Back to Journals » International Journal of Nanomedicine » Volume 15
Extracellular Vesicles – Advanced Nanocarriers in Cancer Therapy: Progress and Achievements
Authors Huyan T, Li H, Peng H, Chen J, Yang R, Zhang W, Li Q
Received 11 November 2019
Accepted for publication 8 July 2020
Published 26 August 2020 Volume 2020:15 Pages 6485—6502
DOI https://doi.org/10.2147/IJN.S238099
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Thomas Webster
Ting Huyan,1,2 Hongduo Li,3 Hourong Peng,1 Jinzhao Chen,4 Ruixin Yang,3 Wei Zhang,5 Qi Li1
1Key Laboratory for Space Biosciences and Biotechnology, Institute of Special Environment Biophysics, School of Life Sciences, Northwestern Polytechnical University, Xi’an 710072, People’s Republic of China; 2Institute of Flexible Electronics (IFE), Northwestern Polytechnical University, Xi’an 710072, People’s Republic of China; 3Xi’an Institute for Food and Drug Control, Xi’an 710054, People’s Republic of China; 4Shanxi Weiqidaguangming Pharmaceutical Co., Ltd, Datong, Shanxi Province 037301, People’s Republic of China; 5Department of Anesthesiology, Henan Provincial People’s Hospital (People’s Hospital of Zhengzhou University), Zhengzhou 450003, People’s Republic of China
Correspondence: Qi Li Key
Laboratory for Space Biosciences and Biotechnology, Institute of Special Environment Biophysics, School of Life Sciences, Northwestern Polytechnical University, Xi’an 710072, People’s Republic of China
Tel/ Fax +86 29 8846 0332
Email [email protected]
Wei Zhang
Department of Anesthesiology, Henan Provincial People’s Hospital (People’s Hospital of Zhengzhou University), Zhengzhou 450003, People’s Republic of China
Tel +86 371 6558 0014
Fax +86 371 6596 4376
Email [email protected]
Abstract: Extracellular vesicles (EVs) are a class of cell-derived, lipid bilayer membrane composed vesicles, and some of them such as exosomes and ectosomes have been proven, playing remarkable roles in transmitting intercellular information, and being involved in each property of cell physiological activities. Nowadays, EVs are considered as potential nanocarriers which could partially resolve the problems of current chemotherapy because of their distinctive advantages. As endogenous membrane encompassed vesicles with nanosize, EVs are able to pass through the natural barriers with prolonged circulation time in vivo and have intrinsic cell targeting properties, they are less toxic, and less immunogenic. Recently, studies focusing on EV-based drug delivery system for cancer therapy have exploded dramatically. This review aims to outline the current applications of EVs as potential nanosized drug carriers in cancer therapy. Firstly, the characteristics and biofunctions of each EV subtype are described. Then the variety of therapeutic cargoes, the loading methods, and the targeting strategy of engineered EVs are emphatically introduced. Thereafter the pros and cons of EVs applied as therapeutic carriers, as well as the future prospects in this field, are discussed.
Keywords: Extracellular vesicles, exosomes, nano-sized carriers, tumor, drug delivery system
Introduction
Cancer is one of the most destructive diseases with a high recurrence and mortality rate and has become the greatest health problem worldwide.1 The global cancer situation is dire due to belated diagnosis, drug resistance, metastasis, and poor prognosis.2 So far chemotherapy is one of the predominant treatments of cancer therapy, which has made numerous progressions and prolonged tens of millions of lives. However, there are still inherent limitations of chemotherapy, including low efficacy, nonspecific distribution, and other undesirable adverse effects.3–5 Hence, methodological improvements, such as developing a targeted, efficient, and safe drug delivery system (DDS) applied in chemotherapy are urgently required.
The key challenge impacting the chemotherapy effect is how to deliver specific drugs to the tumor site and alleviate the side effects to normal tissue and cells. Efforts have been made to solve this problem such as preparing nanosized drug carriers, which are artificial nanoparticles (NPs) embedded with various drugs, including chemotherapeutic drugs, therapeutic proteins or nucleic acids, to deliver the drugs to disease sites.6 Several types of artificial NPs like liposomes, micelles, dendrimer, carbon nanotube, polymeric nanoparticles, magnetic nanoparticles, etc have been made and used in delivering various anticancer drugs.7–11 Compared to traditional administration methods, such as oral administration or intravenous administration of free drug, NPs are able to deliver high doses of therapeutic drugs into the tumor site while bypassing healthy cells because of their enhanced permeability and retention (EPR) effect.12 However, there are inevitable disadvantages of artificial NPs which limited their clinical application. For example, some metal NPs show embryo toxicity after administration;13 artificial NPs have nonuniform particle size and are prone to form agglomerates which can be eliminated rapidly by the mononuclear phagocyte system (MPS), as well as their nonspecificity targeting effort in vivo.2,14,15 Thereupon, endogenous membrane vesicles with nanoscale have received increasing attention. Extracellular vesicles (EVs) are cell-derived lipid bilayer composed vesicles released by almost all living cells. Although EVs were previously considered as cellular trash, they actually are natural information carriers and play important roles in intercellular communication via shuttling biofunctional cargoes between cells. In addition to the physical properties of NP, EVs have additional advantages such as excellent biodegradability, biocompatibility as well as sequence programmability, and have showed ideal prospects as drug delivery carriers in cancer therapy.14
Herein, we review recent progress and achievements of using EVs as nanosized carriers for drug delivery in cancer therapeutics and highlight the challenges and research directions of this field for further promoting their application.
General Concept of EVs
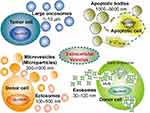
Extracellular vesicle is the generic term to describe cell released membrane vesicles, which covers different types. Currently, according to the guidelines of the International Society for Extracellular Vesicles (ISEV), there are four members in the EV family, which are exosomes, microparticles (or microvesicles, ectosomes), oncosomes, and apoptotic bodies16,17 (Figure 1). Exosomes are the smallest EVs with a diameter between 30 and 100 nm, which are formed by intraluminal vesicles (ILVs). Then ILVs inward budding of endosomal membrane and form multivesicular endosomes (MVEs). After maturation, exosomes are secreted via fusion of MVEs with the cell surface.18 Microvesicles represent a group of medium-size membrane vesicles which are 100 nm to 1000 nm in diameter, including shed membrane vesicles, microparticles and ectosomes. They are generated by outward budding and blebbing from the plasma membrane.19 Oncosomes are used to describe a class of tumor-derived microvesicles that are between 1 and 10 μm in size and could propagate oncogenic information to other cells and tissues in the tumor microenvironment.20–22 Apoptotic bodies are special kinds of vesicles with sizes ranging from 1 μm to 5 μm, which are formed by disassembled apoptotic cells. They contain information and substances from dying cells and are capable of delivering cellular materials to healthy recipient cells.23
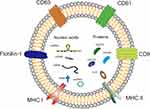
Each type of EV differs in their origin, composition and size, thus presenting heterogeneous properties. Exosomes are the most well studied EVs and found in many kinds of biological fluids, including peripheral blood, cerebrospinal fluid, amniotic fluid, urine, milk, bronchoalveolar lavage fluid, malignant effusions and cell culture medium.24 Exosomes and ectosomes are found enriched in specific lipids, nucleic acids and proteins, after being released into the extracellular environment, they can transfer information from the originating cells to recipient cells and induce phenotypic modulation in recipient cells.25,26 Different kinds of RNAs including mRNA, microRNAs (miRNAs), lncRNA, tRNA and circRNA have been found in exosomes (Figure 2), and most exosomal RNAs play important roles in regulating bioactivity of recipient cells.27 Compared to RNA, people still know little about exosomal DNA.28 Sansone et al reported that DNA fragments originated from either nucleus or mitochondria could be packaged into exosomes by an unknown pathway.29 As containing specific mutation of parental cells, exosomal DNA could emerge as a diagnostic marker for disease.28 However, new evidence indicated there might be no DNA in exosomes. Jeppesen et al reassessed the composition of exosomes and reported that the double-stranded DNA (dsDNA) associated histones were present in the nonvesicular (NV) components rather than in small EVs.30 Therefore, it is still unclear whether there is DNA in exosomes or not, and if there is, the function of exosomal DNA is still waiting to be illuminated.
It is very important to recognize each type of exosomal marker correctly. The potential classical biomarkers for exosomes are major histocompatibility complex (MHC), flotillin-1, CD9, CD63, CD81, HSP70, Alix and TGS101, etc (Figure 2).31 However, some of them like MHC, flotillin-1 and HSP70 have been found presenting in other types of EVs as well.26 To draw the precise biomarker profiles for each type of EV is still a tough challenge. Therefore, researchers were recommended to provide at least three positive protein markers and one negative marker, and better cooperate with electron microscope data to confirm the subtype of EV which they studied.17 In fact, compared to artificial NPs, EVs contain plenty of proteins on or embedded in their membrane, which give them special advantages in delivering drugs in vivo. For example, CD47, an enriched protein in fibroblast-derived EVs can interact with its receptor (SIRPα) on macrophages and release a “do not eat me” signal, therefore help fibroblast-derived EVs escaping from MPS clearance.32 Furthermore, the surface adhesion proteins and vector ligands on EVs such as tetraspanins, integrins, CD11b and CD18 receptors, make EVs prone to being taken up by target cells.33,34 Meanwhile, the specific origin of exosomes could boost them homing to their derived cells or tissue.35 Thus, EVs have the natural advantages in protecting their cargoes, reducing clearance by the MPS and effectively increasing delivery to target tissues with reduced immunogenicity.15,36 Since the isolation, purification and identification of EVs have been well described in recent articles,37–39 here we focus our review on strategies for loading and modifying EVs to target delivering drugs. In addition, the term of EV is used throughout for convenience and general narration, but for the specific EV subsets such as exosomes or microvesicles, we will follow the original statement..
