Back to Journals » Breast Cancer: Targets and Therapy » Volume 13
Extreme Oncoplasty: Breast Conservation in Patients with Large, Multifocal, and Multicentric Breast Cancer
Authors Savioli F , Seth S, Morrow E, Doughty J, Stallard S, Malyon A, Romics L
Received 2 February 2021
Accepted for publication 22 April 2021
Published 25 May 2021 Volume 2021:13 Pages 353—359
DOI https://doi.org/10.2147/BCTT.S296242
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pranela Rameshwar
Francesca Savioli,1,* Subodh Seth,2,* Elizabeth Morrow,1 Julie Doughty,3 Sheila Stallard,3 Andy Malyon,4 Laszlo Romics1,5
1Academic Unit of Surgery, University of Glasgow, Glasgow Royal Infirmary, Glasgow, UK; 2Department of Breast Surgery, Forth Valley Royal Hospital, Larbert, UK; 3Department of Breast Surgery, Gartnavel General Hospital, Glasgow, UK; 4Canniesburn Department of Plastic Surgery, Glasgow Royal Infirmary, Glasgow, UK; 5Department of Breast Surgery, New Victoria Hospital, Glasgow, UK
*These authors contributed equally to this work
Correspondence: Francesca Savioli Email [email protected]
Introduction: Extreme Oncoplastic Breast Conservation Surgery (EOBCS) is offered in selected patients with multifocal or multicentric breast cancer (MFMC). Recent evidence has suggested that EOBCS may be a valuable resource for patients with MFMC who may avoid the risk associated with mastectomy in favour of the benefits of breast conservation without risking their oncological outcomes. Our study examined the practice of EOBCS in two regional breast units in Glasgow, United Kingdom.
Materials and Methods: A prospectively collected database of 50 patients treated with EOBC in two breast units in Glasgow between 2007 and 2018 were evaluated, and clinical outcomes were observed.
Results: Fifty patients (median age 55) underwent EOBCS, of which 43 (86%) had invasive disease. Median tumour size was 55mm (50– 90) and multifocal disease was identified in 22 (44%) patients. Nine patients (18%) were found to have positive margins and underwent a second procedure, with 6 (12%) proceeding to mastectomy. Five-year disease free survival rate was 91.5%, while cancer-specific survival was 95.7%.
Conclusion: EOBCS is oncologically safe in short-term follow-up. Large scale studies are required to confirm these preliminary results, in order to offer EOBCS as a valid option to patients with advanced or multifocal breast cancer.
Keywords: extreme oncoplasty, therapeutic mammoplasty, breast conservation therapy, multifocal breast cancer, multicentric breast cancer
Plain Language Summary
In this study, Savioli et al report how patients from two hospitals in Glasgow, Scotland, with larger and multifocal breast cancers may remain good candidates for more conservative breast surgery. Multifocal and multicentric cancers are terms used to describe breast cancers with more than one central focus, or with larger, complex structured cancers which typically add up to more than 50mm in size. Previously considered to be at risk of poor results, this study adds to current evidence that by avoiding mastectomy and its associated risks, patients with cancers larger than 50mm in size, or with multifocal cancers may also benefit from conservative surgery (often described as “extreme oncoplastic surgery”) without risking their long term risk of recurrent cancer.
