Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
Extrafine Beclometasone Dipropionate/Formoterol Fumarate vs Double Bronchodilation Therapy in Patients with COPD: A Historical Real-World Non-Inferiority Study
Authors Voorham J , Baldi S , Santoro L, Kerkhof M , Contoli M , Fabbri LM , Kerstjens HAM , Luis López-Campos J , Roche N , Singh D, Vogelmeier CF, Price DB
Received 1 July 2020
Accepted for publication 7 October 2020
Published 29 October 2020 Volume 2020:15 Pages 2739—2750
DOI https://doi.org/10.2147/COPD.S269287
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Jaco Voorham,1,2 Simonetta Baldi,3 Luigi Santoro,4 Marjan Kerkhof,1,5 Marco Contoli,6 Leonardo M Fabbri,6 Huib AM Kerstjens,7 Jose Luis López-Campos,8 Nicolas Roche,9 Dave Singh,10 Claus F Vogelmeier,11 David B Price1,12
1Observational & Pragmatic Research Institute Pte Ltd, Singapore, Singapore; 2Data to Insights Research Solutions, Lisbon, Portugal; 3Department of Global Clinical Development, Chiesi SAS, Bois Colombes Cedex, France; 4Department of Global Clinical Development, Chiesi Farmaceutici S.p.A, Parma, Italy; 5Mescio Research, Blauwestad, The Netherlands; 6Section of Respiratory Medicine, Department of Morphology, Surgery and Experimental Medicine, University of Ferrara, Ferrara, Italy; 7Department of Pulmonary Diseases, University of Groningen and University Medical Center Groningen, Groningen, The Netherlands; 8Respiratory Diseases Unit, University Hospital Virgen Del Rocío, Seville, Spain; 9Service de Pneumologie, Hôpital Cochin, APHP, Centre-Université de Paris, Paris, France; 10University of Manchester, Manchester University NHS Foundation Trust, Manchester, UK; 11Department of Internal Medicine, Pulmonary and Critical Care Medicine, University of Marburg, Member of the German Centre for Lung Research (DZL), Marburg, Germany; 12Centre of Academic Primary Care, Division of Applied Health Sciences, University of Aberdeen, Aberdeen, UK
Correspondence: David B Price
Academic Primary Care, Division of Applied Health Sciences University of Aberdeen, Polwarth Building, Foresterhill, Aberdeen AB25 2ZD 198785, UK
Tel +65 6962 3627
Email [email protected]
Purpose: This study aimed to evaluate the non-inferiority of initiating extrafine beclometasone dipropionate/formoterol fumarate (BDP/FF) versus double bronchodilation (long-acting beta-agonists [LABA]/long-acting muscarinic antagonists [LAMA]) among patients with a history of chronic obstructive pulmonary disease (COPD) exacerbations.
Patients and Methods: A historical cohort study was conducted using data from the UK’s Optimum Patient Care Research Database. Patients with COPD ≥ 40 years at diagnosis were included if they initiated extrafine BDP/FF or any LABA/LAMA double therapy as a step-up from no maintenance therapy or monotherapy with inhaled corticosteroids (ICS), LAMA, or LABA and a history of ≥ 2 moderate/severe exacerbations in the previous two years. The primary outcome was exacerbation rate from therapy initiation until a relevant therapy change or end of follow-up. Secondary outcomes included rate of acute respiratory events, acute oral corticosteroids (OCS) courses, and antibiotic prescriptions with lower respiratory indication, modified Medical Research Council score (mMRC) ≥ 2, and time to first pneumonia diagnosis. The non-inferiority boundary was set at a relative difference of 15% on the ratio scale. Five potential treatment effect modifiers were investigated.
Results: A total of 1735 patients initiated extrafine BDP/FF and 2450 patients initiated LABA/LAMA. The mean age was 70 years, 51% were male, 41% current smokers, and 85% had FEV1 < 80% predicted. Extrafine BDP/FF showed non-inferiority to LABA/LAMA for rate of exacerbations (incidence rate ratio [IRR] = 1.01 [95% CI 0.94– 1.09]), acute respiratory events (IRR = 0.98 [0.92– 1.04]), acute OCS courses (IRR = 1.01 [0.91– 1.11]), and antibiotic prescriptions (IRR = 0.99 [0.90– 1.09]), but not for mMRC (OR = 0.93 [0.69– 1.27]) or risk of pneumonia (HR = 0.50 [0.14– 1.73]). None of the a priori defined effect modifier candidates affected the comparative effectiveness.
Conclusion: This study found that stepping up to extrafine BDP/FF from no maintenance or monotherapy was not inferior to stepping up to double bronchodilation therapy in patients with a history of exacerbations.
