Back to Journals » Cancer Management and Research » Volume 12
Extracellular Collagen Mediates Osteosarcoma Progression Through an Integrin α2β1/JAK/STAT3 Signaling Pathway
Authors Wei D, Li C, Ye J, Xiang F, Liu J
Received 26 July 2020
Accepted for publication 29 October 2020
Published 24 November 2020 Volume 2020:12 Pages 12067—12075
DOI https://doi.org/10.2147/CMAR.S273466
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Eileen O'Reilly
Daiqing Wei,1,2 Cui Li,3 Junwu Ye,1,2 Feifan Xiang,1,2 Juncai Liu1,2
1Department of Orthopaedics, The Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, People’s Republic of China; 2Sichuan Provincial Laboratory of Orthopaedic Engineering, Luzhou, Sichuan, People’s Republic of China; 3Department of Nosocomial Infection Control, The Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, People’s Republic of China
Correspondence: Juncai Liu
Department of Orthopaedics, The Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan Province 646000, People’s Republic of China
Tel +86-18015756304
Fax +86-8303165453
Email [email protected]
Background: Osteosarcoma development is a complex set which is determined by various factors. Many patients suffered from sustained osteosarcoma growth and revealed poor response to clinical interventions. However, the underlying mechanisms of osteosarcoma development still remain unclear.
Methods: In our study, we isolated osteosarcoma tissues from clinical patients, which were divided into high degree group (stage G1∼G2) and low degree group (stage G0). The expression of type I collagen, integrin and STAT3 in tumor tissues were analyzed by immunohistochemistry or immunofluorescence. The collagen-induced cells proliferation was detected by CCK8 and colony formation analysis. The activation of JAK/STAT3 signal was examined by Western blotting and immunofluorescence. The anticancer effects of integrin α 2β 1 peptide were analyzed by Sao-2-bearing mice model.
Results: Our results implicated that type I collagen could facilitate malignant osteosarcoma development in patients. In vitro, 2D collagen culture also efficiently mediated the stemness up-regulation of osteosarcoma cells, resulting in the strengthened capability of cells proliferation and tumorigenesis. In mechanism, we found that type I collagen could facilitate the activation of JAK/STAT3 signals through integrin α 2β 1, which elicited tumor-sustained growth and cancer relapse. In tumor-bearing mice model, integrin α 2β 1 signals inhibitor significantly suppressed the osteosarcoma cells proliferation and their tumorigenic ability, which improved the outcome of chemotherapy/radiotherapy.
Conclusion: Our study demonstrated that type I collagen could mediate osteosarcoma development through an integrin α 2β 1/JAK/STAT3 signaling pathway. Blockade of integrin α 2β 1 by α 2β 1 inhibitor efficiently improved outcome of chemotherapy/radiotherapy, which provided new insights for eradicating tumors in clinic.
Keywords: collagen, osteosarcoma, integrin α 2β 1, JAK/STAT3
Introduction
Osteosarcoma is one of the most common bone associated malignant carcinomas with a high risk of invasion and recurrence.1 Currently, localized osteosarcoma was traditionally treated with surgery combined with adjuvant chemotherapy or radiotherapy. However, many osteosarcoma patients still suffer from poor prognosis due to sustained tumor growth and distant metastasis.2,3 The disease also has a high potential of recurrence due to residual tumorigenic cells, or named as cancer stem cells (CSCs).4 Thus, there is an urgent demand to clarify the underlying mechanisms of tumor progression and describe innovative therapeutic approaches, which impairs tumor sustained growth and retards carcinoma recurrence in clinical osteosarcoma treatment.
In recent years, extracellular matrix (ECM) has been considered as an essential culprit for clinical osteosarcoma development and recurrence.5,6 Compelling reports implicate that multiple elements in ECM, such as collagen, fibrinogen and laminin, are tightly associated with tumor growth and cancer cells migration.7–9 Among those ECM compounds, type I collagen have been reported to regulate tumor cells proliferation and migration through activation of pro-survival signaling pathways in several tumor types.10,11 More importantly, increasing evidence has implicated that 2D or 3D collagen culture is capable of mediating the stem-like phenotypes of various cancer cells lines, including osteosarcoma cells, eventually resulting in high potential of tumorigenicity and proliferative characteristic of tumor cells.6,12–15 However, there is a lack of evidence to explain the potential correlation between collagen and osteosarcoma development, and how collagen mediates osteosarcoma progression has yet to be explored.