Introduction
Extreme oncoplasty, first coined by Silverstein et al, is used to describe a subset of breast conservation surgery (BCS) offered to patients who would otherwise be expected to require mastectomy due to their tumour characteristics.1 These patients usually fall into two categories: those with cancers of 50mm or above, and those with multifocal/multicentric cancers (MFMC). The change from mastectomy to breast conservation with apparent survival equivalence between these groups, coupled with improved patient satisfaction and cosmesis, suggest that this method may be considered for more advanced or multicentric disease.1–4 In fact, we offer BCS for potentially poorer prognostic single-tumour patients or in-situ disease but continue to exclude larger or MFMC tumours.5 The introduction of screening coupled with ultrasonography (US) and magnetic resonance imaging (MRI) has allowed us to more sensitively identify multifocal and multicentric cancers, making diagnosis of these “extreme” cases more common, thus surgical options more valid for debate.6–8
Oncoplastic breast conservation (OBC) relies on applying plastic surgery principles within the context of sound oncological resection. Volume-reducing or volume-replacing methods are utilised to optimise postoperative appearances whilst prioritizing negative margin resection, supported by prompt adjuvant therapy.9 In fact, it is agreed that in cases where more than 20% of the breast tissue must be excised for oncological margin clearance, oncoplastic surgery is likely to require more complex techniques.10,11 This may allow larger specimens to be resected than in traditional breast conservation surgery, but with comparable histopathological properties to those who would be offered mastectomy.12,13 Previous work has suggested that as such, OBC should be regarded as a separate entity from breast conservation therapy, and that direct comparison with mastectomy outcomes should be performed to inform decision-making amongst clinicians and with patients when planning for surgery.14,15
EOBCS remains a subject of controversy and strong evidence supporting its application is awaited. Study heterogeneity, differences in terminology and classification between multifocal versus multicentric cancers, and variation in outcome measures examined in small studies means that high-powered and standardised research is essential. The current concern is that limitations associated with small studies may tend towards a bias favouring BCS for MFMC cancers due to patient surgical selection bias, favouring patients with better prognostic indicators.16 Currently, larger, randomised studies such as the MIAMI Trial proposes to look at 50 patients undergoing randomised towards mastectomy or BCS (in the form of multiple lumpectomies) and is projected to be completed in 2021.17 Early data from the ACOSOG Z11102 Trial (Alliance) also suggests that 67.6% of patients with multiple ipsilateral breast cancer achieve margin-negative excision at first surgery.17 Finally, de Lorenzi et al have described the safety in offering Oncoplastic BCS for patients with pT2 cancers in a patient-matched cohort when overall and disease-free survival are examined.18 However, we again see limitation in the retrospective nature of the data, considering that patients undergoing mastectomy had significantly larger tumours, and were also more likely to be multifocal.19 Nonetheless, the time to completion of larger studies can anecdotally be supported thanks to this early work, benefitting patients who are currently undergoing treatment. Previous review of outcomes in standard oncoplastic breast surgery in multiple centres across Scotland has shown that practice is comparable to those in high volume centres,15 suggesting this may translate to extreme OBCS (EOBCS). In addition, recent systematic review suggests that absolute tumour size may not preclude BCS, based on published mean tumour size and available outcome figures.20 It is with this in mind that short and long-term outcomes for these patients have been reported1,2,17,21–23 leading us to examine our own practice within two regional breast units in Glasgow, United Kingdom.
Materials and Methods
Patients who underwent BCS in 2 breast units in Glasgow (Victoria Infirmary and Gartnavel and Western Infirmary in Glasgow) between June 2007 and May 2018 were identified. Patients who had tumours 50mm or greater, or were MFMC, were considered to have an indication for EOBCS, and were therefore included in the analysis. Demographic, histopathologic and surgical data was collected and analysed retrospectively. Medical records were assessed for additional information as necessary. All data was subject to Caldicott approval, anonymised at the point of collection and collected retrospectively, together with access to local Managed Clinical Network data.
The decision to proceed to EOBCS or mastectomy, and whether contralateral surgery was warranted was at the discretion of the Breast surgeon and plastic surgeon (if performed in conjunction) and according to patient wishes, with input from the multidisciplinary team (MDT), reflecting practice dating back to earlier years within the study, during which immediate symmetrisation and joint operations were less commonly performed. Surgical oncoplastic techniques included a variety of reduction mammoplasty or local flap techniques, together with contralateral breast symmetrisation where indicated following consultation with the patient and according to degree of volume resection. The technique used varied based on pre-operative anatomy, patient preference, tumour location and possibility of volume replacement. The surgical procedures were performed by several breast specialty consultants.
As this was a retrospective study, in accordance with our local guidelines and the Declaration of Helsinki, the need for consent was waived, and patient data was anonymised at the point of collection, each patient assigned a study number (stored confidentially in a secure database), and all analysis based on study number from then onwards.
Statistical analysis was performed using Microsoft Excel and IBM Statistical Package for Social Sciences version 24 (SPSS, Chicago, IL, USA).
