allergy Immunol. 2014;25(1):57–63. doi: 10.1111/pai.12142.
9. Bozkurt B, Artac H, Arslan N, et al. Systemic atopy and immunoglobulin deficiency in Turkish patients with vernal keratoconjunctivitis. Ocul Immunol Inflamm. 2013;21(1):28–33. doi: 10.3109/09273948.2012.723110.
10. Leonardi A, DeFranchis G, Zancanaro F et al. Identification of local Th2 and Th0 lymphocytes in vernal conjunctivitis by cytokine flow cytometry. Invest Ophthalmol Vis Sci. 1999;40(12):3036–3040.
11. Micera A, Di Zazzo A, Esposito G, Sgrulletta R, Calder VL, Bonini S. Quiescent and active tear protein profiles to predict vernal keratoconjunctivitis reactivation. Biomed Res Int. 2016; 2016:9672082. doi:10.1155/2016/9672082.
12. Leonardi A, Di Stefano A, Motterle L, Zavan B, Abatangelo G, Brun P Transforming growth factor-β/Smad – signalling pathway and conjunctival remodelling in vernal keratoconjunctivitis. Clin Exp Allergy. 2011;41(1):52–60. doi: 10.1111/j.1365-2222.2010.03626.x.
13. Sacchetti M, Bruscolini A, Abicca I, et al. Current and emerging treatment options for vernal keratoconjunctivitis, Expert Opin Orphan Drugs. 2017;5:4, 343–353. doi: 10.1080/21678707.2017.1300524.
14. Lambiase A, Micera A, Sacchetti M, Mantelli F, Bonini S Toll-like receptors in ocular surface diseases: overview and new findings. Clin Sci (Lond). 2011;120(10):441–450. doi: 10.1042/CS20100425.
15. Lambiase A, Normando EM, Vitiello L, et al. Natural killer cells in vernal keratoconjunctivitis. Mol Vis. 2007;13:1562–1567.
16. Zicari AM, Mora B, Lollobrigida V, et al. Immunogenetic investigation in vernal keratoconjunctivitis. Pediatr Allergy Immunol. 2014;25(5):508–510. doi: 10.1111/pai.12231.
17. Bonini S, Lambiase A, Schiavone M, Centofanti M, Palma LA, Bonini S Estrogen and progesterone receptors in vernal keratoconjunctivitis. Ophthalmology. 1995;102(9):1374–1379. doi:10.1016/S0161-6420(95)30861-5.
18. Sacchetti M, Lambiase A, Moretti C, Mantelli F, Bonini S Sex hormones in allergic conjunctivitis: altered levels of circulating androgens and estrogens in children and adolescents with vernal keratoconjunctivitis. J Immunol Res. 2015;2015:945317. doi: 10.1155/2015/945317.
19. Zicari AM, Cafarotti A, Occasi F et al. Vitamin D levels in children affected by vernal keratoconjunctivitis. Curr Med Res Opin. 2017;33(2):269–274. doi: 10.1080/03007995.2016.1254602.
20. Bantz SK, Zhu Z, Zheng T The role of vitamin D in pediatric asthma. Ann Pediatr Child Health. 2015;3(1):1032.
21. McFadden JP, Thyssen JP, Basketter DA, Puangpet P, Kimber I T helper cell 2 immune skewing in pregnancy/early life: chemical exposure and the development of atopic disease and allergy. Br J Dermatol 2015;172(3):584–591. doi: 10.1111/bjd.13497.
22. Zicari AM, Nebbioso M, Zicari A, et al. Serum levels of IL-17 in patients with vernal keratoconjunctivitis: a preliminary report. Eur Rev Med Pharmacol Sci. 2013;17(9):1242–1244.
23. Kim J, Kang S, Kim J, Kwon G, Koo S Elevated levels of T helper 17 cells are associated with disease activity in patients with rheumatoid arthritis. Ann Lab Med. 2012;33(1):52–59. doi:10.3343/alm.2013.33.1.52.
24. Zicari AM, Nebbioso M, Lollobrigida V et al. Vernal keratoconjunctivitis: atopy and autoimmunity. Eur Rev Med Pharmacol Sci. 2013;17(10):1419–1423.