Keywords: real-world, electronic health records, observational, comparative effectiveness, heterogeneity, chronic obstructive pulmonary disease
Introduction
The aim of chronic obstructive pulmonary disease (COPD) treatment is to reduce symptoms and the frequency and severity of exacerbations, and to improve health status and exercise tolerance. Long-acting bronchodilators (long-acting muscarinic antagonists [LAMAs] and long-acting beta-agonists [LABAs]) are the mainstay of therapeutic management for COPD; their combined use can result in greater benefits than from either therapy alone.1–5 Inhaled corticosteroids (ICS)/LABA combinations are also more effective than each component alone, especially for exacerbation prevention.6 Furthermore, higher blood eosinophil counts is a biomarker associated with an increased benefit from ICS.7 Therefore, in the Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2020 recommendations, ICS/LABA is indicated as a step-up from bronchodilator monotherapy for exacerbating patients (at least two moderate or one severe exacerbation in the previous year) with a blood eosinophil count above 100 cells/µL.8 However, these recommendations are solely based on insights obtained from randomized clinical trials. The real-world evidence on the effectiveness of ICS/LABA in such patients compared with other treatment combinations remains limited. Since patients in randomized trials are not necessarily representative of the patients with COPD in real life, we need real-world evidence added to the trial insights to ensure optimal disease management recommendations.
The FLAME study showed that indacaterol/glycopyrronium (LABA/LAMA) was more effective than fluticasone/salmeterol in exacerbation prevention in patients with a history of exacerbations.9 Only 19.3% of patients in the FLAME study however, had a history of two or more moderate to severe exacerbations in the previous year. In another trial (IMPACT), different results were reported regarding the respective efficacy of these two therapeutic options.10 This discrepancy could relate to variations in study populations (over 50% of the patients included in the IMPACT study had two or more exacerbations) and/or previous treatment history. The abrupt withdrawal of steroids in patients assigned to the LABA/LAMA arm, in addition to the inclusion of patients with asthma in the IMPACT trial, likely contributed to the rapid increase in exacerbations seen in the LABA/LAMA arm.11
Extrafine formulations of inhaled treatments increase drug delivery to the small airways,12 which may improve the benefit of ICS for exacerbation prevention in COPD, where small airway inflammation is prominent. A unique feature of extrafine beclometasone dipropionate/formoterol fumarate (BDP/FF) combination in a single inhaler is that it may be associated with increased effectiveness13 and lower risk of pneumonia.14 There are no real-world evidence studies on the comparative effectiveness of initiating a fixed-dose combination of extrafine BDP/FF in a single inhaler versus LABA/LAMA.
We aimed to determine whether initiating treatment with an extrafine BDP/FF combination in a single inhaler was at least as effective as double bronchodilator therapy (LABA/LAMA) in terms of reducing COPD exacerbations in patients with COPD and a history of exacerbations in a historical cohort extracted from 2002 to 2019. Several patient characteristics have been identified before as risk factors for disease severity.15–17 Therefore, we also aimed to determine how the comparative effectiveness was modified by the exacerbation burden in the baseline year, most recent blood eosinophil count, degree of airflow limitation, COPD GOLD group, and number of concomitant drugs prescribed in the baseline year.
Materials and Methods
Study Design and Data Source
This was a historical cohort study comparing patients with COPD and a history of exacerbations who initiated extrafine BDP/FF (ICS/LABA) to those who initiated LABA/LAMA double therapy in the United Kingdom (UK; Figure 1). The index date, which was the date of therapy initiation, separated a one-year baseline period and the outcome period. Data were extracted for patients who stepped up to extrafine BDP/FF or LABA/LAMA from either no maintenance therapy, or monotherapy with ICS, LAMA, or LABA between 2002 and 2019. The study used primary care data from the Optimum Patient Care Research Database (OPCRD; https://opcrd.co.uk/). OPCRD contains anonymized, longitudinal medical record data for over eight million patients from over 700 primary care practices. The median follow-up duration of medical records is 13 years. These data contain information on demographic and lifestyle parameters, clinical events, referrals to and feedback from specialists, and prescriptions. It is a high-quality data source used regularly in clinical, epidemiological, and pharmaceutical research.18,19 OPCRD is approved by the Health Research Authority for clinical research use and governed by the Anonymised Data Ethics & Protocol Transparency (ADEPT) Committee, an independent body of experts and regulators commissioned by the Respiratory Effectiveness Group (REG, http://www.effectivenessevaluation.org/). This study was approved by the ADEPT committee (approval reference ADEPT0419).