Results
Reports extracted from the our regional (Glasgow) institutional database made within the period June 2007 to May 2018 show a diagnosis of 8580 cases of breast cancer, of whom 4230 had BCS. 304 of these patients had oncoplastic breast surgery. Of these, 50 patients (16%) within this cohort underwent EOBCS for Ct3 or MFMC breast cancer, and were included in this study. These patients were selected if their radiology or pathology results showed multifocal/multicentric cancer, or in which a single lesion was 50mm or more in size. Results are summarised in Table 1. The median age was 55 (range 35–79). Of these, 32 patients were screen-detected, 27 patients were symptomatic, and one was identified via the family history service. Twenty-eight patients had cT3 disease on pre-operative imaging, with a median tumour size of 55 (50–90) mm. Twenty-two patients had MFMC cancers, with the largest lesion being less than 50mm in size, but overall radiological abnormality exceeding 50mm in largest diameter within each patient. The mean BMI of the patients was 31.3kg/m2 (21–44), with 12 patients being considered overweight (BMI 25–30 kg/m2) and 22 patients being obese (BMI >30 kg/m2). Eight patients were current smokers, and 9 patients were ex-smokers.
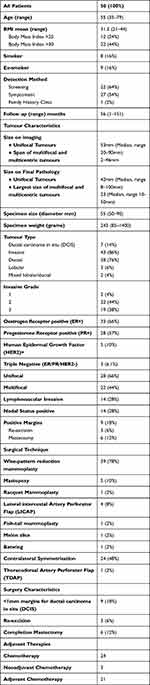 |
Table 1 Population and Tumour Characteristics for Study Cohort |
Forty-five patients were treated with volume displacement reduction mammoplasty. Reduction mammoplasties were carried out using a “Wise” pattern incision in 39 patients, Benelli-type reduction was done in two patients, while tennis-racquet, melon slice, batwing and fish-tail mammoplasties were done in one patient each. Immediate contralateral symmetrisation was carried out in 24 patients. Five patients were treated with volume replacement oncoplastic conservation, of which lateral intercostal artery perforator (LICAP) flap was used in four cases and thoraco-dorsal artery perforator (TDAP) flap in one patient. The median excised specimen weight was 243 (85–1400) grams.
Forty-three patients had invasive cancer and seven patients had ductal carcinoma in situ (DCIS) on final postoperative pathology. Of the invasive cancers, 38 patients had invasive ductal cancer, 3 patients had invasive lobular cancer, and two patients had mixed ductal/lobular cancer. Twenty-two patients had grade 2, while 19 had grade 3 invasive cancers. Thirty-three patients had ER positive disease, and five patients were HER-2 positive. Fourteen patients were node positive.
Fourteen (28%) patients developed surgical complications, but only two of them required reoperation for haematoma (Table 2). Within the patient who developed complications, 5 patients developed haematoma in the breast, 5 had delayed wound healing or skin breakdown, 3 patients developed fat necrosis and 1 patient had cellulitis.
 |
Table 2 Surgical Complication Following Extreme Oncoplastic Breast Conservation and Interventions Required - Major Surgical Complication Identifies Patients Requiring Further Surgery |
Nine patients had incomplete margins (18%), of which three underwent re-excision and six required completion mastectomy. Those who required mastectomy either had multiple margin involvement, or had multiple previous attempts at breast conservation without successful clearance of margins, or in whom there were concerns in confidently recognising the original tumour bed following re-shaping. Twenty-three patients received adjuvant chemotherapy and all received radiotherapy. Three patients received neoadjuvant treatment with no radiological response prior to surgery. Patients who developed complications did not have a delay in the commencement of their adjuvant therapy.
Median follow-up time of all patients was 62 (6–165) months. Forty-nine patients had a minimum follow-up of 13 months. During this follow-up period 5 patients developed distant metastases, of which one also developed local recurrence diagnosed at the time of metastatic presentation. Overall recurrence was therefore 10%. Four (8%) patients had died at time of follow up, of which 3 were due to recurrence of disease, with one further patient who died due to metastatic ovarian cancer. All of the patients with recurrence had tumours >50mm in size rather than a diagnosis of MFMC breast cancer.
Discussion
Extreme oncoplasty must strike a balance between oncological clearance and satisfactory aesthetic outcome. This should not come at the cost of postoperative complications, or survival/disease recurrence disadvantage. Historically, guidance has suggested that MFMC cancers, (together with T4 cancers) should be considered a contraindication to BCS, however recent international consensus unanimously voted that oncoplastic surgery should allow the broadening of indication for BCS for larger or multifocal tumours.4,24 In this study, we describe the short-term outcomes for patients who would normally be offered mastectomy due to the clinical size or multifocal/multicentric nature of their breast cancer and demonstrate that EOBCS is oncologically safe.