Methods for Loading EVs with Therapeutic Cargoes
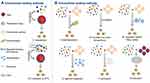
EVs have tight and ordered phospholipid bilayers, destruction of EV integrity may lead to their elimination by the MPS, thus to load EVs efficiently without disrupting their membranes is very important. The current strategies can be typically classified as endocellular loading methods and extracellular loading methods (Figure 3).
Endocellular loading methods mean incubating or transfecting donor cells with the therapeutic cargo, and then the cargo is enriched in donor cells and packed into EVs via endogenous cargo-selecting machinery during EV generation.40–44 Subsequently, the EVs with therapeutic cargo entrapped are isolated from culture medium of donor cells. This method mainly is applied to load nucleic acid drugs like miRNAs, small interfering RNAs (siRNAs) and small molecule drugs. Nevertheless, the loading efficiency of incubation or transfection are still unsatisfactory, on top of that the cargo or transfection reagents themselves might be toxic to donor cells. The methods to load cargo into EVs directly in extracellular environment are called extracellular loading methods, which include using transfection reagents, extrusion, electroporation, saponin treatment, sonication and freeze–thaw method.45 Extracellular loading methods which mainly make transitory apertures on EVs, such as sonication, extrusion, and saponin, generally showed greater drug loading efficiency than endocellular loading methods,46–48 however, they require additional purification steps that may disrupt EV structural integrity.49 The appropriate loading method should be considered carefully according to the physical and chemical properties of the specific cargo. For hydrophobic agents, such as paclitaxel and curcumin, efficient loading can be achieved through simple co-incubation of EVs and drugs at room temperature.50,51 For hydrophilic drugs, the lipid bilayer will restrain their passive penetration into EVs.52 Hence, it is necessary to make transitory apertures on EV membranes to promote the hydrophilic drugs entering into the EVs. Recently, Haney et al studied the efficiency of loading catalase into EVs by different strategies. Their results confirmed that sonication is the most efficient method for loading proteins into EVs. The loading efficiency of these methods increased as follow: incubation, freeze–thaw cycles, extrusion, and sonication.47 However each method has its advantages and disadvantages. For instance, using transfection reagent can improve the loading efficiency, but additional complicated purification processes are needed. Electroporation is another typically used method, which involves making a transient electrical field to generate pores on the EV lipid bilayer membrane. But electroporation needs the special equipment and testing optimum working conditions before experiment. The study focused on the horizontal comparison of drug loading efficiency between endocellular and extracellular methods, which is still rare. There is also an urgent need to develop a method that can detect the loading capacity of EVs precisely and conveniently to monitor delivery efficiency of EVs.
Delivery of Nucleic Acid Cargo by EVs
EVs have been considered as endogenous delivery carriers in delivering nucleic acids to cells. Thereafter, many of the studies have used EVs to deliver nucleic acid drugs. siRNAs are a class of double-stranded RNAs, which could interfere with the expression of specific genes by causing mRNA excision or restraining mRNA translation.53 However, siRNA is unstable in circulation, and easy to eliminate as well as difficult to enter target cells.54 Therefore, a specific delivery carrier is needed to bring siRNA to the disease site and tha alleviates adverse effects.54 EVs were first documented and engineered to deliver siRNA between cells in 2011. In this pioneering work, Alvarez-Erviti et al designed an experiment to express a neuron-targeting protein which is rabies virus glycoprotein (RVG)-derived peptide on the surface of exosomes derived from mouse dendritic cells. Then the siRNA of BACE1 gene was loaded into these engineered exosomes. By injecting these exosomes into the bloodstream of mice, siRNA was specifically delivered to target cells in mice brains and effectively knocked down BACE1.35 In another representative study, by using electroporation strategy, fibroblast-derived exosomes were loaded with nucleic acid cargo targeting KRASG12D, a common oncogenic in pancreatic cancer. These nucleic acid cargos could be delivered into tumors in pancreatic ductal adenocarcinoma (PDAC) mice by exosomes. This study further confirmed exosomes have superior ability to deliver either siRNA or shRNA and suppress tumor growth in vivo.32 miRNA is a type of noncoding RNA with a length of 18 to 22 nucleotides, which has been proved to play a critical role in regulating gene expression by combining with the 3ʹUTR of target mRNA sequences to restrain mRNA translation or induce degradation.55 The miRNAs that negatively regulate oncogene expression are potential tumor suppressors.55.Theoretically, nanosized carriers designed for delivering siRNA can be easily introduced to miRNAs because of their similar physicochemical properties. In a recent study, Liu et al used miR-128-3p expression lentivirus to transfect normal intestinal FHC cells and got miR-128-3p packaging FHC cell exosomes. These exosomes could efficiently deliver miR-128-3p to oxaliplatin-resistant colorectal cancer (CRC) cells, suppress epithelial-mesenchymal transition (EMT) and increase intracellular oxaliplatin accumulation by suppressing the expression of Bmi1, an E-cadherin and ATP-dependent glutathione S-conjugate export pump, and a drug transporter MRP5.56 In another study, Baldari et al constructed a lentiviral vector which expressed miR-125b linked with a specific ExoMotif sequence tag that was used to target miRNA into EVs. By transfecting mesenchymal stromal/medicinal signaling cells (ASCs) derived from human adipose tissue, miR-125b was efficiently loaded into ASC EVs, and specifically reduced cell proliferation in vitro by modulating a series of miR-125b target genes in hepatocellular carcinomas.57 Similar studies emerged, by using lentiviral vector transfected into the donor cells as well, Zhang et al prepared miR-101 packaging EVs, which were able to inhibit osteosarcoma cell invasion and migration in vitro as well as inhibited osteosarcoma metastasis in vivo.58
In addition to delivery of nucleic acid directly, EVs were employed as an advanced tool for genomic editing. CRISPR-Cas9 is a novel implement to accurately incise DNA sequences with the aid of a single guide RNA (sgRNA), which is complementary to the target gene.59 In Lin et al’s study, exosomes carrying sgRNA were prepared by transfecting sgRNA expressing vector into HEK293 FT cells. Results showed that this kind of nanoparticles could deliver CRISPR/Cas9 system to mesenchymal stem cells (MSCs) and regulate the target gene expression.60 In Kanada et al’s study, microvesicles derived from breast cancer cell lines 4T1 were used as the delivery vehicles. The authors cloned the gene of a thymidine kinase (TK)/nitroreductase (NTR) fusion protein into a minicircle (MC) DNA vector. Through transfecting this MC DNA vector to 4T1 cell, they obtained the MC DNA vector loaded microvesicles. These microvesicles effectively delivered TK-NTR-encoding vector into the tumor tissue of breast cancer xenograft mice, and showed effective prodrug conversion and antitumor effect.61 Most studies focused on using EVs to deliver nucleic acid cargo via endocellular loading methods. However, although some specific sequence motifs of RNA appear to promote preferential EV sorting, such as the sequence motifs including “ACCAGCCU”, “CAGUGAGC”, “UAAUCCCA”, or zipcode-like 25-nt sequence with a “CTGCC” code domain,62,63 it is still unclear whether cells can selectively package target nucleic acid into EVs or not, and it is ambiguous how much transfected nucleic acid cargoes can be loaded into EVs. Moreover, lentiviral vector is the most popular transfection tool in these studies, and it should be very carefully evaluated whether the lentiviral vectors themselves have potential biosecurity risk before being used in vivo.
Delivery of Protein Cargo by EVs
Because proteins are relatively big in size and prone to loss activity, it is difficult to load any proteins into EVs directly. In an early study, cargo protein was overexpressed in donor cells to make them accumulate in EVs. Lee et al first overexpressed MHC class II (MHC-II) molecules in the murine melanoma cell line by transducting the Class II trans activator (CIITA) gene. The exosomes derived from transfected cells packaged a high amount of MHC-II and tumor antigen TRP2, which could significantly inhibit tumor growth by activating the immune response of DCs in vitro and inducing antitumor immune responses including promoting splenocyte proliferation and activation as well as IL-2 secretion in tumor-bearing mice.64 In a subsequent study, a protein cargo, protein-cytosine deaminase (CD) fused to uracil phosphoribosyltransferase (UPRT), was overexpressed in donor cells (HEK-293T cells) by Mizrak et al. Then the microvesicles (MVs) carrying CD-UPRT-EGFP mRNA and protein were isolated from donor cells and intratumorally injected into tumor-bearing mice. These MVs were capable of transferring their cargo and achieving a high level expression of functional protein in recipient cells, and further inhibiting schwannoma tumor growth in vivo.65 Based on this method, Sterzenbach et al developed a novel approach of effectively packing protein into EVs. By labeling with a WW tag that leads to late-domain (L-domain) pathway dependent recognition by the L-domain-containing protein Ndfip1, and resulting in cargo protein ubiquitination and loading into exosomes, Cre recombinase was ubiquitinated and subsequently loaded into EVs via transfecting corresponding vector to donor cells. The WW-Cre embed EVs could be taken up by recipient neurons in brain regions in the mouse model, indicating the potential usage of engineered EVs to deliver biologically active proteins crossthe BBB and treat brain tumor.66 A few studies loaded protein cargo via simply co-incubation therapeutic protein with donor cells. In Barok et al’s work, trastuzumab emtansine (T-DM1), an antibody drug, was used to treat the donor cells, HER2-positive tumor cells. Then the exosomes with T-DM1 embed could be obtained from HER2-positive tumor cells. These exosomes were able to deliver T-DM1 to other cancer cells and reduce their viability.67 In general, before applying EVs to packaging protein cargo, some issues should be carefully considered. Firstly, what is the upper limit of the protein size that can be loaded into EVs, and how to load macromolecular cargo into EVs effectively? Moreover, whether the transmembrane proteins expressed on EVs for targeting or therapy purposes can be folded and modified correctly to maintain their biological activity. Noguchi et al found that the biological activity of proteins encapsulated in the EVs was highly affected by lyophilization. Thus, the proper preservation method of EVs carried proteins needs to be further considered.68 Finally, whether the engineering and modification processes on donor cells would affect the morphology and composition of EVs remain unclear.