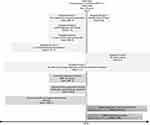 |
Figure 1 Study design. |
Study Population
Patients were included in the study if they met the following criteria: 1) initiated extrafine fixed dose combination ICS/LABA (extrafine BDP/FF) or any LABA/LAMA double therapy, either as a fixed-dose combination or two separate inhalers, as a step-up from no maintenance therapy or monotherapy with ICS, LAMA, or LABA; 2) received the initiated therapy for at least 90 days (to avoid the inclusion of artefacts, temporary medical records, and temporary interruption of one medication class); 3) had a diagnostic Read code for COPD, not followed by a COPD “resolved” code; 4) were aged ≥40 years at index date; 5) had at least one year of continuous practice data prior to the index date (baseline year); and 6) had two or more moderate/severe exacerbations (see Table 1 for definition) in the two years prior to and including the index date. With this 2-year period we optimize the use of available longitudinal data, resulting in greater ability to assess effect modification by exacerbation burden.
 |
Table 1 Study Outcomes and Definitions |
Patients who had active asthma at or after the index date (defined as ≥1 diagnostic Read code for asthma or ≥1 asthma monitoring or review Read code recorded), a Read code for Asthma-COPD overlap syndrome as the COPD diagnostic code, a diagnostic code for other chronic lower respiratory conditions ever recorded, or no evidence of smoking ever were excluded from the analyses. All code lists are available from the authors upon request. Code lists for COPD and asthma were based on version 38 of the Quality and Outcomes Framework business rules, a pay-for-performance scheme active in the UK since 2004, which has resulted in highly accurate recording of diagnoses.20 In line with a recently suggested framework of graphical representation of studies done in health care databases,21 we provide such detailed information in Figure 1.
Patients were divided into two exposure groups: those who initiated extrafine BDP/FF in a single inhaler and those who initiated LABA/LAMA. Where a patient was eligible for both groups, the LABA/LAMA initiation date was used, to avoid a patient being used twice in the analyses. Where multiple initiation dates of the same drug group occurred, the first date was used as the index date in the analyses.
Study Outcomes
The primary outcome was the annualized rate of moderate/severe COPD exacerbations (see Table 1 for definition) during follow-up. Follow-up began on the index date (ie therapy initiation) and continued until the patient left the practice, had a relevant therapy change, or until the last date of the practice’s data collection. In the extrafine BDP/FF group, a relevant therapy change comprised a switch to triple therapy (ICS/LABA/LAMA), a switch to another ICS/LABA, or a switch to no maintenance therapy, LAMA or LABA monotherapy, or LABA/LAMA double therapy when extrafine BDP/FF was not switched back to within six months. This tolerance period of six months was used to avoid exposure misclassification due to apparent small changes in therapy which could be the result of artefacts in the data. In the LABA/LAMA group, a relevant therapy change comprised a switch to an ICS-containing therapy or a switch to no maintenance therapy or LAMA or LABA monotherapy when LABA/LAMA was not switched back to within six months. The secondary outcomes were rate of acute respiratory events, acute oral corticosteroids courses, and antibiotic prescriptions with lower respiratory indication, modified Medical Research Council score (mMRC) within 18 months after index date, and time to first pneumonia diagnosis. Details on the primary and secondary outcomes are presented in Table 1.
Data Analyses
Analyses were performed using Stata MP/6 version 15.1 (StataCorp, College Station, TX). The type and duration of prior maintenance therapies were assessed for all patients included.
Continuous variables were summarized using descriptive statistics of central tendency (mean and median) and dispersion (standard deviation [SD] and interquartile range [IQR]). Categorical variables were described with frequencies and percentages. The standardized mean difference (SMD) was used to quantify differences in both continuous and categorical variables between the groups at baseline. We considered an SMD ≤10% to indicate sufficient balance between the groups.
Inverse probability of treatment (IPT) weighting was used to account for potential biases such as indication bias, as attempts to match the study groups resulted in 29–50% loss in patient numbers and selection of less severely ill patients. A propensity score was generated with a logistic regression model using all available baseline characteristics which had shown an association to the outcome of interest. The propensity score was used to weight the data with the inverse of the treatment probability. Weights above the 99th percentile and below the 1st percentile were truncated at these values. Weighted SMDs were calculated to verify the balancing effect of the IPT weighting approach.
The number of patients who improved (ie, fewer number of exacerbations in the one-year follow-up period compared to the baseline period), remained stable (same number), and worsened (more in the one-year follow-up period than the baseline period) was calculated in those patients with at least one year of follow-up. Unadjusted and IPT weighted changes are reported.