Our results are comparable to those in the literature. Previous work by Rosenkranz et al in 2018 suggested that in the case of multiple ipsilateral breast cancers, breast conservation is possible, suggesting rates of 67.6% achievement of negative margins.17 Despite this, conversion to mastectomy remained low (7.1%). It remains to be seen how this impacts on long term survival and recurrence rates, the results of which are as yet awaited as part of the ACOSOG Z11102 (Alliance) Trial.17 Koppiker et al looked at 39 cases of extreme oncoplasty in which routine cavity shaving with frozen section were performed, although follow up is limited to 12 months, and suggested that EOBCS may be an option in patients with larger breasts, particularly when standard BCS may not yield satisfactory results.9,23,25
Within our study cohort, postoperative complications, although affecting more than a quarter of patients, only required significant intervention in 4%. The variability in standard nomenclature for oncoplastic surgeries, and the “tailored” nature of each procedure make direct comparison difficult.4 The complication rates for therapeutic mammaplasty in the literature vary greatly amongst a very heterogeneous group of studies, reviews quoting between 10% and 90% risk of complication.19,26–28 Nevertheless, the complication rate here is comparable to the one we reported earlier in a population-based audit in Scotland.14
Thanks to EOBCS, 88% patients within this study were spared mastectomy, with margin positivity comparable to other studies which have reported rates from 5%–37.8%.20,22,23,28,29 In previous work by Silverstein, the examination of 66 patients with multi-focal/multi-centric cancer or cancers measuring 50mm or above suggests that clear margins could be achieved 83% of the time.1,30 Re-excision was required in 9.1% (six) cases, and mastectomy was eventually required in 6.1% (four) cases. In another study clear margins were achieved in 78.3% (n=87) of patients, while 37.8% (n=42) and 13.5% (n=15) required re-excision for DCIS or invasive cancer in the margins, respectively.28 Mean follow up of 24 months suggested 1.5% (n=1) of patients developed early recurrence, although long term follow up data is still required.1
To reduce the inherent risk associated with advanced, larger tumours, any delay to adjuvant treatment must be avoided.31 BCS does not impact on commencement of adjuvant therapy, including in the case of larger tumours.13,14,21,25,32 This mirrors reports that when compared to BCS and mastectomy (with and without reconstruction) no delays resulted from the use of oncoplastic procedures, although the results are limited by variability in reporting within the literature.21,25,33 Although not formally assessed as part of this study, we did not identify significant delays to adjuvant treatment in this cohort.
Due to the retrospective nature of our data, cosmetic assessment was not available within our study. However, evidence suggests that satisfactory outcomes are possible in extreme oncoplasty. When viewing cosmetic outcomes, Nebril et al report significantly greater satisfaction and quality of life in patients undergoing extreme oncoplastic procedures when compared to non-extreme oncoplastic surgery.20 In a study by Crown et al, 111 patients undergoing extreme oncoplasty were examined and cosmetic outcomes scored and 95% (n=85) patients reported good to excellent cosmesis.28 Complications were reported in 18 patients (16%), but within those, good cosmesis was reported in 93.3% (n=14) of the 15 who were assessed. Future study should regularly evaluate patient reported outcome measures (PROMs) in order to assess not only feasibility and oncological outcomes but also cosmesis to inform decision-making and patient selection.19
Pearce et al have described the use of Latissimus dorsi (LD) miniflaps and therapeutic mammaplasty in patients in the “extreme” subset.29 They describe similar practices of frozen section and intra-operative specimen radiology in 90% and 50% of their LD mini-flap and TM cases respectively. Based on their local recurrence rates at mean follow up, predicted recurrence-free 5-year survival was estimated at as 98% for the entire study cohort, with predicted 5-year and 10-year recurrence rates of 1.1% and 16%. The longer follow up, although limited by the size of the cohort, begins to indicate that the long-term outcomes for these patients may prove to be comparable to those undergoing mastectomy.29
Conclusion
Our study echoes the findings of previous research suggesting that EOBCS should be offered to patients who would usually be exclusively offered mastectomy, with good outcomes at 5-year follow up. Further research should come as part of a large, multi-centre prospective randomised trial, with stratification of pre-operative indications for BCS, perioperative (neo)adjuvant therapy (and factors contributing to delays to adjuvant therapy), and supported by robust, validated assessment of PROMs and long-term survival outcomes. Only then can robust evidence of the feasibility and safety of EOBCS be proven, and this subset of surgery be offered for breast cancer patients and inform the dialogue between patient and the multi-disciplinary care team.