Delivery of Chemotherapy Drugs by EVs
Conventional chemotherapy drugs such as paclitaxel (PTX) and doxorubicin (DOX) have been most studied since the EV-based drug delivery strategy was established. PTX is a powerful antimitotic drug that has been approved by the Food and Drug Administration (FDA) to treat solid tumors. Due to its low aqueous solubility, PTX needs to be dissolved by Cremophor EL and ethanol before being used in patients.69 In study from Kim et al, PTX was loaded into exosomes to treat multiple drug resistant (MDR) cancer. Through sonication treatment, PTX was loaded into exosomes derived from RAW 264.7 cells. These PTX-exosomes have shown ideal antitumor effects in several tumor cells and produced strong antineoplastic effect in lung metastases in mice models.70 Then the same research team further improved the EV delivery system in the following study. By mixing RAW 264.7 exosomes with aminoethylanisamide-polyethylene glycol (AA-PEG) and PTX under sonication condition, AA-PEG-exoPTX was prepared. As AA-PEG can target the sigma receptor, which is overexpressed by lung cancer cells, thus AA-PEG-exoPTX could accurately deliver PTX to lung cancer cells and show superior antimetastatic efficacy in murine pulmonary metastasis mice model.71
DOX, a powerful topoisomerase II inhibitor, is another popularly used chemotherapy drug. Unfortunately, DOX-based therapy has severe side effects like cardiotoxicity, nephrotoxicity, and neurotoxicity, hence further improvement on its target ability is needed urgently. In a study from Wei et al, exosomes derived from bone marrow MSCs (BM-MSCs) with tumor-homing features were isolated and mixed with DOX-HCl in a specific buffer (desalinizing with triethylamine), and then dialyzed overnight. The prepared Exo-DOX, is able to enhance cellular uptake efficiency and shows antitumor effects on osteosarcoma MG63 cell lines, but low cytotoxicity on myocardial cells.72 In another work, an exosome delivery strategy was developed to treat triple-negative breast cancer. A disintegrin and metalloproteinase 15 (A15) is the protein containing Arg-Gly-Asp (RGD) motif, which is a widely expressed membrane protein on tumor cells and involved in tumor progression and suppression. Gong et al reported that phorbol 12-myristate 13-acetate (PMA) stimulating could effectively increase the content of A15 in exosomes derived from THP-1 cells. By mixing DOX with A15 exosomes in triethylamine solution overnight, the DOX packaged A15-exo (A15-Exo/DOX) were obtained. Then the authors further co-incubated cholesterol-modified miR-159 (Cho-miR-159) with A15-Exo/DOX to form a co-delivery system. This co-delivery system showed therapeutic effects on triple-negative breast cancer both in vitro and in vivo.73 Several nano materials for drug loading such as liposomes, chitosan and inorganic NPs have been employed to deliver chemotherapy drugs to tumors. As successor vehicles, EVs have become the new focus in this field. However, the origin of EVs might affect their uptake efficiency by recipient cells,74 because EVs have an inherent homing property. Consequently, when delivering drugs to tumors in vivo, the donor cells should be carefully selected because EVs derived from normal cells may induce undesired drug accumulation in normal tissue. Thus, if the exosomes derived from heterologous normal cells can be used in chemotherapy drug delivery to avoid the drug accumulating in normal tissue becomes an interesting question waiting to be answered.
EVs Engineering Strategies
In addition, to further enhance the efficiency of loading cargo into EVs and/or targeting delivery to the tumor site, many strategies have been established to engineer EVs by genetically engineering donor cells and/or modifying EVs directly via nongenetic engineering methods.
Indirect EV Engineering via Donor Cell Modification
Through genetically engineering donor cells, EVs can be modified including expressing new protein on their membrane or increasing the packaging efficiency of therapeutic protein cargo loading by specific localized peptide. In a pioneering work, a transmembrane domain of platelet-derived growth factor receptor was fused to the GE11 peptide which can specifically bind to EGFR on HEK293 cells, and then GE11 can be located on the exosomes of HEK293 cells. These engineered HKE293 cells were further transfected with synthetic let-7a miRNA. Through interacting between GE11 and EGFR expressed on tumors, this let-7a carrying exosomes was able to target tumor cells and deliver let-7a efficiently. This delivery system showed hypothetical effect on treating EGFR-expressing breast cancer in a xenograft breast cancer mouse model.75 Also via targeting EGFR, Kooijmans et al anchored glycosylphosphatidylinositol (GPI) signal peptides with anti-EGFR nanobodies. By transfected donor cells with the vectors encoding fusion protein, anti-EGFR nanobodies enriched EVs were obtained, which could efficiently target EGFR-expressing tumor cells.76 In addition to EGFR, Her2 is another commonly used tumor-associated membrane protein in related studies. Gomari et al expressed ankyrin repeated proteins (DARPins), a specific ligand against Her2 in MSCs, subsequently MSCs exosomes with Her2 ligand were isolated and loaded with DOX by electroporation. Through treating Her2+ and Her2- tumor cell lines respectively, these DOX-loaded exosomes showed better uptake efficiency by Her2+ target cells compared to the other one, which highlighted their target delivery ability.77 In addition to utilizing guide peptides, cargo protein was fused with the protein associated with EV biogenesis to enhance the EVs loading efficiency in some studies. For example, by fusing the fragment of interleukin-3 (IL-3) ligand with EV marker protein Lamp2b, Bellavia et al improved the loading efficiency of IL-3 ligand into EVs of HEK 293T cells. These IL-3 ligand-expressed EVs (IL3-L EVs) which carried imatinib or BCR-ABL siRNA, could target chronic myelogenous leukemia (CML) cells and inhibit cancer cell growth in vitro and in vivo.78 As a tetraspanin, CD63 is a classical EV marker protein that plays a role in the sorting of EV cargos. And apolipoprotein A1 (Apo-A1) is a target of the scavenger receptor class B type 1 (SR-B1) receptor, which is highly expressed on the liver cancer cells surface. Liang et al transfected HEK 293T cells with a fusion protein which constructed both CD63 and Apo-A1 genes to obtain engineered EVs. These EVs, which were loaded with miR-26a via electroporation, could be taken up efficiently by HepG2 cells and decrease their proliferation and migration in vitro.79 For tracking purposes, the fluorescent dyes that specifically label lipids such as PKH67, PKH26, and near-infrared lipophilic dye were used to label exosomes in many studies.80–83 However, it is difficult to eliminate the interference from the debris of plasma membrane and organelle by using these fluorescent dyes to label EVs as well because it is still unknown if fluorescent dye affects the size and structure of EVs. Therefore, the improved strategy is to integrate the luminescent proteins such as eGFP, tdTomato and luciferase with EV membrane proteins to obtain luminous EVs in EV membrane to tracing uptake of EVs in vitro and biodistribution of EVs in vivo in some studies.83–85 The peptides that have been used in recent studies for anchoring cargo protein to EV surfaces or targeting tumor cells are summarized in Table 1.
