Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Extragenital Bullous Lichen Sclerosus Treated with Fractional CO2 Laser (FxCO2) and Wet Dressing of Halcinonide Solution: A Case Report
Authors Yuan Y, Wang CJ , Li H
Received 21 December 2021
Accepted for publication 1 March 2022
Published 10 March 2022 Volume 2022:15 Pages 427—431
DOI https://doi.org/10.2147/CCID.S355111
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Jeffrey Weinberg
Yunyan Yuan, Caroline J Wang, Houmin Li
Department of Dermatology, Peking University People’s Hospital, Beijing, People’s Republic of China
Correspondence: Houmin Li, Tel +86 10 88325472, Email [email protected]
Abstract: Lichen sclerosis (LS) is an insidious, chronic, relapsing skin disease characterized by atrophic, porcelain-appearing plaques. It usually arises in the anogenital area, but some cases can present in extragenital regions with a variety of presentations, including a bullous variant. Topical corticosteroids are a first-line therapy and are usually the most effective treatment to induce remission of LS. However, there is a subset of patients that does not respond well to topical steroids. Herein, we report an extragenital bullous LS case successfully treated with a fractional CO2 laser (FxCO2) and subsequent wet dressing of halcinonide solution.
Keywords: extragenital, bullous, lichen sclerosus, fractional CO2 laser
Introduction
Lichen sclerosus is a chronic inflammatory skin disease that typically presents as polygonal papules that coalesce into ivory-white sclerotic atrophic plaques.1 The true incidence of LS is unknown and possibly underestimated due to providers’ lack of familiarity, asymptomatic presentations, or patient embarrassment.2 Lichen sclerosus affects people of all ages. Male to female ratios have been reported to range between 1:1 and 1:10. Women exhibit a bimodal age distribution, with LS more common in prepubescent and postmenopausal females. In men, the disease typically manifests itself in the fourth decade.3 Classic LS tends to occur in the anogenital region and is characterized by severe pruritus, soreness, dysuria, and dyspareunia. In addition, an increased risk of squamous cell carcinoma has been reported in such patients.1 Isolated extragenital lesions account for only 6% of LS. Unlike typical LS, the extragenital variant is prone to be asymptomatic and has no association with an increased risk of malignancy.4,5 While most patients can be effectively managed with ultrapotent topical steroids, a small proportion of patients with extragenital LS remains recalcitrant to therapy. It has been reported in the literature that FxCO2 therapy may be a sure remedy for patients with genital LS.6–8
Case Report
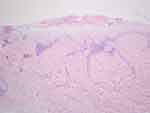
A 41-year-old male presented with asymptomatic sclerotic plaque for 2 years and bullae for 6 months on the right back. Since discovering the lesion, the patient had frequently scratched it automatically. Physical examination revealed an irregular, ill-defined, wrinkled whitish atrophic plaque. A thick-walled intact bulla with an uneven surface and a creased margin was located on the upper part of the lesion. Follicular plugs and desquamation were observed [Figure 1]. The Nikolsky sign was negative. Dermoscopic examination revealed bright white patches, yellowish-white keratotic follicular plugs, linear irregular vessels, and fine white scales [Figure 2]. An incisional biopsy of the bulla revealed a missing epidermis, pale-stained homogenized collagen in the upper dermis, and perivascular inflammatory infiltration of predominantly lymphocytes below the hyalinized area [Figure 3]. ANA testing and the anti-ENA antibody spectrum were negative. Based on the clinical evaluation and biopsy results, the patient was diagnosed with extragenital bullous lichen sclerosus. The patient was treated with mucopolysaccharide polysulfate cream, compound econazole nitrate cream (which contains triamcinolone acetonide 0.1% and econazole nitrate 1%), and tacrolimus cream twice daily for 2 months. Tacrolimus cream was then continued on a daily basis as maintenance therapy. The lesion had improved [Figure 1]. Nonetheless, the patient was not satisfied with the therapeutic outcome and required additional intervention. A fractional CO2 laser with deep FX (fluency of 12.5 mJ/pixel and density of 10%) followed by Active FX (fluency of 50 mJ/pixel and density of 40%), in conjunction with subsequent cold and wet application of Halcinonide Solution for 30 minutes, was then used. The lesion’s visual appearance improved dramatically [Figure 1].