Disclosure
The authors declare no conflicts of interest.
References
1. Silverstein MJ, Savalia N, Khan S, Ryan J. Extreme oncoplasty: breast conservation for patients who need mastectomy. Breast J. 2015;21(1):52–59. doi:10.1111/tbj.12356
2. Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347(16):1227–1232. doi:10.1056/NEJMoa020989
3. Veronesi U, Banfi A, Salvadori B, et al. Breast conservation is the treatment of choice in small breast-cancer - long-term results of a randomized trial. Eur J Cancer. 1990;26(6):668–670. doi:10.1016/0277-5379(90)90113-8
4. Weber WP, Soysal SD, El-Tamer M, et al. First international consensus conference on standardization of oncoplastic breast conserving surgery. Breast Cancer Res Treat. 2017;165(1):139–149. doi:10.1007/s10549-017-4314-5
5. Masannat Y, Agrawal A, Maraqa L, et al. Multifocal and multicentric breast cancer, is it time to think again? AnnRoyal Coll Surg Engl. 2019;1–5.
6. Sardanelli F, Giuseppetti GM, Panizza P, et al. Sensitivity of MRI versus mammography for detecting foci of multifocal, multicentric breast cancer in fatty and dense breasts using the whole-breast pathologic examination as a gold standard. Am J Roentgenol. 2004;183(4):1149–1157. doi:10.2214/ajr.183.4.1831149
7. Coombs B. Multifocal and multicentric breast cancer: does each focus matter? (vol 23, pg 7497, 2005). J Clin Oncol. 2006;24(10):1648.
8. Wilkinson LS, Given-Wilson R, Hall T, Potts H, Sharma AK, Smith E. Increasing the diagnosis of multifocal primary breast cancer by the use of bilateral whole-breast ultrasound. Clin Radiol. 2005;60(5):573–578.
9. Munhoz AM, Montag E, Gemperli R. Oncoplastic breast surgery: indications, techniques and perspectives. Gland Surg. 2013;2(3):143–157. doi:10.3978/j.issn.2227-684X.2013.08.02
10. Mansfield L, Agrawal A, Cutress RI. Oncoplastic breast conserving surgery. Gland Surg. 2013;2(3):158–162. doi:10.3978/j.issn.2227-684X.2013.08.04
11. Mansell J, Weiler-Mithoff E, Martin J, et al. How to compare the oncological safety of oncoplastic breast conservation surgery - To wide local excision or mastectomy? Breast. 2015;24(4):497–501. doi:10.1016/j.breast.2015.05.003
12. Mansell J, Weiler-Mithoff E, Stallard S, Doughty JC, Mallon E, Romics L. Oncoplastic breast conservation surgery is oncologically safe when compared to wide local excision and mastectomy. Breast. 2017;32:179–185. doi:10.1016/j.breast.2017.02.006
13. Morrow ES, Stallard S, Doughty J, et al. Oncoplastic breast conservation occupies a niche between standard breast conservation and mastectomy - A population-based prospective audit in Scotland. EJSO. 2019;45(10):1806–1811. doi:10.1016/j.ejso.2019.03.014
14. Romics L, Macaskill EJ, Fernandez T, et al. A population-based audit of surgical practice and outcomes of oncoplastic breast conservations in Scotland - An analysis of 589 patients. EJSO. 2018;44(7):939–944. doi:10.1016/j.ejso.2018.04.004
15. Winters ZE, Horsnell J, Elvers KT, et al. Systematic review of the impact of breast-conserving surgery on cancer outcomes of multiple ipsilateral breast cancers. Bjs Open. 2018;2(4):162–174. doi:10.1002/bjs5.53
16. Winters Z, Roberts N, McCartan N, et al. 16. Can patients with multiple breast cancers in the same breast avoid mastectomy by having multiple lumpectomies to achieve equivalent rates of local breast cancer recurrence? A randomized controlled feasibility trial called MIAMI UK (NCT03514654). Eur J Surg Oncol. 2019;45(5):881. doi:10.1016/j.ejso.2019.01.202