 |
Table 1 The Guide Peptide and Targeting Proteins Employed in Studies |
At least two studies are devoted to controlling cargo protein anchored on the EVs membrane or released into the internal space as required. Yim et al developed a method to load cargo proteins into EVs during EV biogenesis. Two specific proteins, CRY-interacting basic-helix-loop-helix 1 (CIB1) and photoreceptor cryptochrome 2 (CRY2) were used in this study. Both of these proteins were from Arabidopsis thaliana and can regulate floral initiation through blue light-dependent phosphorylation. There are reversible protein–protein interactions that can be controlled by blue light between the two proteins. Using this module, a truncated CIB1 was conjugated to CD9 (an EV-associated tetraspanin protein), while cargo protein was conjugated to CRY2. Along with blue light turning on or turning off, the two proteins combined or separated, and the cargo protein could be controlled to anchor on the EV membrane or be released into the intraluminal space.86 Based on similar concepts, Pi et al developed RNA nanotechnology that could control placing of RNA nanoparticles on the outer surface of the EV membrane, or loading into the EV lumen, for cargo loading purposes. In their strategy, membrane-anchoring cholesterol was placed at the arrowtail or arrowhead of the RNA aptamer, resulting in anchoring the RNA nanoparticles to the EV outer surface or loading the RNA into the EVs, respectively. These ligand-displaying EVs could specifically deliver siRNA to target cells and effectively inhibit tumor growth in three cancer models.87
Direct EV Modification
Although the authors claimed they got the modified EVs by engineering the donor cells, the specific modification efficiency is still unclear. Direct modification strategy provides another simple and convenient way to engineer EVs. Smyth et al reported a strategy that can conjugate biomolecules to the EV surface directly via the click chemistry method, which is a copper-catalyzed azide-alkyne cycloaddition. In this study, alkyne groups were conjugated to EVs through a copper-catalyzed azide-alkyne cycloaddition. This process neither changed EV size nor affected its adherence or internalization by recipient cells. In addition, this method could bioconjugate the small molecules and macromolecules onto the surface of EVs successfully with several advantages, including fast high specificity and compatibility with aqueous buffers.88 However, whether this method can be introduced to conjugate proteins such as receptors, ligands, or antibodies to EVs and keep their therapeutic activity is still to be revealed. A modified method that aimed to extend the in vivo circulation time of EVs was also developed. In the work from Kooijmans et al, EVs modified with EGFR conjugated to polyethylene glycol (PEG) were prepared. By mixing micelles with EVs of neuro2A cells, a temperature-dependent transfer process of nano-PEG-lipids to the EV membranes was achieved. This modification process did not affect EV morphology, size distribution, and protein composition but prolonged circulation times of the EVs in an animal model, possibly increasing EV accumulation in targeted tissues and improving cargo delivery efficiency.89 However, there is an opposite view that EVs carrying PEGylation might trigger an anti-PEG IgM response, and lead to rapid clearance from the circulation.90 In another study, Zhu et al mixed EV suspensions with special micelles formed by the reaction of 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy (polyethylene glycol)-2000]-c(RGDyK) (DSPE-PEG2000-cRGDyK) with 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffer to obtain cRGD-modified EVs. These EVs could effectively deliver the antitumor drug PTX to glioblastoma cells.91 A33 is a uniformly expressed antigen in CRC. Li et al coated surface of carboxyl superparamagnetic iron oxide nanoparticles with A33 antibodies, which can bind to the A33 antigen on the exosomes derived from A33-positive CRC cells to prepare A33Ab carrying exosomes. Through multiple mixing steps in a special buffer system, DOX was loaded into this exosome to form A33Ab-US-Exo/DOX complex (A33 antibodies-nanoparticles-exosomes). This complex could be efficiently taken up by A33-positive colorectal cancer cells and exerted high binding affinity and antiproliferative effects in vitro and suppress tumor growth and extend the survival in the CRC mice model with lower cardiotoxicity.92 Endocytosis has been considered as the main pathway for the cellular uptake of EVs, and the macropinocytosis of the recipient cells will reorganize the actin, ruffle the plasma membrane, and engulf the large volumes of extracellular fluid.93 Therefore, the activation of macropinocytosis will greatly help the cellular uptake of EVs. Nakase et al reported that it was helpful to functionalize EVs by enhancing the macropinocytosis induction for improving EV-based intracellular delivery of therapeutic molecules. In their studies, they demonstrated some useful methodologies for achieving enhanced cellular EV uptake by the epidermal growth factor (EGF) receptor, oncogenic K-Ras, pH-sensitive fusogenic peptide, and arginine-rich cell-penetrating peptides (CPPs).93–97
Along with the rapid development of EV-based drug delivery in cancer treatment, various strategies have been constantly developed. However, it is still immature to apply EV- based DDS to clinical application. Based on a few clinical trials, therapeutic EVs only showed safety and feasibility in non-small cell lung cancer (NSCLC) patients (ClinicalTrials.gov identifier: NCT01159288) or a limited beneficial clinical response in lung cancer patients with malignant pleural effusion.98 The major hindrance of applying an EV delivery system clinically includes the relative low yield of available EV carriers and unsatisfactory drug loading efficiency. To address these challenges, exosomes originated from a novel source could be tested in future.
Novel EVs in Antitumor Drug Delivery
Natural Product Derived EVs for Antitumor Drug Delivery
Natural derived EVs such as EVs derived from milk, fruit, and vegetables, etc may be the potential resources of available EVs in subsequent utilization to obtain a large number of EVs is to use milk as a very common food ingredient with large and stable source. The study of Aqil et al100 proved that exosomes derived from bovine milk have properties of biocompatibility, low-cost, accessibility, stability in acidic environment and lack of toxicity when used as drug carriers.99 In their subsequent work, exosomes isolated from raw milk were loaded with siRNA against specific genes including VEGF, EGFR, AKT, MAPK and KRAS by electroporation or chemical transfection. Results showed that these milk exosomes carrying siRNA can be taken up and showed the silent rate of target gene from near 20% to over 80% in different tumor cell lines. Among them, exosomes carrying siKRASG12S showed significant antitumor effect on lung tumor bearing mice.100 Li et al reported that ginger derived exosome-like nanovesicles (GDENs) displayed similar size, density, and morphology to human derived exosomes. These ginger oriented nanovesicles have folic acid (FA) on their surface and can be targeted to deliver to KB cancer cells. By using exosomes transfection reagent, GDENs were mixed with arrow-tail RNA nanoparticle to prepare GDENs carrying the siRNA targeting BIRC5 (baculoviral inhibitor with apoptosis repeat-containing 5) gene. These exosome-like nanovesicles from ginger were able to knockdown the target gene efficiently in vitro and inhibit tumor growth on a xenograft model by intravenous administration.101 Not limited to laboratory study, EVs derived from plants have been introduced into clinical trials. The researchers from the James Graham Brown Cancer Center used fruit-derived EVs to deliver curcumin to treat cancer patients. Curcumin has powerful inhibiting effect on colon cancer cell lines, however, because of low solubility, poor stability and is prone to be metabolized, oral curcumin has showed only limited bioavailability even at very high doses. Through using fruit-derived EVs transmitting, most of the major obstacles of curcumin application could be solved, including increased solubility, bioavailability, and stability. In this trial, the effect of curcumin on cellular metabolism, immune modulation, and phospholipid profiles of normal and malignant colon cells from colon cancer patients was characterized. Because it is still an ongoing work, the conclusion needs to be further clarified (NCT01294072). Furthermore, some plant originated exosomes, such as lemon,102 grape,103 and carrot104 have been used in treating diseases. Because of the natural superiority, including numerous, they are inexpensive and easy to be obtained, exosomes derived from milk or plants are the potential candidates as drug delivery vehicles in cancer therapy.
EVs-mimetic Nanovesicles for Antitumor Drug Delivery
Other novel strategies to solve the main obstacles of lacking enough quantity of EVs are the prepared EV mimics. In a pioneering work, Jang et al developed a platform to prepare bioinspired exosome-mimetic nanovesicles rapidly and conveniently. The monocytes or macrophages were broken down by using a serial extrusion through filters with diminishing pore sizes (10, 5, and 1 μm) in the DOX contained buffer to prepare DOX carrying nanovesicles (NVs). These DOX loaded NVs showed similar antitumor activity as DOX loaded exosomes and have better performance than DOX-loaded liposomes in vivo.46 In a further study, Zhu et al prepared generous amounts of EVs like nanovesicles by extruding NK cells through filters with progressively smaller pore size. These NK cell derived EV mimetics have significant antitumor effect in vitro and in vivo.105 By utilizing mechanical forces as well, a microfluidic device was fabricated by Jo et al to prepare EV mimetic nanovesicles. This microfluidic device mainly composed by microchannels, when cells flowed through slits in microchannels, they were stretched and generated nanovesicles due to their elongated shape caused by sheer stress. These nanovesicles incorporated membrane proteins and were similar to exosomes in shape and content, and they were able to deliver RNA to target cells.106 Some other devices based on squeezing cells by using mechanical force have also been developed recently,107–109 which can generate a great quantity of exosome mimetic nanovesicles and they are valuable for applications in drug delivery. However, further study is needed to investigate whether these devices of prepared nanovesicles contain organelle or cell fragments, and whether these by-products would induce undesirable inflammatory response in vivo.