Non-inferiority of initiating extrafine BDP/FF versus double bronchodilators was tested in per protocol analyses. The upper boundary of non-inferiority was set at a relative difference of 15% (corresponding to a hazard ratio [HR], incidence rate ratio [IRR], or odds ratio [OR] for outcomes with extrafine BDP/FF versus LABA/LAMA of 1.15) based on clinical and statistical considerations. The margin was defined on the limit of the 95% confidence interval that is closest to the null effect.22 Clinical judgement was applied to choose the fraction of the null effect that must be preserved by the active drug, in this case 50%. Event rates over the entire follow-up period were analyzed using conditional negative binomial regression, weighted by the logarithm of the duration of follow-up to account for differences in length of follow-up between patients and adjusted for covariates that caused a change in estimate of >2% in the IPT weighted analyses. Given the number of extrafine BDP/FF and LABA/LAMA initiators in this study, there was 90% power, using 5% significance, to detect a minimal difference in exacerbation rate of 10%. Time-to-event analyses were used to analyze the association between treatment and time to first pneumonia diagnosis with right censoring at the time of loss to follow-up or treatment change, adjusted for residual confounders following IPT weighting. To account for multiple testing, Holm’s method23 was used to adjust significance levels for the six primary and secondary outcomes. Patients with a history of asthma and/or a diagnosis of allergic rhinitis were excluded in sensitivity analyses to assess the robustness of the findings with regards to incomplete exclusion of patients with concomitant asthma.
Effect modification was assessed for the following five candidates: exacerbation burden in the baseline year, most recent blood eosinophil count (within five years), degree of airflow limitation (FEV1% predicted), COPD GOLD group (A-D), and number of distinct drugs (apart from COPD drugs) prescribed in the baseline year. Effect modification was tested by introducing an interaction term between exposure and the candidate modifier into the regression models. Significance levels for the interaction terms were adjusted using Holm’s method.
The study population was created in 2018, and thus a large majority of patients had their index date before the changes in COPD treatment recommendations after 2018. More specifically, the 2019 GOLD Report version recommended incorporating the use of peripheral blood eosinophil counts to predict the efficacy of ICS for exacerbation prevention.8 Therefore, we further assessed the effect of blood eosinophil count on the comparative effectiveness, as post hoc analyses, in the following COPD subgroups: a) patients with ≥1 severe or ≥2 moderate exacerbations in the one-year baseline period (1597 LABA/LAMA and 1244 extrafineBDP/FF initiators), b) patients with ≥3 moderate/severe exacerbations in the one-year baseline period (734 LABA/LAMA and 681 extrafine BDP/FF initiators), and c) patients who stepped up from either LAMA or LABA monotherapy (669 LABA/LAMA and 283 extrafine BDP/FF initiators). Please refer to the online Supplementary Materials for further details regarding methodology.
Role of the Funding Source
The funder of the study participated in the study design. All authors, including those employed by the funder of the study, participated in the data interpretation, and writing of the manuscript. All authors had full access to study results and had final responsibility for the decision to submit for publication.
Results
A total of 1735 eligible patients initiated extrafine BDP/FF and 2450 patients initiated LABA/LAMA. A patient flow chart is displayed in Figure 2. Weighted summary statistics of demographic and clinical characteristics of patients who initiated extrafine BDP/FF and those who initiated LABA/LAMA are presented in Table 2 (and Table e1 in the Online Supplementary Materials). Ninety-four percent (73/78) of characteristics were well balanced (SMD≤10%) between treatment groups following weighting. The mean age in both groups was 70 years and around half of patients were male. Over 30% of patients in both groups belonged to GOLD group D at baseline. Unweighted baseline characteristics are presented in supplementary Table e2. Notably, in the baseline year a higher percentage of extrafine BPD/FF initiators experienced from 3 to >5 exacerbations (43.6%) in comparison to LABA/LAMA initiators (33.9%). In the meantime, a higher percentage of LABA/LAMA initiators experienced from 0 to 2 exacerbations (66.1%) in comparison to extrafine BDP/FF initiators (56.6%). This imbalance was confirmed by the standardized mean difference of 23.8.
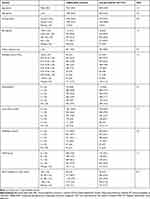 |
Table 2 Baseline Characterization of IPT Weighted Population |
 |
Figure 2 Patient flowchart. |
The duration of therapies prior to the index date and the time patients stayed on the index therapy was similar between patients who initiated extrafine BDP/FF and those who initiated LABA/LAMA. Approximately 38% of patients in each group received refill prescriptions for their index therapy for at least one year. The duration of follow-up was 1.5 years for both groups.