In our study, we further provided evidence to suggest that type I collagen is tightly correlated to the tumor development in osteosarcoma patients. Additionally, we identified the mechanisms of collagen mediated osteosarcoma development, which depends on an integrin α2β1/JAK/STAT3 signaling pathway. Here, we further took advantages from the combination of integrin α2β1 inhibitor and chemotherapy/radiotherapy, which efficiently suppressed tumorigenesis and tumor growth, providing innovative therapeutic revenues for osteosarcoma treatment.
Materials and Methods
Cell Culture and Reagents
Human osteosarcoma cell lines Saos-2 and MG-63 were purchased from the American Type Culture Collection (ATCC, USA). All tumor cells were cultured using RPMI1640 complete medium, containing 10% fetal bovine serum (Gibco, MA, USA) under 37°C and 5% CO2. For 2D collagen culture, type I collagen (Solarbio, Beijing, China) was diluted to 2.5 mg/mL with sterile water. Then, 25μL 10×phosphate-buffered saline (PBS) and 20μL 1N NaOH solution (Solarbio, Beijing, China) were subsequently added into the mixture. The 295μL of the collagen mixture was seeded into each well of a 24-well plate and mixed thoroughly. After 37°C incubation for 2 hours, the collagen mixtures became solid and cancer cells were seeded on the 2D collagen gel for 2D cells culture. α2β1 Integrin Ligand Peptide and STAT3 inhibitor FLLL32 were purchased from Med Chem Express (NJ, USA). Other reagents are of HPLC standard and purchased from Transgene biological Company (Beijing, China).
Collection of Patient Tumor Cells
Human osteosarcoma tissues were collected at the Affiliated Hospital of Southwest Medical University in this study. Patients were divided into high degree group (HD, stage G1~G2) and low degree group (LD, stage G0). Primary osteosarcoma tissues were sterilely obtained after the core-needle biopsy. Samples were stored at −80°C immediately. This study was approved by the local institutional review board of the Affiliated Hospital of Southwest Medical University. The internal review board’s approval and patients’ informed consent for this study were also obtained.
Cell Proliferation Experiment
For cell proliferation analysis, 2000 cells were inoculated in a 96-well plate and cultured at incubator. After 72 hours, 10ul CCK8 solution (Thermo, MA, USA) was added to each well, following with incubating for 2 hours. The absorbance at 450nm was detected with a microplate reader (Bio-rad, CA, USA). Each experiment was performed independently for at least 3 times.
Cell Colony Formation
For colony formation analysis, 200 tumor cells were seeded in a 6-well plate. After 14 days, cells were fixed by methanol and stained by giemsa staining solution (Solarbio, Beijing, China). The colony numbers were counted for further analysis. Each experiment was conducted for at least independent 3 times.
Western Blot
The total proteins of osteosarcoma cells were extracted by 1× SDS gel protein buffer (Solarbio, Beijing, China). Then, samples were cooked at 95°C to denature the proteins. The protein was transferred to PVDF membrane (Solarbio, Beijing, China) using an electrotransfer system according to standard procedures. After transferring the membrane, the PVDF membranes were blocked with 0.5% skimmed milk powder (diluted in PBS solution) for 1 hour at room temperature, and the primary antibody was incubated at 4°C overnight. The primary antibodies are list: integrin α2 (1:100, Santa Cruz, Texas, USA), integrin β1 (1:1000, CST, Massachusetts, USA), JAK1 (1:1000, Abcam, Cambridge, UK), p-JAK1 (1:1000, Abcam, Cambridge, UK), JAK2 (1:1000, Abcam, Cambridge, UK), p-JAK2 (1:1000, Abcam, Cambridge, UK), STAT3 (1:1000, Abcam, Cambridge, UK), p-STAT3 (1:1000, Abcam, Cambridge, UK) and actin (1:1000, Abcam, Cambridge, UK). After that, the HRP secondary antibody (1:5000, Abcam, Cambridge, UK) were incubated at room temperature for 1 hour, and finally treated with super-sensitive luminescent solution (Solarbio, Beijing, China).
Immunofluorescence Staining
Tumor samples were fixed in a 2% paraformaldehyde ice bath (Solarbio, Beijing, China), incubated in 0.2% PBST at 37°C for 15 minutes, and blocked with 2.5% BSA (Solarbio, Beijing, China). Then, the samples were treated with type I collagen antibody (1:400, Abcam, Cambridge, UK) at 4°C for 3 hours, following with secondary antibody (1:1000, Abcam, Cambridge, UK) for 1 hour. Samples were analyzed by confocal microscope (Olympus, Tokyo, Japan).