EVs-liposome Hybrid Nanovesicles for Antitumor Drug Delivery
Liposomes are artificial vesicles composed of a lipid bilayer being widely used as nanocarriers in clinical trials.110–112 As the pioneer in nanobased DDS, liposomes have been certified to reduce the side effects relative to free drugs.113 However, this kind of DDS has critical limitations: first, the chemical solvents and additives during the liposome generation procedure may cause toxicity for clinical use; second, the membrane of the liposome lacked biofunctional proteins which can initiate the signal transduction.107 EVs have exhibited better cell targeting ability and low toxicity, therefore they were considered a promising nanocarrier in DDS.35,114 Despite being alternatives to conventional DDS, EVs still face drawbacks in low yield and the drug loading capacity limitation.115 Therefore, a novel hybrid approach was established recently. In this hybrid system, EVs with targeting ability and biocompatibility were combined with liposome production and drug loading. As in an aforementioned study, both fibroblast-derived exosomes and liposomes were loaded with nucleic acid cargo targeting KRASG12D, showing that this hybrid nanocarrier has better antitumor effect than liposomes.32 In another representative study, exosomes were incubated with a mixture of liposomes and pEGFP-C1 plasmids for 12 h at 37°C to prepare hybrid nanovesicles, which could overcome the volumetric restriction.60 Other novel hybrid methods have been developed subsequently. Piffoux et al fused cell line-derived EVs and liposomes by using PEG. This strategy could efficiently enrich EVs with exogenous hydrophilic and lipophilic compounds while maintaining their intrinsic content and biological properties.116
Discussion
As emerging nanocarriers, EVs have many advantages in targeting delivery of drugs to tumor site; however, this DDS is still at an initial stage. How to choose appropriate EVs for drug delivery is the first challenge waiting to be solved. For instance, tumor cell derived EVs contain oncogenic drivers that may induce unexpected side effects in antitumor therapy. Substantial evidence demonstrated that EVs derived from MSCs or immunocytes may be ideal candidates. MSCs are well known to produce large amounts of EVs117 and show pleiotropic biological activity in treating multiple diseases, including cancer.118–122 Hence, MSC-derived EVs are a considerable option for drug delivery carriers in future studies. The donor cells used in the mentioned drug delivery studies are summarized in Table 2. Other potential candidates for clinical application are EVs derived from immunocytes. The protein composition of EVs depends on the donor cell type, somehow reflecting the specific functions of their parent cell. For instance, EVs derived from B cells carrying B cell receptors (BCR);123 T cells secrete EVs bearing T cell receptor (TCR), Src-like tyrosine kinases and adhesion molecules,124 as well as DC-derived EVs containing the DC-associated proteins.125 These specific immunoreceptors drive these EVs to target tumors exclusively, which make them excellent carriers for antitumor drug delivery studies. It is particularly worth mentioning that EVs derived from natural killer (NK) cells express NK-associated receptors, such as FASL, NKG2D, and cytotoxic proteins including perforin and granulysin.126,127 NK cells are one of the most essential antitumor immune cells, which could recognize and kill tumor cells without prior immunization and subsequently trigger the adaptive immune responses via secretion of various chemokines and cytokines.128 NK cells have been widely used in clinics to treat cancers.129–131 Recent evidence indicated that NK cell-derived EVs have a cytotoxic effect on tumor cells and perform an antineoplastic immune response by activating other immune cells.132 Therefore, NK cell-derived EVs are ideal carriers to deliver antitumor drugs and have natural antitumor activity simultaneously in cancer therapy.133 However, some obstacles of using immunocytes derived EV- delivery antitumor drugs should be considered. There are still biosafety risks remaining when using EVs derived from immune cell lines, but EVs derived from neither autologous nor allogenic primary immune cells may not meet the required quantity. A promising solution is preparing EVs like engineered nanovesicles carrying immunocyte-associated proteins or receptors. For instance, by incubating NK cell exosomes (NKEXO) with biomimetic core-shell nanoparticles (NNs) carrying let-7a mimics, Wang et al developed a NN/NKEXO cocktail DDS, and these cocktail nanoparticles have targeting and antitumor capabilities both in vitro and in vivo.134 It is worth trying to ascertain whether these approaches could be utilized to acquire enough quantities of EV-like vesicles for clinical therapy. Furthermore, more novel trials have been reported including enhancing the release of bioactive EVs by interfering with the endolysosomal trafficking,135 culturing cells at low pH conditions to increase the release of EVs136 and developing a 3-D culture system with tangential flow filtration to enhance the yield and improve the activity of EVs.137
 |
Table 2 The Donor Cells Used in the Mentioned Drug Delivering Studies |
Furthermore, there are other issues should be considered seriously in developing the EV-based DDS in future. First, what are the optimum sizes and the types of EV for delivering cargo to specific tissue? The size distribution of EVs depended on the extraction method, for instance, ultracentrifugation-isolated EVs are generally bigger than the EVs extracted by the ExoQuick precipitation method.138 There is evidence that the size of EVs could affect the interaction between EVs and recipient cells; smaller EVs can be taken up more by recipient cells and induce higher response to the “EV cargo”.138 In the case of NPs, 40–50 nm is the optimum diameter range with the greatest cell uptake rate.139 In regard to EVs, what is the optimal size for EV uptaking by recipient cells is a question worth investigating.
Second, during EV isolation, the impurities that have similar sizes or sedimentation coefficients with EVs, such as debris from the cell membrane, large heteroproteins, or organelles, could not be excluded. These impurities may disturb the effect of the EV-based drug delivery. Recently, the recommended method for EV isolation has been published in the Minimal Information for Studies of Extracellular Vesicles 2018 guidelines.17 Subsequent studies should follow the standard protocol to obtain EVs with stable quality and make the drug delivery study scalable and reproducible. Furthermore, more economical and convenient methods for EV isolation are still urgently needed.
Third, it is known that there are at least three ways for EVs to interact with recipient cells, including engulfment by recipient cells, fusing EVs with the cytoplasmic membrane of recipient cells and releasing their intraluminal contents, and transmitting signaling molecules and inducing signaling cascades through interactions between the receptor and ligand located on the cytomembranes of recipient cells and EVs.19 Which is the most efficient way for EVs to affect recipient cells and delivering their cargo, including the cargo loaded into EVs or linked on their outer membrane to recipient cells, is stillto be deciphered?
Last but not least, although studies have proven that intraperitoneal injection of EVs into experimental animals cannot generate both toxicity and immune response,140 several crucial issues should be settled before the EV-based DDS is translated to clinical therapeutics. Evidence showed that in animals, unmodified EVs would preferentially accumulate in liver, kidney, and spleen and are easily eliminated by bile excretion, renal filtration, or phagocytosis in the reticuloendothelial system, leading to very low concentrations of exogenous EVs in target tissues.141 Moreover, the results obtained from animal models cannot be directly applied to humans because of the differences in physiological structure and immune response between human and animals. Finally, as an endogenous mediator for intercellular communication in vivo, EVs play a very important role in every aspect of the biological process. Whether there are any potential risks that the exogenous EVs disturb the normal function of endogenous EVs by competition or some unknown mechanism should be assessed carefully.
Conclusion
The EVs based drug delivering system is being developed. Various cargoes such as nucleic acid, proteins, and chemotherapeutics have been loaded into EVs and tested in multifarious in vitro and in vivo antitumor models as well as clinical treatment of cancer (Figure 4). Several key points, including increased loading efficiency, increased targeting efficiency, increased circulation time in vivo, reduced side effects, and obtaining stable and abundant EVs are still the main development directions which deserve the researchers in this field to devote their vigor in.
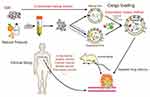 |
Figure 4 EVs as targeted transport nanocarriers in cancer therapy. |
Funding
This work was funded by the Natural Science Basic Research Program of Shaanxi (grant no. 2019JM-046 and 2019JM-360), Henan Provincial Health System Training Program for Study Abroad (grant no. 2017060), Science and Technology Project Henan Province (grant no. 182102310167), Medical Science Research Project of Henan Province (grant no. 201602227), the Guangzhou Science and Technology Program (grant no. 0.201803010094) and the Natural Science Foundation of Guangdong (grant no. 2020A151501158) and the National Training Program of Innovation and Entrepreneurship for Undergraduates (grant no. 201910699168).
Disclosure
J Chen is an employee of Shanxi Weiqidaguangming Pharmaceutical Co., Ltd. The authors report no other possible conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Maeda H, Khatami M. Analyses of repeated failures in cancer therapy for solid tumors: poor tumor-selective drug delivery, low therapeutic efficacy and unsustainable costs. Clin Transl Med. 2018;7(1):11. doi:10.1186/s40169-018-0185-6
3. Magdy T, Burmeister BT, Burridge PW. Validating the pharmacogenomics of chemotherapy-induced cardiotoxicity: what is missing? Pharmacol Ther. 2016;168:113–125. doi:10.1016/j.pharmthera.2016.09.009
4. Marchetti C, De Felice F, Di Pinto A, et al. Dose-dense weekly chemotherapy in advanced ovarian cancer: an updated meta-analysis of randomized controlled trials. Crit Rev Oncol Hematol. 2018;125:30–34. doi:10.1016/j.critrevonc.2018.02.016
5. Tang J, Zhu N, Rao S, Carlson KS. Stem cell damage after chemotherapy- can we do better? Best Pract Res Clin Haematol. 2019;32(1):31–39. doi:10.1016/j.beha.2019.02.001
6. Davoodi P, Lee LY, Xu Q, et al. Drug delivery systems for programmed and on-demand release. Adv Drug Deliv Rev. 2018;132:104–138. doi:10.1016/j.addr.2018.07.002
7. Nikravan G, Haddadi-Asl V, Salami-Kalajahi M. Synthesis of dual temperature - and pH-responsive yolk-shell nanoparticles by conventional etching and new deswelling approaches: DOX release behavior. Colloids Surf B Biointerfaces. 2018;165:1–8. doi:10.1016/j.colsurfb.2018.02.010
8. La-Beck NM, Liu X, Wood LM. Harnessing liposome interactions with the immune system for the next breakthrough in cancer drug delivery. Front Pharmacol. 2019;10:220. doi:10.3389/fphar.2019.00220
9. Das M, Huang L. Liposomal nanostructures for drug delivery in gastrointestinal cancers. J Pharmacol Exp Ther. 2019;370(3):647–656. doi:10.1124/jpet.118.254797
10. Li G, Lei Q, Wang F, et al. Fluorinated polymer mediated transmucosal peptide delivery for intravesical instillation therapy of bladder cancer. Small. 2019;15(25):e1900936. doi:10.1002/smll.201900936
11. Wang H, Agarwal P, Zhao G, et al. Overcoming ovarian cancer drug resistance with a cold responsive nanomaterial. ACS Cent Sci. 2018;4(5):567–581. doi:10.1021/acscentsci.8b00050
12. Kalyane D, Raval N, Maheshwari R, Tambe V, Kalia K, Tekade RK. Employment of enhanced permeability and retention effect (EPR): nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater Sci Eng C Mater Biol Appl. 2019;98:1252–1276. doi:10.1016/j.msec.2019.01.066
13. Brun NR, Lenz M, Wehrli B, Fent K. Comparative effects of zinc oxide nanoparticles and dissolved zinc on zebrafish embryos and eleuthero-embryos: importance of zinc ions. Sci Total Environ. 2014;476–477:657–666. doi:10.1016/j.scitotenv.2014.01.053
14. Pullan JE, Confeld MI, Osborn JK, Kim J, Sarkar K, Mallik S. Exosomes as drug carriers for cancer therapy. Mol Pharm. 2019;16(5):1789–1798. doi:10.1021/acs.molpharmaceut.9b00104
15. Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release. 2015;219:396–405. doi:10.1016/j.jconrel.2015.07.030
16. Lotvall J, Hill AF, Hochberg F, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3:26913. doi:10.3402/jev.v3.26913
17. Thery C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. doi:10.1080/20013078.2018.1535750
18. Harding C, Heuser J, Stahl P. Endocytosis and intracellular processing of transferrin and colloidal gold-transferrin in rat reticulocytes: demonstration of a pathway for receptor shedding. Eur J Cell Biol. 1984;35(2):256–263.