The number of patients who improved, remained stable, or worsened from the baseline year to the first follow-up year for each outcome variable is displayed in Table 3. Both treatment groups showed a reduction in the number of exacerbations from the baseline to the outcome year (61% of extrafine BDP/FF and 58% of LABA/LAMA initiators). The percentage of extrafine BDP/FF initiators who worsened was 21.5% compared to 23.5% of LABA/LAMA initiators (Table 3).
 |
Table 3 Number of Patients Who Improved, Remained Stable, or Worsened from 1-Year Baseline to the First Outcome Year |
Multivariable Outcome Models
Extrafine BDP/FF was non-inferior to LABA/LAMA for the primary outcome in the weighted model; the upper confidence limit for exacerbation rate of 1.09 was below the prespecified non-inferiority margin of 1.15 (IRR = 1.01 [95% CI 0.94–1.09]; Figure 3). Extrafine BDP/FF was also non-inferior to LABA/LAMA for secondary outcomes including rate of acute respiratory events (IRR = 0.98 [0.92–1.04]), acute OCS courses (IRR = 1.01 [0.91–1.11]), and antibiotic prescriptions with evidence of lower respiratory indication (IRR = 0.99 [0.90–1.09]; Figure 3). However, non-inferiority was not achieved for the secondary outcomes of having an mMRC score ≥2 (HR = 0.96 [0.74–1.23]) and risk of a pneumonia infection (HR = 0.50 [0.14–1.73]). See Table e3 in the Online Supplementary Materials for unadjusted data on follow-up and outcomes. Results were similar when patients with a history of asthma and/or rhinitis were excluded in sensitivity analyses (see Figure e1 in the Online Supplementary Materials).
 |
Figure 3 Model results, all patients. |
Effect Modification
There was no evidence that any of the a priori candidate modifiers (blood eosinophil count, exacerbations, GOLD group, FEV1% predicted, other medication burden) significantly changed the comparative effectiveness between extrafine BDP/FF and LABA/LAMA. The results from assessment of effect modification of the modifier candidates using the primary and secondary outcomes is shown in Table e4 as p-values of the interaction term in the adjusted outcome models corrected for multiple testing. In subgroup analyses, no indication that blood eosinophil count was predictive of the rate of exacerbations during follow-up in the extrafine BDP/FF group was found. However, LABA/LAMA tended to lose efficacy in reducing exacerbations with increasing eosinophil count. In the subgroup of patients that came from LAMA or LABA monotherapy, blood eosinophil count showed a clear trend (p=0.051) of comparative effectiveness based on the number of SABA inhalers used in favor of extrafine BDP/FF with increasing eosinophil count. See the Online Supplementary Materials for results of effect modification in the subgroup analyses (Figures e2–e3).
Discussion
This historical real-world observational study showed similar exacerbation reduction from the baseline to the first outcome year in patients who initiated extrafine BDP/FF and those who initiated LABA/LAMA from no maintenance or monotherapy. Stepping up to extrafine BDP/FF was not inferior to stepping up to double bronchodilation therapy in patients with a history of exacerbations.
Our non-inferiority finding is in agreement with another real-world study comparing LABA/LAMA to any ICS/LABA initiators.24 Our results are in contrast to the finding of superiority of indacaterol/glycopyrronium (LABA/LAMA) over fluticasone/salmeterol (ICS/LABA) in exacerbation reduction in the FLAME study.9 Our study population had a comparable exacerbation burden (≥2 in a 2-year baseline vs ≥1 in a 1-year period in the trial), but we compared different compounds. This might suggest that the extrafine particle size of BDP/FF needs to be taken into account when evaluating the choice between double bronchodilation therapy and ICS/LABA.
For all outcomes (rates of exacerbations, acute OCS courses, antibiotics courses, and acute respiratory events) non-inferiority of extrafine BDP/FF could be claimed with the exception of having an mMRC score ≥2 and time until a pneumonia infection. The number of pneumonia cases during follow-up was low (n = 15); four with BDP/FF and 11 with LABA/LAMA. This could relate to the molecular characteristics of FF or to the extrafine BDP/FF formulation increasing the volume of drug distribution within the bronchial tree. The pneumonia incidence rate was 0.25 cases per 100 patient-years (in the IMPACT study there was an incidence rate of between 6–10 cases per 100 patient-years10), resulting in low statistical power as well as the inability to check models for residual confounding. Similarly, for MRC dyspnoea scale the OR is 0.96. However, the 95% CI is wide, and its upper limit crosses the non-inferiority margin. The result is not unexpected as MRC scores are variably associated with patients’ perceptions of respiratory symptom burden or disease severity.25 None of the candidate modifiers (blood eosinophil count, exacerbations, GOLD group, FEV1% predicted, other medication burden) significantly changed the comparative effectiveness. Although extrafine BDP/FF is licensed for patients with COPD and a FEV1% predicted below 50% and a history of repeated exacerbations,26 we did not find evidence that the comparative effectiveness was different depending on the FEV1% predicted and exacerbation rate in the one-year baseline period. Missing FEV1 data may have resulted in loss of power and dilution of any possible effect modification of baseline FEV1 on the outcomes after initiation of treatments.