Animal Protocols
All animal experiments were approved by the Animal Protection Committee of the Affiliated Hospital of Southwest Medical University and carried out in accordance with the guidelines and regulations of the Ethics Committee of the Affiliated Hospital of Southwest Medical University for the welfare of animals in cancer research. Animal Ethics Number: 201801–050. Female NOD-SCID mice (6–8 weeks old) were purchased from Huafukang (Beijing, China). All mice were housed in a specific pathogen-free (SPF) facility. For in vivo cells proliferation analysis, 2×106 Saos-2 or MG-63 in 50uL PBS were subcutaneously injected into mice. The tumor volume was recorded every day until day 50. The calculation formula of tumor volume is: tumor volume = length × width2/2. For tumorigenesis analysis, 1×105 Saos-2 or MG-63 in 50uL PBS were subcutaneously injected into mice. After 30 days, the tumor-bearing mice were recorded for further analysis. For tumor suppression analysis, 2×106 Saos-2 or MG-63 in 50uL PBS were subcutaneously injected into mice. On day 14, 100ul of PBS, IFO (2 mg/kg), and α2β1 integrin ligand peptide (0.5 mg/kg) were injected into the mice by tail vein injection twice a week. The treatment was performed for 2 weeks. As a blank control, mice were injected with an equal volume of low-key saline. For radiotherapy, mice were treated with radiotherapy (0.5 Gy per mouse) twice a week. The treatment was performed for 2 weeks. The tumor volume and survival time of tumor-bearing mice were recorded. The calculation formula of tumor volume is: tumor volume = length × width2/2. All animal experiments were monitored by the Affiliated Hospital of Southwest Medical University.
Statistical Analysis
Each experiment is repeated for at least 3 times. The results are expressed as mean ± SEM, and GraphPad 6.0 software is used for significance analysis and statistics. Statistical significance between groups was calculated by Student’s t test for two groups or by one-way ANOVA for more than two groups. The survival rates were determined by Kaplan–Meier survival analysis (*p<0.05; **p<0.01; ***p<0.001; ns, no significant difference).
Results
Type I Collagen Promoted Osteosarcoma Cells Proliferation
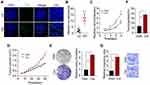
To explore the role of type I collagen in osteosarcoma development, we collected osteosarcoma tissues from patients and divided tumor tissues into high degree group (HD, stage G1~G2) and low degree group (LD, stage G0). Notably, elevated expression of type I collagen was observed in HD osteosarcoma tissues (Figure 1A and B), reminding us the potential role of type I collagen in osteosarcoma progression. Next, we further added soluble type I collagen into the culture medium of osteosarcoma cells Saos-2/MG-63. However, no significant difference of cells proliferation was observed after addition of collagen (Supplementary Figure 1A and B). Given the presence of insoluble collagen in mammalian tissues, we further seeded Saos-2 and MG-63 cells on the 2D type I collagen gel as described in materials. After 4 days of 2D collagen culture, Saos-2 and MG-63 cells were collected for proliferation analysis. Intriguingly, pre-culture in 2D collagen system significantly strengthened the proliferative characteristic of osteosarcoma cells compared to dish cultured group (Figure 1C and Supplementary Figure 1C). The same results were duplicated in vivo (Figure 1D and Supplementary Figure 1D). More importantly, 2D collagen culture also mediated enhanced capability of colony formation (Figure 1E and Supplementary Figure 1E) and tumorigenesis (Figure 1F and Supplementary Figure 1F) of Saos-2 and MG-63 cells, reminding that the presence of type I collagen might facilitate the neoplasm recurrence or tumor development in osteosarcoma. Meanwhile, the transwell analysis indicated that 2D collagen culture also promoted the capability of cells migration (Figure 1G). Together, we suggested that type I collagen could facilitate osteosarcoma progression.