19. van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–228. doi:10.1038/nrm.2017.125
20. Minciacchi VR, Freeman MR, Di Vizio D. Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin Cell Dev Biol. 2015;40:41–51. doi:10.1016/j.semcdb.2015.02.010
21. Bordeleau F, Chan B, Antonyak MA, Lampi MC, Cerione RA, Reinhart-King CA. Microvesicles released from tumor cells disrupt epithelial cell morphology and contractility. J Biomech. 2016;49(8):1272–1279. doi:10.1016/j.jbiomech.2015.10.003
22. Morello M, Minciacchi VR, de Candia P, et al. Large oncosomes mediate intercellular transfer of functional microRNA. Cell Cycle. 2013;12(22):3526–3536. doi:10.4161/cc.26539
23. Xu X, Lai Y, Hua ZC. Apoptosis and apoptotic body: disease message and therapeutic target potentials. Biosci Rep. 2019;39(1). doi:10.1042/BSR20180992
24. Kowal J, Tkach M, Thery C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116–125. doi:10.1016/j.ceb.2014.05.004
25. Choi D, Lee TH, Spinelli C, Chennakrishnaiah S, D’Asti E, Rak J. Extracellular vesicle communication pathways as regulatory targets of oncogenic transformation. Semin Cell Dev Biol. 2017;67:11–22. doi:10.1016/j.semcdb.2017.01.003
26. Kowal J, Arras G, Colombo M, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A. 2016;113(8):E968–977. doi:10.1073/pnas.1521230113
27. Jiang L, Vader P, Schiffelers RM. Extracellular vesicles for nucleic acid delivery: progress and prospects for safe RNA-based gene therapy. Gene Ther. 2017;24(3):157–166. doi:10.1038/gt.2017.8
28. Sharma A, Johnson A. Exosome DNA: critical regulator of tumor immunity and a diagnostic biomarker. J Cell Physiol. 2020;235(3):1921–1932. doi:10.1002/jcp.29153
29. Sansone P, Savini C, Kurelac I, et al. Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer. Proc Natl Acad Sci U S A. 2017;114(43):E9066–E9075. doi:10.1073/pnas.1704862114
30. Jeppesen DK, Fenix AM, Franklin JL, et al. Reassessment of exosome composition. Cell. 2019;177(2):428–445 e418. doi:10.1016/j.cell.2019.02.029
31. Tkach M, Thery C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016;164(6):1226–1232. doi:10.1016/j.cell.2016.01.043
32. Kamerkar S, LeBleu VS, Sugimoto H, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546(7659):498–503. doi:10.1038/nature22341
33. Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;30:3–22. doi:10.1002/0471143030.cb0322s30
34. Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9(8):581–593. doi:10.1038/nri2567
35. Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–345. doi:10.1038/nbt.1807
36. Das CK, Jena BC, Banerjee I, et al. Exosome as a novel shuttle for delivery of therapeutics across biological barriers. Mol Pharm. 2019;16(1):24–40. doi:10.1021/acs.molpharmaceut.8b00901
37. Monguio-Tortajada M, Galvez-Monton C, Bayes-Genis A, Roura S, Borras FE. Extracellular vesicle isolation methods: rising impact of size-exclusion chromatography. Cell Mol Life Sci. 2019;76(12):2369–2382. doi:10.1007/s00018-019-03071-y
38. Coumans FAW, Brisson AR, Buzas EI, et al. Methodological guidelines to study extracellular vesicles. Circ Res. 2017;120(10):1632–1648. doi:10.1161/CIRCRESAHA.117.309417
39. Xu R, Greening DW, Zhu HJ, Takahashi N, Simpson RJ. Extracellular vesicle isolation and characterization: toward clinical application. J Clin Invest. 2016;126(4):1152–1162. doi:10.1172/JCI81129
40. Tang K, Zhang Y, Zhang H, et al. Delivery of chemotherapeutic drugs in tumour cell-derived microparticles. Nat Commun. 2012;3:1282. doi:10.1038/ncomms2282
41. Votteler J, Ogohara C, Yi S, et al. Designed proteins induce the formation of nanocage-containing extracellular vesicles. Nature. 2016;540(7632):292–295. doi:10.1038/nature20607
42. Garcia-Manrique P, Matos M, Gutierrez G, Pazos C, Blanco-Lopez MC. Therapeutic biomaterials based on extracellular vesicles: classification of bio-engineering and mimetic preparation routes. J Extracell Vesicles. 2018;7(1):1422676. doi:10.1080/20013078.2017.1422676
43. Li Z, Zhou X, Wei M, et al. In vitro and in vivo RNA inhibition by CD9-HuR functionalized exosomes encapsulated with miRNA or CRISPR/dCas9. Nano Lett. 2018;19(1):19–28. doi:10.1021/acs.nanolett.8b02689
44. Lv P, Liu X, Chen X, et al. Genetically engineered cell membrane nanovesicles for oncolytic adenovirus delivery: a versatile platform for cancer virotherapy. Nano Lett. 2019;19(5):2993–3001. doi:10.1021/acs.nanolett.9b00145
45. Zhang YF, Shi JB, Li C. Small extracellular vesicle loading systems in cancer therapy: current status and the way forward. Cytotherapy. 2019;21(11):1122–1136. doi:10.1016/j.jcyt.2019.10.002
46. Jang SC, Kim OY, Yoon CM, et al. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano. 2013;7(9):7698–7710. doi:10.1021/nn402232g
47. Haney MJ, Klyachko NL, Zhao Y, et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release. 2015;207:18–30. doi:10.1016/j.jconrel.2015.03.033
48. Wan Y, Wang L, Zhu C, et al. Aptamer-conjugated extracellular nanovesicles for targeted drug delivery. Cancer Res. 2018;78(3):798–808. doi:10.1158/0008-5472.CAN-17-2880
49. Baek G, Choi H, Kim Y, Lee HC, Choi C. Mesenchymal stem cell-derived extracellular vesicles as therapeutics and as a drug delivery platform. Stem Cells Transl Med. 2019;8(9):880–886. doi:10.1002/sctm.18-0226
50. Sun D, Zhuang X, Xiang X, et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18(9):1606–1614. doi:10.1038/mt.2010.105
51. Saari H, Lazaro-Ibanez E, Viitala T, Vuorimaa-Laukkanen E, Siljander P, Yliperttula M. Microvesicle- and exosome-mediated drug delivery enhances the cytotoxicity of Paclitaxel in autologous prostate cancer cells. J Control Release. 2015;220(Pt B):727–737. doi:10.1016/j.jconrel.2015.09.031
52. Walker S, Busatto S, Pham A, et al. Extracellular vesicle-based drug delivery systems for cancer treatment. Theranostics. 2019;9(26):8001–8017. doi:10.7150/thno.37097
53. Apparailly F, Jorgensen C. siRNA-based therapeutic approaches for rheumatic diseases. Nat Rev Rheumatol. 2013;9(1):56–62. doi:10.1038/nrrheum.2012.176
54. Tatiparti K, Sau S, Kashaw SK, Iyer AK. siRNA delivery strategies: a comprehensive review of recent developments. Nanomaterials. 2017;7(4):77. doi:10.3390/nano7040077
55. Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16(3):203–222. doi:10.1038/nrd.2016.246
56. Liu T, Zhang X, Du L, et al. Exosome-transmitted miR-128-3p increase chemosensitivity of oxaliplatin-resistant colorectal cancer. Mol Cancer. 2019;18(1):43. doi:10.1186/s12943-019-0981-7
57. Baldari S, Di Rocco G, Magenta A, Picozza M, Toietta G. Extracellular vesicles-encapsulated microRNA-125b produced in genetically modified mesenchymal stromal cells inhibits hepatocellular carcinoma cell proliferation. Cells. 2019;8(12):1560. doi:10.3390/cells8121560
58. Zhang K, Dong C, Chen M, et al. Extracellular vesicle-mediated delivery of miR-101 inhibits lung metastasis in osteosarcoma. Theranostics. 2020;10(1):411–425. doi:10.7150/thno.33482
59. Garneau JE, Dupuis ME, Villion M, et al. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature. 2010;468(7320):67–71. doi:10.1038/nature09523
60. Lin Y, Wu J, Gu W, et al. Exosome-liposome hybrid nanoparticles deliver CRISPR/Cas9 system in MSCs. Adv Sci. 2018;5(4):1700611. doi:10.1002/advs.201700611
61. Kanada M, Kim BD, Hardy JW, et al. Microvesicle-mediated delivery of minicircle DNA results in effective gene-directed enzyme prodrug cancer therapy. Mol Cancer Ther. 2019;18(12):2331–2342. doi:10.1158/1535-7163.MCT-19-0299
62. Batagov AO, Kuznetsov VA, Kurochkin IV. Identification of nucleotide patterns enriched in secreted RNAs as putative cis-acting elements targeting them to exosome nano-vesicles. BMC Genomics. 2011;12(Suppl 3):S18. doi:10.1186/1471-2164-12-S3-S18
63. Bolukbasi MF, Mizrak A, Ozdener GB, et al. miR-1289 and “Zipcode”-like sequence enrich mRNAs in microvesicles. Mol Ther Nucleic Acids. 2012;1:e10. doi:10.1038/mtna.2011.2
64. Lee YS, Kim SH, Cho JA, Kim CW. Introduction of the CIITA gene into tumor cells produces exosomes with enhanced anti-tumor effects. Exp Mol Med. 2011;43(5):281–290. doi:10.3858/emm.2011.43.5.029
65. Mizrak A, Bolukbasi MF, Ozdener GB, et al. Genetically engineered microvesicles carrying suicide mRNA/protein inhibit schwannoma tumor growth. Mol Ther. 2013;21(1):101–108. doi:10.1038/mt.2012.161
66. Sterzenbach U, Putz U, Low LH, Silke J, Tan SS, Howitt J. Engineered exosomes as vehicles for biologically active proteins. Mol Ther. 2017;25(6):1269–1278. doi:10.1016/j.ymthe.2017.03.030
67. Barok M, Puhka M, Vereb G, Szollosi J, Isola J, Joensuu H. Cancer-derived exosomes from HER2-positive cancer cells carry trastuzumab-emtansine into cancer cells leading to growth inhibition and caspase activation. BMC Cancer. 2018;18(1):504. doi:10.1186/s12885-018-4418-2
68. Noguchi K, Hirano M, Hashimoto T, Yuba E, Takatani-Nakase T, Nakase I. Effects of lyophilization of arginine-rich cell-penetrating peptide-modified extracellular vesicles on intracellular delivery. Anticancer Res. 2019;39(12):6701–6709. doi:10.21873/anticanres.13885
69. Liu B, Gordon WP, Richmond W, Groessl T, Tuntland T. Use of solubilizers in preclinical formulations: effect of Cremophor EL on the pharmacokinetic properties on early discovery compounds. Eur J Pharm Sci. 2016;87:52–57.