We did not find a significant impact of difference of eosinophil count on the comparative effectiveness, despite a trend when comparing the two treatments in a subpopulation of patients stepping up from LAMA or LABA monotherapy, the patients the treatment guidelines8 refer to. In addition, in these patients a trend in favor of extrafine BDP/FF on SABA reduction with increasing eosinophil count exists. The limited number of patients analyzed is preventing us reaching the conclusions provided by Suissa et al, who analyzed a population-based cohort of 12,366 initiators of LAMAs (mainly tiotropium) matched to 12,366 initiators of LABA-ICS.27 They found that initial treatment with ICS/LABAs was only more effective than with LAMAs in patients with high blood eosinophil counts.
We used the most recently recorded blood eosinophil count within five years. Almost 45% of the eosinophil counts were recorded within 6 months prior to the index date, 66% within one year, and only 16% were recorded more than two years earlier. Some studies on stability of blood eosinophil counts over time have shown that values remain reasonably stable over a period of two years.28,29 Therefore, we do not think this has affected our results.
Our data indicate that treatment exposure lasted 1-year in approximately 38% of patients. It is a higher percentage in comparison to figures reported by Suissa et al, who found that 67% of patients in the LABA/LAMA group and 72% in the ICS/LABA group discontinued the treatment after 3.3 months.24
The strengths of our study include the large cohort of patients from a real-world setting, representative of the UK population, making our results likely generalizable to the wider COPD population. Some limitations however also need consideration. The OPCRD dataset represents information collected for clinical and routine use rather than specifically for research purposes but reflects real-world prescribing practices. However, extensive quality control and validity checks are conducted at practice level. Also, the study had limited power to detect differences in pneumonia events due to low incidence rates. Finally, we could only adjust for confounding by measured and considered baseline characteristics of the patients. Thus, we cannot guarantee that our results are unbiased; however, as the most important disease severity indicators were used for propensity score estimation, it is unlikely that any residual bias is large.
Conclusion
In summary, this observational study found that stepping up to extrafine BDP/FF from no maintenance or monotherapy was not inferior to stepping up to double bronchodilation therapy in patients with a history of COPD exacerbations. The study did not identify patient factors that relevantly modified the comparative effectiveness. Notably, the finding that the FEV1% predicted did not modify the comparative effectiveness calls for studies to explore the possibility to indicate extrafine BDP/FF for a broader target population. Overall, these real-world findings confirm how extrafine ICS/LABA fits into maintenance therapy of COPD patients at risk of exacerbations. Eosinophilia should be considered a continuum in therapeutic decision making; however, more studies are needed to better understand how blood eosinophil count interacts with exacerbation burden and treatment choice on exacerbation reduction in larger real-world cohorts.
Abbreviations
ADEPT, Anonymised Data Ethics & Protocol Transparency; BDP, beclometasone dipropionate; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in one second; FF, formoterol fumarate; HR, hazard ratio; ICS, inhaled corticosteroids; IPT, inverse probability of treatment; IQR, interquartile range; IRR, incidence rate ratio; LABA, long-acting beta-agonists; LAMA, long-acting muscarinic antagonists; mMRC, Modified Medical Research Council Dyspnea Scale; NNB, number needed to benefit; OCS, oral corticosteroids; OPCRD, Optimum Patient Care Research Database; OR, odds ratio; SABA, short-acting beta-2 agonists; SD, standard deviation; SMD, standardized mean difference.
Data Sharing Statement
The dataset supporting the conclusions of this article was derived from the Optimum Patient Care Research Database (www.opcrd.co.uk). The OPCRD has ethical approval from the National Health Service (NHS) Research Authority to hold and process anonymised research data (Research Ethics Committee reference: 15/EM/0150). This study was approved by the Anonymised Data Ethics Protocols and Transparency (ADEPT) committee – the independent scientific advisory committee for the OPCRD. The authors do not have permission to give public access to the study dataset; researchers may request access to OPCRD data for their own purposes. Access to OCPRD can be made via the OCPRD website (https://opcrd.co.uk/our-database/data-requests/) or via the enquiries email [email protected].
Ethics Approval
OPCRD has received a favorable opinion for clinical research use from the Health Research Authority (REC reference: 15/EM/0150). Governance is provided by the Anonymous Data Ethics Protocols and Transparency (ADEPT) committee, an independent body of experts and regulators commissioned by the Respiratory Effectiveness Group (REG, http://www.effectivenessevaluation.org/) to govern the standard of research conducted on internationally recognized databases. This study was approved by the ADEPT committee (approval reference ADEPT0419) and registered with the European Union electronic Register of Post-Authorization Studies (EUPAS Register number 29223).
Acknowledgments
Dave Singh is supported by the National Institute for Health Research (NIHR). Writing and editorial support was provided by Dr Julia Granerod, supported by the Observational and Pragmatic Research Institute Pte. Ltd (OPRI).