25. Nebbioso M, Zicari AM, Celani C, Lollobrigida V, Grenga R, Duse M Pathogenesis of vernal keratoconjunctivitis and associated factors. Semin Ophthalmol. 2015; 30 (5–6): 340–344. doi: 10.3109/08820538.2013.874483.
26. Malagola R, Arrico L, Migliorini R, D’Ambrosio EM, Grenga R Ocular traumatology in children. A retrospective study. G Chir. 2012; 33 (11–12): 423–428.
27. Gokhale NS Systematic approach to managing vernal keratoconjunctivitis in clinical practice: severity grading system and a treatment algorithm. Indian J Ophthalmol. 2016;64(2):145–148. doi:10.4103/0301-4738.179727.
28. Gokhale NS, Samant R, Sharma V Oral cyclosporine therapy for refractory severe vernal keratoconjunctivitis. Indian J Ophthalmol. 2012;60:220–223. doi: 10.4103/0301-4738.95878.
29. Sacchetti M, Lambiase A, Mantelli F, Deligianni V, Leonardi A, Bonini S Tailored approach to the treatment of vernal keratoconjunctivitis. Ophthalmology. 2010;117(7):1294–1299. doi: 10.1016/j.ophtha.2009.11.043.
30. Leonardi A, Lazzarini D, La Gloria Valerio A, Scalora T, Fregona I Corneal staining patterns in vernal keratoconjunctivitis: the new VKC-CLEK scoring scale. Br J Ophthalmology. 2018;102:1448–1453. doi: 10.1136/bjophthalmol-2017-311171.
31. Mantelli F, Santos MS, Petitti T, et al. Systematic review and meta analysis of randomised clinical trials on topical treatments for vernal keratoconjunctivitis. Br J Ophthalmol. 2007;91(12):1656–1661. doi: 10.1136/bjo.2007.122044.
32. Labcharoenwongs P, Jirapongsananuruk O, Visitsunthorn N, Kosrirukvongs P, Saengin P, Vichyanond P A double-masked comparison of 0.1% tacrolimus ointment and 2% cyclosporine eye drops in the treatment of vernal keratoconjunctivitis in children. Asian Pac J Allergy Immunol. 2012;30:177–184.
33. Borel JF, Feurer C, Magnee C, et al. Effects of the new anti-lymphocytic peptide cyclosporin A in animals. Immunology. 1977;32:1017–1025.
34. Borel JF, Feurer C, Gubler HU, et al. Biological effects of cyclosporin A: a new antilymphocytic agent. Agents Actions. 1976;6:468–475. doi:10.1007/BF01973261
35. Calne RY Immunosuppression for organ grafting – observations on cyclosporin A. Immunol Rev. 1979;46:113–124.
36. Ahern MJ, Harrison W, Hollingsworth P, Bradley J, Laing B, Bauliss C A randomised double-blind trial of cyclosporin and azathioprine in refractory rheumatoid arthritis. Aust N Z J Med. 1991;21(6):844–849.
37. Amor B, Dougados M Cyclosporine: therapeutic effects in rheumatic diseases. Transplant Proc. 1988;20(3 Suppl 4):218–223.
38. Laupacis A, Keown PA, Ulan RA, McKenzie N, Stiller CR, Cyclosporin A. A powerful immunosoprressant. Can Med Assoc J. 1982;126(9):1041–1046.
39. Halloran F, Madrenas J The mechanism of action of cyclosporine: a perspective for the 90’s. Clin Biochem. 1991;24:3–7 doi: 10.1016/0009-9120(91)90063-K.
40. Liu J FK506 and ciclosporin: molecular probes for studying intracellular signal transduction. Trends Pharmacol Sci. 1993;14(5):182–188. doi: 10.1016/0165-6147(93)90206-Y.
41. Ho S, Clipstone N, Timmermann L, et al. The mechanism of action of cyclosporin A and FK506. Clin Immunol Immunopathol. 1996; 80 (3 Pt 2): S40–S5.
42. Cocco L, Rubbini S, Manzoli L, et al. Inositides in the nucleus: presence and characterisation of the isozymes of phospholipasebeta family in NIH 3T3 cells. Biochim Biophys Acta 1999;1438:295–299. doi:10.1016/s1388-1981(99)00061-x
43. Schreiber SL, Crabtree GR The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992;13(4):136–142 doi: 10.1016/0167-5699(92)90111-J.