 |
Figure 1 The lesion at time of presentation (A), after topical treatment (B), after FxCO2 in combination with subsequent cold and wet application (C), respectively. |
 |
Figure 2 Dermoscopy reveals bright white patches, yellowish-white keratotic follicular plugs, linear irregular vessels and white scales. |
Discussion
Although the exact pathogenesis of LS remains obscure, autoimmune mechanisms, genetic factors, trauma and chronic irritation, infections of Borrelia burgdorferi or Epstein–Barr virus, and hormonal influences are all considered possible causes.4
Isolated extragenital lichen sclerosus is rare. Clinical manifestations such as blisters may occur infrequently.9 Extragenital lesions tend to occur on the trunk and proximal extremities and are prone to koebnerization in areas of chronic pressure, physical trauma, and scarring.10 Histologic characteristics of LS include epidermal atrophy, follicular plugging, hydropic degeneration of the basal cells, dilated blood vessels, lymphocytic infiltration in the dermis, and edematous homogenized superficial dermis, which may evolve to hyalinization and sclerosus of the superficial dermis. In severe cases, similar to clinical characteristics, a subepidermal blister or hemorrhagic vesicle may be present. The mechanism of bulla development has not been fully explained. Disruption of the dermal-epidermal junction and marked edema in the papillary dermis resulting from intensive vacuolar degeneration of the basal layer contribute to a weakened state. With only minimal trauma or shearing forces from normal movement, the fragile condition coupled with epidermal atrophy may lead to a bullous or hemorrhagic variant of LS.3,11 Dermoscopic evaluation may reveal bright white/white–yellowish patches and yellowish-white keratotic follicular plugs, which correspond to histologically diffuse, dense, homogenous fibrosis/hyalinization of the superficial dermis and follicular plugging, respectively. Additionally, LS may exhibit white scaling, hemorrhagic spots, erythematous areas (focally/localized or diffusely distributed), and red focused vessels, especially linear-irregular or dotted ones.12
A clinical diagnosis can be made when the patient presents with suggestive signs and symptoms. A confirmatory biopsy is recommended if the following conditions exist: atypical features or diagnostic ambiguity, and suspicion of neoplasm.10 Extragenital bullous LS should be differentiated from bullous morphea, bullous lichen planus, cicatricial bullous pemphigoid, and circumscribed lymphangioma.1
However, LS cannot be cured, and treatment can be frustrating. A great number of treatment options have been tried to achieve remission and prevent the progression of the disease. Extragenital bullous LS is a rare variant of LS that is treated similarly to the classic form. The recommended first-line therapies are ultrapotent topical corticosteroids with or without maintenance topical calcineurin inhibitors, intralesional corticosteroids, or oral corticosteroids. Oral agents such as acitretin, methotrexate, and other immunomodulators such as cyclosporine can be used if the patient does not achieve clinical control.3 Along with platelet-rich plasma therapy, the following energy-based modalities have been studied recently in vulvar LS: photodynamic therapy (PDT), fractional CO2 laser (FxCO2) therapy, and high-intensity focused ultrasound (HIFU). However, more studies to determine the efficacy and safety of these emerging treatments are necessary.13 In contrast to classic lichen sclerosus, long-term follow-up is not required in patients with extragenital lichen sclerosus unless systemic medications or phototherapy are used.10
In this case, fractional CO2 laser (FxCO2) combined with subsequent wet dressing of halcinonide solution achieved a good result, indicating that this regimen seems to be a suitable treatment option. Our review of the literature may reveal the mechanism of action for the treatment approach. The CO2 laser ablates the epidermis and superficial dermis. Numerous microscopic wounds on ablated skin not only stimulate collagen formation and remodeling but also allow for better absorption of topical corticosteroids.14,15 However, since the patient was lost to follow-up, more sessions of CO2 laser were not administered. Hence, further studies to validate the efficacy of this treatment option are needed.
Conclusion
The patient reported here presented with isolated bullous lichen sclerosus. It is important for clinicians to be mindful of this rare form of LS. Histological examination is essential when a clear diagnosis cannot be made or the patient fails to respond to treatment. Early detection and treatment are critical for a favorable prognosis. Meanwhile, to achieve satisfactory outcomes, emerging therapies for vulvar LS are worthwhile choices for refractory extragenital LS.