Disclosure
JV and MK were employees of the Observational and Pragmatic Research Institute at the time of the study, which conducted this study and conducted paid research in respiratory disease on behalf of the following other organisations in the past 5 years: Aerocrine, AKL Research and Development Ltd, Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Mylan, Mundipharma, Napp, Novartis, Orion, Takeda, Teva, Zentiva (a Sanofi company). SB is a full time employee at CHIESI SAS France. LS is a full-time employee at Chiesi Farmaceutici, Parma, Italy. MC reports grants from Chiesi and University of Ferrara, Italy, outside the submitted work, and personal fees from Chiesi, AstraZeneca, Boehringer Ingelheim, Alk-Abello, GlaxoSmithKline, Novartis, Zambon. LMF reports lecture fees and/or consultancies from Alfasigma, AstraZeneca, Chiesi, Boehringer Ingelheim, GlaxoSmithKline, Lusofarmaco, Merck, Novartis, Zambon, and Verona Pharma. HAMK reports grants and consultancy/advisory board participation from/for AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, and Novartis, all outside the submitted work. All were paid to his institution. JLLC has received over the last 3 year honoraria for lecturing, scientific advice, participation in clinical studies or writing for publications for: AstraZeneca, Boehringer Ingelheim, Chiesi, CSL Behring, Esteve, Ferrer, Gebro, GlaxoSmithKline, Grifols, Menarini, Novartis, Rovi, and Teva. NR reports grants and personal fees from Boehringer Ingelheim, Novartis, and Pfizer, and personal fees from Teva, GSK, AstraZeneca, Chiesi, Sanofi, Trudell, and Zambon. DS has received personal fees from GSK, Cipla, Genentech and Peptinnovate, and personal fees and grant support from AstraZeneca, Boehringer Ingelheim, Chiesi, Glenmark, Gossamerbio, Menarini, Mundipharma, Novartis, Pfizer, Pulmatrix, Theravance and Verona. CFV gave presentations at symposia and/or served on scientific advisory boards sponsored by AstraZeneca, Boehringer Ingelheim, CSL Behring, Chiesi, GlaxoSmithKline, Grifols, Menarini, Novartis, Nuvaira, OmniaMed, and MedUpdate. DBP has board membership with Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia, Mylan, Mundipharma, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, Teva Pharmaceuticals, Thermofisher; consultancy agreements with Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Mylan, Mundipharma, Novartis, Pfizer, Teva Pharmaceuticals, Theravance; grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia, Mylan, Mundipharma, Novartis, Pfizer, Regeneron Pharmaceuticals, Respiratory Effectiveness Group, Sanofi Genzyme, Teva Pharmaceuticals, Theravance, UK National Health Service; payment for lectures/speaking engagements from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Mylan, Mundipharma, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, Teva Pharmaceuticals; payment for the development of educational materials from Mundipharma, Novartis; payment for travel/accommodation/meeting expenses from AstraZeneca, Boehringer Ingelheim, Mundipharma, Mylan, Novartis, Thermofisher; funding for patient enrolment or completion of research from Novartis; stock/stock options from AKL Research and Development Ltd which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd (Australia and UK) and 74% of Observational and Pragmatic Research Institute Pte Ltd (Singapore); 5% shareholding in Timestamp which develops adherence monitoring technology; is peer reviewer for grant committees of the Efficacy and Mechanism Evaluation programme and Health Technology Assessment; and was an expert witness for GlaxoSmithKline. The authors report no other conflicts of interest in this work.
References
1. Tashkin DP, Pearle J, Iezzoni D, et al. Formoterol and tiotropium compared with tiotropium alone for treatment of COPD. COPD. 2009;6(1):17–25.
2. van Noord JA, Aumann JL, Janssens E, et al. Combining tiotropium and salmeterol in COPD: effects on airflow obstruction and symptoms. Respir Med. 2010;104(7):995–1004. doi:10.1016/j.rmed.2010.02.017
3. van Noord JA, Aumann JL, Janssens E, et al. Effects of tiotropium with and without formoterol on airflow obstruction and resting hyperinflation in patients with COPD. Chest. 2006;129(3):509–517. doi:10.1378/chest.129.3.509
4. Price D, Ostrem A, Thomas M, et al. Dual bronchodilation in COPD: lung function and patient-reported outcomes – a review. Int J Chron Obstruct Pulmon Dis. 2017;12:141–168. doi:10.2147/COPD.S116719
5. Nici L, Mammen MJ, Charbek E, et al. Pharmacologic management of chronic obstructive pulmonary disease: an official American thoracic society clinical practice guideline. Am J Respir Crit Care Med. 2020;201(9):e56–e69. doi:10.1164/rccm.202003-0625ST
6. Tashkin DP, Strange C. Inhaled corticosteroids for chronic obstructive pulmonary disease: what is their role in therapy? Int J Chron Obstruct Pulmon Dis. 2018;13:2587–2601. doi:10.2147/COPD.S172240
7. Siddiqui SH, Pavord ID, Barnes NC, et al. Blood eosinophils: a biomarker of COPD exacerbation reduction with inhaled corticosteroids. Int J Chron Obstruct Pulmon Dis. 2018;13:3669–3676. doi:10.2147/COPD.S179425
8. GOLD. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease - 2019 report. Chronic Obstr Pulm Dis. 2019.
9. Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med. 2016;374(23):2222–2234. doi:10.1056/NEJMoa1516385
10. Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–1680.