17. Rosenkranz KM, Ballman K, McCall L, et al. The feasibility of breast-conserving surgery for multiple ipsilateral breast cancer: an initial report from ACOSOG Z11102 (alliance) trial. Ann Surg Oncol. 2018;25(10):2858–2866. doi:10.1245/s10434-018-6583-6
18. De Lorenzi F, Loschi P, Bagnardi V, et al. Oncoplastic breast-conserving surgery for tumors larger than 2 centimeters: is it oncologically safe? A matched-cohort analysis (vol 23, pg 1852, 2016). Ann Surg Oncol. 2016;23:S1056–S. doi:10.1245/s10434-016-5152-0
19. McIntosh J, O’Donoghue JM. Therapeutic mammaplasty–a systematic review of the evidence. Eur J Surg Oncol. 2012;38(3):196–202. doi:10.1016/j.ejso.2011.12.004
20. Nebril BA, Novoa AG, Polidorio N, Garea CC, Alejandro AB, Oses JM. Extreme oncoplasty: the last opportunity for breast conservation-Analysis of its impact on survival and quality of life. Breast J. 2019;25(3):535–536. doi:10.1111/tbj.13267
21. Khan S, Epstein M, Savalia N, Silverstein M. Extreme oncoplasty: breast conservation for patients who traditionally require mastectomy. Ann Surg Oncol. 2018;25:361–362.
22. Khan S, Ryan J, Savalia N, Silverstein JM. Extreme oncoplasty: breast conservation for patients who need mastectomy. Ann Surg Oncol. 2015;22:67–68.
23. Koppiker CB, Ul Noor A, Dixit S, et al. Extreme oncoplastic surgery for multifocal/multicentric and locally advanced breast cancer. Int J Breast Cancer. 2019;2019:1–8. doi:10.1155/2019/4262589
24. Association of Breast Surgery at BASO. Oncoplastic breast surgery–a guide to good practice. Eur J Surg Oncol. 2007;33(Suppl 1):S1–23. doi:10.1016/j.ejso.2007.04.014
25. Campbell EJ, Romics L. Oncological safety and cosmetic outcomes in oncoplastic breast conservation surgery, a review of the best level of evidence literature. Breast Cancer. 2017;9:521–530. doi:10.2147/BCTT.S113742
26. Caruso F, Catanuto G, De Meo L, et al. Outcomes of bilateral mammoplasty for early stage breast cancer. Eur J Surg Oncol. 2008;34(10):1143–1147. doi:10.1016/j.ejso.2007.09.012
27. Giacalone PL, Roger P, Dubon O, et al. Comparative study of the accuracy of breast resection in oncoplastic surgery and quadrantectomy in breast cancer. Ann Surg Oncol. 2007;14(2):605–614. doi:10.1245/s10434-006-9098-5
28. Crown A, Laskin R, Rocha FG, Grumley J. Extreme oncoplasty: expanding indications for breast conservation. Am J Surg. 2019;217(5):851–856. doi:10.1016/j.amjsurg.2019.01.004
29. Pearce BCS, Fiddes RN, Paramanathan N, Chand N, Laws SAM, Rainsbury RM. Extreme oncoplastic conservation is a safe new alternative to mastectomy. Eur J Surg Oncol. 2019;45:913. doi:10.1016/j.ejso.2019.01.129
30. Silverstein MJ. Radical mastectomy to radical conservation (extreme oncoplasty): a revolutionary change. J Am Coll Surg. 2016;222(1):1–9. doi:10.1016/j.jamcollsurg.2015.10.007
31. Chang EI, Peled AW, Foster RD, et al. Evaluating the feasibility of extended partial mastectomy and immediate reduction mammoplasty reconstruction as an alternative to mastectomy. Ann Surg. 2012;255(6):1151–1157. doi:10.1097/SLA.0b013e31824f9769
32. Bamford R, Sutton R, McIntosh J. Therapeutic mammoplasty allows for clear surgical margins in large and multifocal tumours without delaying adjuvant therapy. Breast. 2015;24(2):171–174. doi:10.1016/j.breast.2015.01.003
33. McCulley SJ, Macmillan RD. Therapeutic mammaplasty–analysis of 50 consecutive cases. Br J Plast Surg. 2005;58(7):902–907. doi:10.1016/j.bjps.2005.03.007
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