70. Kim MS, Haney MJ, Zhao Y, et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine. 2016;12(3):655–664. doi:10.1016/j.nano.2015.10.012
71. Kim MS, Haney MJ, Zhao Y, et al. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: in vitro and in vivo evaluations. Nanomedicine. 2018;14(1):195–204. doi:10.1016/j.nano.2017.09.011
72. Wei H, Chen J, Wang S, et al. A nanodrug consisting of doxorubicin and exosome derived from mesenchymal stem cells for osteosarcoma treatment in vitro. Int J Nanomedicine. 2019;14:8603–8610. doi:10.2147/IJN.S218988
73. Gong C, Tian J, Wang Z, et al. Functional exosome-mediated co-delivery of doxorubicin and hydrophobically modified microRNA 159 for triple-negative breast cancer therapy. J Nanobiotechnology. 2019;17(1):93. doi:10.1186/s12951-019-0526-7
74. Huyan T, Du Y, Huang Q, Huang Q, Li Q. Uptake characterization of tumor cell-derived exosomes by natural killer cells. Iran J Public Health. 2018;47(6):803–813.
75. Ohno S, Takanashi M, Sudo K, et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther. 2013;21(1):185–191. doi:10.1038/mt.2012.180
76. Kooijmans SA, Aleza CG, Roffler SR, van Solinge WW, Vader P, Schiffelers RM. Display of GPI-anchored anti-EGFR nanobodies on extracellular vesicles promotes tumour cell targeting. J Extracell Vesicles. 2016;5:31053. doi:10.3402/jev.v5.31053
77. Gomari H, Forouzandeh Moghadam M, Soleimani M. Targeted cancer therapy using engineered exosome as a natural drug delivery vehicle. Onco Targets Ther. 2018;11:5753–5762. doi:10.2147/OTT.S173110
78. Bellavia D, Raimondo S, Calabrese G, et al. Interleukin 3- receptor targeted exosomes inhibit in vitro and in vivo chronic myelogenous leukemia cell growth. Theranostics. 2017;7(5):1333–1345. doi:10.7150/thno.17092
79. Liang G, Kan S, Zhu Y, Feng S, Feng W, Gao S. Engineered exosome-mediated delivery of functionally active miR-26a and its enhanced suppression effect in HepG2 cells. Int J Nanomedicine. 2018;13:585–599. doi:10.2147/IJN.S154458
80. Yoshida K, Tsuda M, Matsumoto R, et al. Exosomes containing ErbB2/CRK induce vascular growth in premetastatic niches and promote metastasis of bladder cancer. Cancer Sci. 2019;110(7):2119–2132. doi:10.1111/cas.14080
81. Li Y, Liu Y, Xiu F, et al. Characterization of exosomes derived from Toxoplasma gondii and their functions in modulating immune responses. Int J Nanomedicine. 2018;13:467–477. doi:10.2147/IJN.S151110
82. Liu H, Shen M, Zhao D, et al. The effect of triptolide-loaded exosomes on the proliferation and apoptosis of human ovarian cancer SKOV3 cells. Biomed Res Int. 2019;2019:2595801.
83. Wiklander OP, Nordin JZ, O’Loughlin A, et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles. 2015;4:26316. doi:10.3402/jev.v4.26316
84. Kanada M, Bachmann MH, Hardy JW, et al. Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proc Natl Acad Sci U S A. 2015;112(12):E1433–1442. doi:10.1073/pnas.1418401112
85. Lai CP, Kim EY, Badr CE, et al. Visualization and tracking of tumour extracellular vesicle delivery and RNA translation using multiplexed reporters. Nat Commun. 2015;6:7029. doi:10.1038/ncomms8029
86. Yim N, Ryu SW, Choi K, et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module. Nat Commun. 2016;7:12277. doi:10.1038/ncomms12277
87. Pi F, Binzel DW, Lee TJ, et al. Nanoparticle orientation to control RNA loading and ligand display on extracellular vesicles for cancer regression. Nat Nanotechnol. 2018;13(1):82–89. doi:10.1038/s41565-017-0012-z
88. Smyth T, Petrova K, Payton NM, et al. Surface functionalization of exosomes using click chemistry. Bioconjug Chem. 2014;25(10):1777–1784. doi:10.1021/bc500291r
89. Kooijmans SAA, Fliervoet LAL, van der Meel R, et al. PEGylated and targeted extracellular vesicles display enhanced cell specificity and circulation time. J Control Release. 2016;224:77–85. doi:10.1016/j.jconrel.2016.01.009
90. Ichihara M, Shimizu T, Imoto A, et al. Anti-PEG IgM response against PEGylated liposomes in mice and rats. Pharmaceutics. 2010;3(1):1–11. doi:10.3390/pharmaceutics3010001
91. Zhu Q, Ling X, Yang Y, et al. Embryonic stem cells-derived exosomes endowed with targeting properties as chemotherapeutics delivery vehicles for glioblastoma therapy. Adv Sci. 2019;6(6):1801899. doi:10.1002/advs.201801899
92. Li Y, Gao Y, Gong C, et al. A33 antibody-functionalized exosomes for targeted delivery of doxorubicin against colorectal cancer. Nanomedicine. 2018;14(7):1973–1985. doi:10.1016/j.nano.2018.05.020
93. Nakase I, Kobayashi NB, Takatani-Nakase T, Yoshida T. Active macropinocytosis induction by stimulation of epidermal growth factor receptor and oncogenic Ras expression potentiates cellular uptake efficacy of exosomes. Sci Rep. 2015;5:10300. doi:10.1038/srep10300
94. Nakase I, Futaki S. Combined treatment with a pH-sensitive fusogenic peptide and cationic lipids achieves enhanced cytosolic delivery of exosomes. Sci Rep. 2015;5:10112. doi:10.1038/srep10112
95. Nakase I, Noguchi K, Fujii I, Futaki S. Vectorization of biomacromolecules into cells using extracellular vesicles with enhanced internalization induced by macropinocytosis. Sci Rep. 2016;6:34937. doi:10.1038/srep34937
96. Nakase I, Ueno N, Katayama M, et al. Receptor clustering and activation by multivalent interaction through recognition peptides presented on exosomes. Chem Commun. 2017;53(2):317–320. doi:10.1039/C6CC06719K
97. Nakase I, Noguchi K, Aoki A, Takatani-Nakase T, Fujii I, Futaki S. Arginine-rich cell-penetrating peptide-modified extracellular vesicles for active macropinocytosis induction and efficient intracellular delivery. Sci Rep. 2017;7(1):1991. doi:10.1038/s41598-017-02014-6
98. Guo M, Wu F, Hu G, et al. Autologous tumor cell-derived microparticle-based targeted chemotherapy in lung cancer patients with malignant pleural effusion. Sci Transl Med. 2019;11(474). doi:10.1126/scitranslmed.aav5519
99. Melnik BC, John SM, Schmitz G. Milk: an exosomal microRNA transmitter promoting thymic regulatory T cell maturation preventing the development of atopy? J Transl Med. 2014;12:43. doi:10.1186/1479-5876-12-43
100. Aqil F, Munagala R, Jeyabalan J, et al. Milk exosomes - Natural nanoparticles for siRNA delivery. Cancer Lett. 2019;449:186–195. doi:10.1016/j.canlet.2019.02.011
101. Li Z, Wang H, Yin H, Bennett C, Zhang HG, Guo P. Arrowtail RNA for ligand display on ginger exosome-like nanovesicles to systemic deliver siRNA for cancer suppression. Sci Rep. 2018;8(1):14644. doi:10.1038/s41598-018-32953-7
102. Baldini N, Torreggiani E, Roncuzzi L, Perut F, Zini N, Avnet S. Exosome-like nanovesicles isolated from citrus limon L. exert antioxidative effect. Curr Pharm Biotechnol. 2018;19(11):877–885. doi:10.2174/1389201019666181017115755
103. Ju S, Mu J, Dokland T, et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol Ther. 2013;21(7):1345–1357. doi:10.1038/mt.2013.64
104. Mu J, Zhuang X, Wang Q, et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol Nutr Food Res. 2014;58(7):1561–1573. doi:10.1002/mnfr.201300729
105. Zhu L, Gangadaran P, Kalimuthu S, et al. Novel alternatives to extracellular vesicle-based immunotherapy - exosome mimetics derived from natural killer cells. Artif Cells Nanomed Biotechnol. 2018;46(sup3):S166–S179. doi:10.1080/21691401.2018.1489824