44. Coster DJ, Shepherd WF, Fook TC, Rice NS, Jones BR Prolonged survival of corneal allografts in rabbits treated with cyclosporin A. Lancet 1979;2:68–89.
45. Hunter PA, Wilhelmus KR, Rice NS, Jones BR Cyclosporin A applied topically to the recipient eye inhibits corneal graft rejection. Clin Exp Immunol 1981;45:173–177.
46. U.S. Food and Drug Administration. [updated April 08, 2005]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/21-023_Restasis.cfm.
47. Donnenfeld E, Pflugfelder SC Topical ophthalmic cyclosporine: pharmacology and clinical uses. Surv Ophthalmol 2009;54:321–338. doi: 10.1016/j.survophthal.2009.02.002
48. Gupta V, Sahu PK Topical cyclosporin A in the management of vernal keratoconjunctivitis. Eye. 2001;15(Pt 1):39–41. doi: 10.1038/eye.2001.10.
49. Levy O, Labbé A, Borderie V, Laroche L, Bouheraoua N La ciclosporine topique en ophtalmologie: farmacologie et indications thérapeutiques. J Fr Ophtalmol. 2016;39(3):292–307. doi:10.1016/j.jfo.2015.11.008
50. Mihatsch MJ, Kyo M, Morozumi K, Yamaguchi Y, Nickeleit V, Ryffel B The side-effects of ciclosporine-A and tacrolimus. Clin.Nephrol. 1998;49:356–363.
51. Ganesan V, Milford DV, Taylo CM, Hulton SA, Parvaresh S, Ramani P Cyclosporin-related nephrotoxicity in children with nephrotic syndrome. Pediatr Nephrol 2002;17:225–226. doi: 10.1007/s00467-001-0810-1.
52. Lallemand F, Felt-Baeyens O, Besseghir K, Behar-Cohen F, Gurny R Cyclosporine A delivery to the eye: a pharmaceutical challenge. Eur J Pharm Biopharm. 2003;56:307–318. doi: 10.1016/S0939-6411(03)00138-3.
53. Tang-Liu DD, Acheampong A Ocular pharmacokinetics and safety of ciclosporin, a novel topical treatment for dry eye. Clin Pharmacokinet. 2005;44:247–261. doi: 10.2165/00003088-200544030-00003.
54. Baiza-Duran LM, González-Villegas AC, Contreras-Rubio Y, et al. Safety and efficacy of topical 0.1% and 0.05% cyclosporine A in an aqueous solution in steroid-dependent vernal keratoconjunctivitis in a population of Mexican children. J Clin Exp Ophthalmol. 2010;1:115. doi:10.4172/2155-9570.1000115.
55. Lallemand F, Schmitt M, Bourges JL, Gurny R, Benita S, Garrigue JS. Cyclosporine A delivery to the eye: a comprehensive review of academic and industrial efforts. Eur J Pharm Biopharm. 2017;117:14–28. doi: 10.1016/j.ejpb.2017.03.006.
56. Kauss Hornecker M, Charles Weber S, Brandely Piat M-L, Darrodes M, Jomaa K, Chast F Cyclosporine eye drops: a 4-year retrospective study (2009–2013). J Fr Ophtalmol 2015;38:700–708. doi: 10.1016/j.jfo.2015.02.008.
57. Labbé A, Baudouin C, Ismail D et al. Pan-European survey of the topical ocular use of cyclosporine A. J Fr Ophtalmol. 2017;40(3):187–195. doi: 10.1016/j.jfo.2016.12.004.
58. Esposito S, Fior G, Mori A, Osnaghi S, Ghiglioni D. An update on the therapeutic approach to vernal keratoconjunctivitis. Pediatric Drugs. 2016;18(5):347–355. doi: 10.1007/s40272-016-0185-1.
59. Leonardi A Management of vernal keratoconjunctivitis. Ophthalmol Ther. 2013;2(2):73–88. doi:10.1007/s40123-013-0019-y.
60. Singhal D, Sahay P, Maharana PK, Raj N, Sharma N, Titiyal JS Vernal keratoconjunctivitis. Surv Ophthalmol. 2018;12.
61. Uchio E, Kimura R, Migita H, Kozawa M, Kadonosono K Demographic aspects of allergic ocular diseases and evaluation of new criteria for clinical assessment of ocular allergy. Graefes Arch Clin Exp Ophthalmol. 2008;246(2):291–296. doi: 10.1007/s00417-007-0697-z.