Ethics and Consent Statements
Written informed consent was provided by the patient to have the case details and any accompanying images published. Institutional approval was not required to publish the case details.
Funding
Project 81772160 was supported by the National Natural Science Foundation of China.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Arnold N, Manway M, Stephenson S, Lipkin H. Extragenital bullous lichen sclerosus on the anterior lower extremities: report of a case and literature review. Dermatol Online J. 2017;23(6). doi:10.5070/D3236035393
2. Fergus KB, Lee AW, Baradaran N, et al. Pathophysiology, clinical manifestations, and treatment of lichen sclerosus: a Systematic Review. Urology. 2020;135:11–19. doi:10.1016/j.urology.2019.09.034
3. Sauder MB, Linzon-Smith J, Beecker J. Extragenital bullous lichen sclerosus. J Am Acad Dermatol. 2014;71(5):981–984. doi:10.1016/j.jaad.2014.06.037
4. Fistarol SK, Itin PH. Diagnosis and treatment of lichen sclerosus: an update. Am J Clin Dermatol. 2013;14(1):27–47. doi:10.1007/s40257-012-0006-4
5. Kirtschig G. Lichen sclerosus-presentation, diagnosis and management. Dtsch Arztebl Int. 2016;113(19):337–343. doi:10.3238/arztebl.2016.0337
6. Stewart K, Javaid S, Schallen KP, Bartlett S, Carlson NA. Fractional CO2 laser treatment as adjunctive therapy to topical steroids for managing vulvar lichen sclerosus. Lasers Surg Med. 2022;54(1):138–151. doi:10.1002/lsm.23476
7. Pagano T, Conforti A, Buonfantino C, et al. Effect of rescue fractional microablative CO2 laser on symptoms and sexual dysfunction in women affected by vulvar lichen sclerosus resistant to long-term use of topic corticosteroid: a prospective longitudinal study. Menopause. 2020;27(4):418–422. doi:10.1097/GME.0000000000001482
8. Filippini M, Sozzi J, Farinelli M, Verdelli A. Effects of fractional CO2 laser treatment on patients affected by vulvar lichen sclerosus: a Prospective Study. Photobiomodul Photomed Laser Surg. 2021;39(12):782–788. doi:10.1089/photob.2021.0053
9. Lima RS, Maquiné GÁ, Schettini AP, Santos M. Bullous and hemorrhagic lichen sclerosus–Case report. An Bras Dermatol. 2015;90(3 Suppl 1):118–120. doi:10.1590/abd1806-4841.20153502
10. Lewis FM, Tatnall FM, Velangi SS, et al. British Association of Dermatologists guidelines for the management of lichen sclerosus. Br J Dermatol. 2018;178(4):839–853. doi:10.1111/bjd.16241
11. Shiver M, Papasakelariou C, Brown JA, Wirges M, Kincannon J. Extragenital bullous lichen sclerosus in a pediatric patient: a case report and literature review. Pediatr Dermatol. 2014;31(3):383–385. doi:10.1111/pde.12025
12. Errichetti E, Lallas A, Apalla Z, Di Stefani A, Stinco G. Dermoscopy of morphea and cutaneous lichen sclerosus: clinicopathological correlation study and comparative analysis. Dermatology. 2017;233(6):462–470. doi:10.1159/000484947
13. Krapf JM, Mitchell L, Holton MA, Goldstein AT. Vulvar lichen sclerosus: current perspectives. Int J Womens Health. 2020;12:11–20. doi:10.2147/IJWH.S191200
14. Patel SP, Nguyen HV, Mannschreck D, Redett RJ, Puttgen KB, Stewart FD. FractionalCO2 laser treatment outcomes for pediatric hypertrophic burn scars. J Burn Care Res. 2019;40(4):386–391. doi:10.1093/jbcr/irz046
15. Lee A, Lim A, Fischer G. Fractional carbon dioxide laser in recalcitrant vulval lichen sclerosus. Australas J Dermatol. 2016;57(1):39–43. doi:10.1111/ajd.12305
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.