11. Suissa S, Drazen JM. Making sense of triple inhaled therapy for COPD. N Engl J Med. 2018;378(18):1723–1724. doi:10.1056/NEJMe1716802
12. Bousquet J, Poli G, Acerbi D, et al. Systemic exposure and implications for lung deposition with an extra-fine hydrofluoroalkane beclometasone dipropionate/formoterol fixed combination. Clin Pharmacokinet. 2009;48:347–358.
13. Postma DS, Roche N, Colice G, et al. Comparing the effectiveness of small-particle versus large-particle inhaled corticosteroid in COPD. Int J Chron Obstruct Pulmon Dis. 2014;9:1163–1186.
14. Sonnappa S, Martin R, Israel E, et al. Risk of pneumonia in obstructive lung disease: a real-life study comparing extrafine and fine-particle inhaled corticosteroids. PLoS One. 2017;12:e0178112.
15. Kerkhof M, Sonnappa S, Postma DS, et al. Blood eosinophil count and exacerbation risk in patients with COPD. Eur Respir J. 2017;50(1).
16. Bafadhel M, Peterson S, De Blas MA, et al. Predictors of exacerbation risk and response to budesonide in patients with chronic obstructive pulmonary disease: a post-hoc analysis of three randomised trials. Lancet Respir Med. 2018;6(2):117–126. doi:10.1016/S2213-2600(18)30006-7
17. Kerkhof M, Freeman D, Jones R, Chisholm A, Price DB. Predicting frequent COPD exacerbations using primary care data. Int J Chron Obstruct Pulmon Dis. 2015;10:2439–2450.
18. Belhassen M, Nibber A, Van Ganse E, et al. Inappropriate asthma therapy-a tale of two countries: a parallel population-based cohort study. NPJ Prim Care Respir Med. 2016;26(1):16076. doi:10.1038/npjpcrm.2016.76
19. Halpin DM, Kerkhof M, Soriano JB, et al. Eligibility of real-life patients with COPD for inclusion in trials of inhaled long-acting bronchodilator therapy. Respir Res. 2016;17(1):120. doi:10.1186/s12931-016-0433-5
20. Quint JK, Mullerova H, DiSantostefano RL, et al. Validation of chronic obstructive pulmonary disease recording in the clinical practice research datalink (CPRD-GOLD). BMJ Open. 2014;4(7):e005540. doi:10.1136/bmjopen-2014-005540
21. Schneeweiss S, Rassen JA, Brown JS, et al. Graphical depiction of longitudinal study designs in health care databases. Ann Intern Med. 2019;170(6):398–406. doi:10.7326/M18-3079
22. Althunian TA, de Boer A, Groenwold RH, et al. Defining the noninferiority margin and analysing noninferiority: an overview. Br J Clin Pharmacol. 2017;83(8):1636–1642. doi:10.1111/bcp.13280
23. Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70.
24. Suissa S, Dell’Aniello S, Ernst P. Comparative effectiveness and safety of LABA-LAMA vs LABA-ICS treatment of COPD in real-world clinical practice. Chest. 2019;155(6):1158–1165. doi:10.1016/j.chest.2019.03.005
25. Rennard S, Decramer M, Calverley PM, et al. Impact of COPD in North America and Europe in 2000: subjects’ perspective of confronting COPD international survey. Eur Respir J. 2002;20(4):
26. (eMC) TeMC. Foster 100/6 Inhalation Solution - Summary of Product Characteristics. 2018.
27. Suissa S, Dell’Aniello S, Ernst P. Comparative effectiveness of LABA-ICS versus LAMA as initial treatment in COPD targeted by blood eosinophils: a population-based cohort study. Lancet Respir Med. 2018;6(11):855–862. doi:10.1016/S2213-2600(18)30368-0
28. Southworth T, Beech G, Foden P, et al. The reproducibility of COPD blood eosinophil counts. Eur Respir J. 2018;52(1):1800427. doi:10.1183/13993003.00427-2018
29. Landis SH, Suruki R, Hilton E, Compton C, Galwey NW. Stability of blood eosinophil count in patients with COPD in the UK clinical practice research datalink. COPD. 2017;14(4):382–388. doi:10.1080/15412555.2017.1313827
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