62. Ebihara N, Ohashi Y, Uchio EA et al. large prospective observational study of novel cyclosporine 0.1% aqueous ophthalmic solution in the treatment of severe allergic conjunctivitis. J Ocul Pharmacol Ther. 2009;25(4):365–372. doi: 10.1089/jop.2008.0103.
63. Quintana-Hau JD, Cruz-Olmos E, López-Sánchez MI, et al. Characterization of the novel ophthalmic drug carrier sophisen in two of its derivatives: 3A ofteno and modusik-A ofteno. Drug Dev Ind Pharm. 2005;31(3):263–269. doi: 10.1081/DDC-52058.
64. Takamura E, Uchio E, Ebihara N, et al. A prospective, observational, all-prescribed-patients study of cyclosporine 0.1% ophthalmic solution in the treatment of vernal keratoconjunctivitis. Nihon Ganka Gakkai Zasshi. 2011;115(6):508–515.
65. Bron AJ, Evans VE, Smith JA Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22(7):640–650.
66. Sacchetti M, Baiardini I, Chini L, Moschese V, Bruscolini A, Lambiase A Development and preliminary validation of a new screening questionnaire for identifying atopic children. Pediatric Health Med Ther. 2017;8:99–105. doi:10.2147/PHMT.S142271.
67. Leonardi A, Doan S, Amrane M, et al. A randomized, controlled trial of cyclosporine A cationic emulsion in pediatric vernal keratoconjunctivitis: the VEKTIS study. Ophthalmology. 2018;27.
68. Kiliç A, Gürler B Topical 2% cyclosporine A in preservative-free artificial tears for the treatment of vernal keratoconjunctivitis. Can J Ophthalmol. 2006;41(6):693–698. doi:10.3129/i06-061.
69. Pucci N, Novembre E, Cianferoni A, et al. Efficacy and safety of cyclosporine eyedrops in vernal keratoconjunctivitis. Ann Allergy Asthma Immunol. 2002;89(3):298–303. doi:10.1016/S1081-1206(10)61958-8.
70. Spadavecchia L, Fanelli P, Tesse R et al. Efficacy of 1.25% and 1% topical cyclosporine in the treatment of severe vernal keratoconjunctivitis in childhood. Pediatr Allergy Immunol. 2006;17(7):527–532. doi: 10.1111/j.1399-3038.2006.00427.x.
71. Lambiase A, Leonardi A, Sacchetti M, et al. Topical cyclosporine prevents seasonal recurrences of vernal keratoconjunctivitis in a randomized, double-masked, controlled 2-year study. J Allergy Clin Immunol. 2011;128(4):896–897. doi: 10.1016/j.jaci.2011.07.004.
72. Keklikci U, Dursun B, Cingu AK Topical cyclosporine a 0.05% eyedrops in the treatment of vernal keratoconjunctivitis-randomized placebo-controlled trial. Adv Clin Exp Med. 2014;23(3):455–461. doi:10.17219/acem/37145
73. Daniell M, Constantinou M, Vu HT, Taylor HR Randomised controlled trial of topical ciclosporin A in steroid dependent allergic conjunctivitis. Br J Ophthalmol. 2006;90(4):461–464. doi:10.1136/bjo.2005.082461.
74. Pescosolido N, Barbato A, Pascarella A, Giannotti R, Genzano M, Nebbioso M Role of protease-inhibitors in ocular diseases. Molecules. 2014;19(12):20557–20569. doi:10.3390/molecules191220557
75. Nebbioso M, Sacchetti M, Bianchi G, et al. Tear ferning test and pathological effects on ocular surface before and after topical cyclosporine in vernal keratoconjunctivitis patients. J Ophthalmol. 2018;2018:1061276. doi:10.1155/2018/1061276.
76. Sacchetti M, Macchi I, Tiezzi A, La Cava M, Massaro-Giordano G, Lambiase A Pathophysiology of corneal dystrophies: from cellular genetic alteration to clinical findings. J Cell Physiol. 2016;231(2):261–269. doi: 10.1002/jcp.25082
77. Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapyreport. Ocul Surf. 2017;15(3):575–628. doi: 10.1016/j.jtos.2017.05.006.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.