106. Jo W, Jeong D, Kim J, et al. Microfluidic fabrication of cell-derived nanovesicles as endogenous RNA carriers. Lab Chip. 2014;14(7):1261–1269. doi:10.1039/C3LC50993A
107. Yoon J, Jo W, Jeong D, Kim J, Jeong H, Park J. Generation of nanovesicles with sliced cellular membrane fragments for exogenous material delivery. Biomaterials. 2015;59:12–20. doi:10.1016/j.biomaterials.2015.04.028
108. Goh WJ, Zou S, Ong WY, et al. Bioinspired cell-derived nanovesicles versus exosomes as drug delivery systems: a cost-effective alternative. Sci Rep. 2017;7(1):14322. doi:10.1038/s41598-017-14725-x
109. Goh WJ, Zou S, Czarny B, Pastorin G. nCVTs: a hybrid smart tumour targeting platform. Nanoscale. 2018;10(15):6812–6819. doi:10.1039/C7NR08720A
110. Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65(1):36–48. doi:10.1016/j.addr.2012.09.037
111. Bulbake U, Doppalapudi S, Kommineni N, Khan W. Liposomal formulations in clinical use: an updated review. Pharmaceutics. 2017;9(2):12. doi:10.3390/pharmaceutics9020012
112. Farooq MA, Aquib M, Farooq A, et al. Recent progress in nanotechnology-based novel drug delivery systems in designing of cisplatin for cancer therapy: an overview. Artif Cells Nanomed Biotechnol. 2019;47(1):1674–1692. doi:10.1080/21691401.2019.1604535
113. Cheng R, Liu L, Xiang Y, et al. Advanced liposome-loaded scaffolds for therapeutic and tissue engineering applications. Biomaterials. 2020;232:119706. doi:10.1016/j.biomaterials.2019.119706
114. Hoshino A, Costa-Silva B, Shen TL, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–335. doi:10.1038/nature15756
115. Gudbergsson JM, Jonsson K, Simonsen JB, Johnsen KB. Systematic review of targeted extracellular vesicles for drug delivery - Considerations on methodological and biological heterogeneity. J Control Release. 2019;306:108–120. doi:10.1016/j.jconrel.2019.06.006
116. Piffoux M, Silva AKA, Wilhelm C, Gazeau F, Tareste D. Modification of extracellular vesicles by fusion with liposomes for the design of personalized biogenic drug delivery systems. ACS Nano. 2018;12(7):6830–6842. doi:10.1021/acsnano.8b02053
117. Yeo RW, Lai RC, Zhang B, et al. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev. 2013;65(3):336–341. doi:10.1016/j.addr.2012.07.001
118. Cui X, He Z, Liang Z, Chen Z, Wang H, Zhang J. Exosomes from adipose-derived mesenchymal stem cells protect the myocardium against ischemia/reperfusion injury through Wnt/β-catenin signaling pathway. J Cardiovasc Pharmacol. 2017;70(4):225–231. doi:10.1097/FJC.0000000000000507
119. Wang B, Yao K, Huuskes BM, et al. Mesenchymal stem cells deliver exogenous microRNA-let7c via exosomes to attenuate renal fibrosis. Mol Ther. 2016;24(7):1290–1301. doi:10.1038/mt.2016.90
120. Bai L, Shao H, Wang H, et al. Effects of mesenchymal stem cell-derived exosomes on experimental autoimmune uveitis. Sci Rep. 2017;7(1):4323. doi:10.1038/s41598-017-04559-y
121. Chen Z, Wang H, Xia Y, Yan F, Lu Y. Therapeutic potential of mesenchymal cell-derived miRNA-150-5p-expressing exosomes in rheumatoid arthritis mediated by the modulation of MMP14 and VEGF. J Immunol. 2018;201(8):2472–2482. doi:10.4049/jimmunol.1800304
122. Ding Y, Cao F, Sun H, et al. Exosomes derived from human umbilical cord mesenchymal stromal cells deliver exogenous miR-145-5p to inhibit pancreatic ductal adenocarcinoma progression. Cancer Lett. 2019;442:351–361. doi:10.1016/j.canlet.2018.10.039
123. Rialland P, Lankar D, Raposo G, Bonnerot C, Hubert P. BCR-bound antigen is targeted to exosomes in human follicular lymphoma B-cells. Biol Cell. 2006;98(8):491–501. doi:10.1042/BC20060027
124. Blanchard N, Lankar D, Faure F, et al. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/ζ complex. J Immunol. 2002;168(7):3235–3241. doi:10.4049/jimmunol.168.7.3235
125. Del Cacho E, Gallego M, Lee SH, et al. Induction of protective immunity against Eimeria tenella infection using antigen-loaded dendritic cells (DC) and DC-derived exosomes. Vaccine. 2011;29(21):3818–3825. doi:10.1016/j.vaccine.2011.03.022
126. Lugini L, Cecchetti S, Huber V, et al. Immune surveillance properties of human NK cell-derived exosomes. J Immunol. 2012;189(6):2833–2842. doi:10.4049/jimmunol.1101988
127. Jong AY, Wu CH, Li J, et al. Large-scale isolation and cytotoxicity of extracellular vesicles derived from activated human natural killer cells. J Extracell Vesicles. 2017;6(1):1294368. doi:10.1080/20013078.2017.1294368
128. Baginska J, Viry E, Paggetti J, et al. The critical role of the tumor microenvironment in shaping natural killer cell-mediated anti-tumor immunity. Front Immunol. 2013;4:490. doi:10.3389/fimmu.2013.00490
129. Ishikawa E, Tsuboi K, Saijo K, et al. Autologous natural killer cell therapy for human recurrent malignant glioma. Anticancer Res. 2004;24(3b):1861–1871.
130. Koehl U, Sorensen J, Esser R, et al. IL-2 activated NK cell immunotherapy of three children after haploidentical stem cell transplantation. Blood Cells Mol Dis. 2004;33(3):261–266. doi:10.1016/j.bcmd.2004.08.013
131. Passweg JR, Tichelli A, Meyer-Monard S, et al. Purified donor NK-lymphocyte infusion to consolidate engraftment after haploidentical stem cell transplantation. Leukemia. 2004;18(11):1835–1838. doi:10.1038/sj.leu.2403524
132. Fais S. NK cell-released exosomes: natural nanobullets against tumors. Oncoimmunology. 2013;2(1):e22337. doi:10.4161/onci.22337
133. Li Q, Wang H, Peng H, Huyan T, Cacalano NA. Exosomes: versatile nano mediators of immune regulation. Cancers. 2019;11(10):1557. doi:10.3390/cancers11101557
134. Wang G, Hu W, Chen H, Shou X, Ye T, Xu Y. Cocktail strategy based on NK cell-derived exosomes and their biomimetic nanoparticles for dual tumor therapy. Cancers. 2019;11(10):1560. doi:10.3390/cancers11101560
135. Ortega FG, Roefs MT, de Miguel Perez D, et al. Interfering with endolysosomal trafficking enhances release of bioactive exosomes. Nanomedicine. 2019;20:102014. doi:10.1016/j.nano.2019.102014
136. Ban JJ, Lee M, Im W, Kim M. Low pH increases the yield of exosome isolation. Biochem Biophys Res Commun. 2015;461(1):76–79. doi:10.1016/j.bbrc.2015.03.172
137. Haraszti RA, Miller R, Stoppato M, et al. Exosomes produced from 3D cultures of MSCs by tangential flow filtration show higher yield and improved activity. Mol Ther. 2018;26(12):2838–2847. doi:10.1016/j.ymthe.2018.09.015
138. Caponnetto F, Manini I, Skrap M, et al. Size-dependent cellular uptake of exosomes. Nanomedicine. 2017;13(3):1011–1020. doi:10.1016/j.nano.2016.12.009
139. Shang L, Nienhaus K, Nienhaus GU. Engineered nanoparticles interacting with cells: size matters. J Nanobiotechnology. 2014;12:5. doi:10.1186/1477-3155-12-5
140. Zhu X, Badawi M, Pomeroy S, et al. Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. J Extracell Vesicles. 2017;6(1):1324730. doi:10.1080/20013078.2017.1324730
141. Barile L, Vassalli G. Exosomes: therapy delivery tools and biomarkers of diseases. Pharmacol Ther. 2017;174:63–78. doi:10.1016/j.pharmthera.2017.02.020
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.