Type I Collagen Mediated Osteosarcoma Development Through Integrin α2β1
Our previous data have implicated that insoluble 2D collagen, instead of soluble collagen, could mediate osteosarcoma cells proliferation, which provides evidence to suggest that collagen might facilitate osteosarcoma development through biomechanical force signals rather than chemical signals.16 Integrin α2β1, which belongs to integrin family, is involved in the biomechanical force signals transduction in tumor cells. Here, we observed enhanced expression of integrin α2 and integrin β1 in 2D collagen cultured Saos-2 and MG-63 cells (Figure 2A and Supplementary Figure 2A). To further explore the role of integrin α2β1 in osteosarcoma development, we used α2β1 integrin ligand peptide to block the integrin α2β1 signals. As anticipated, blockade of integrin α2β1 efficiently suppressed the proliferation (Figure 2B and Supplementary Figure 2B) and tumor growth (Figure 2C and Supplementary Figure 2C) of 2D collagen cultured Saos-2 and MG-63 cells. Additionally, the integrin α2β1 inhibition also weakened the capability of colony formation of tumor cells (Figure 2D and Supplementary Figure 2D) or tumorigenesis in vivo (Figure 2E and Supplementary Figure 2E), implicating that blockade of integrin α2β1 could suppress the osteosarcoma development induced by collagen. Meanwhile, blockade of integrin α2β1 suppressed the cells migration of 2D collagen-cultured Sao-2 cells (Figure 2F). Additionally, we also detected the integrin α2 and integrin β1 expression in those osteosarcoma tissues (divided into high collagen expression group and low collagen expression group). Consistent to our previous results, increasing expression of integrin α2 and integrin β1 was observed in high collagen expression group (Figure 2G). Additionally, we also found a high correlation (R2>0.5) between type I collagen expression and integrin α2/integrin β1 in our osteosarcoma tissues (Figure 2H and I). Together, those results implicated that type I collagen mediates osteosarcoma progression through integrin α2β1 receptor.
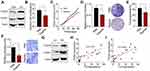 |
Figure 2 Type I collagen mediates tumor progression through integrin α2β1. (A) Western blotting of integrin α2 and integrin β1 in Saos-2 and Collagen-cultured Saos-2 cells. (B) Relative cell proliferation of collagen-cultured Saos-2 cells treated with α2β1 integrin ligand peptide (0.5mM, 48 hours). (C) tumor volume of collagen-cultured Saos-2 cells tumor-bearing mice pre-treated with α2β1 integrin ligand peptide (0.5 mM, 48 hours treatment before injection). (D) Relative colony formation of collagen pre-cultured Saos-2 cells pre-treated with α2β1 integrin ligand peptide (0.5 mg/kg, 48 hours). (E) Tumorigenesis of collagen-cultured Saos-2 cells pre-treated with α2β1 integrin ligand peptide (0.5 mM, 48 hours treatment before injection) in NOS-SCID mice. (F) Relative transwell cells number of 2D collagen-cultured Sao-2 cells treated with PBS or α2β1 integrin ligand peptide (0.5mM, 48 hours). (G) the expression of integrin α2 and integrin β1 in the osteosarcoma tissues from HD and LD patients. (H) The correlation analysis of type I collagen expression and integrin α2 expression detected by Western blotting (R2=0.5934). (I) The correlation analysis of type I collagen expression and integrin β1 expression detected by Western blotting (R2=0.7431). *Indicates P <0.05. |
Type I Collagen Mediated the JAK1/2-STAT3 Signals Activation
Next, we further explored the downstream signaling molecules of integrin α2β1. Here, our Western blotting analysis showed that 2D collagen culture could facilitate the phosphorylation of JAK1/2 and STAT3 in Saos-2 and MG-63 cells, whereas blockade of integrin α2β1 suppressed the expression of JAK1/2 and STAT3 (Figure 3A and Supplementary Figure 3A), suggesting that 2D collagen culture mediated the JAK1/2-STAT3 signal activation through integrin α2β1. To further explore the role of JAK1/2-STAT3 signal in osteosarcoma progression, we used FLLL32, an STAT3 inhibitor, to suppress the JAK1/2-STAT3 signal. Intriguingly, blockade of JAK1/2-STAT3 obviously suppressed the proliferation (Figure 3B and Supplementary Figure 3B) and tumor growth (Figure 3C and Supplementary Figure 3C) of 2D collagen-cultured Saos-2 and MG-63 cells. Additionally, blockade of JAK1/2-STAT3 signals inhibited the colony formation (Figure 3D and Supplementary Figure 3D) and tumorigenesis (Figure 3E and Supplementary Figure 3E) of 2D collagen-cultured Saos-2 and MG-63 cells, indicating that collagen regulated osteosarcoma development through JAK1/2-STAT3 signals. Next, we further examined the JAK1/2-STAT3 expression in osteosarcoma tissues from patients. Consistently, HD osteosarcoma tissues revealed a higher level of phosphorylated JAK1/2-STAT3 (Figure 3F). Those results suggested that Type I collagen mediated the JAK1/2-STAT3 signals activation to promote osteosarcoma growth.
Suppression of Integrin α2β1 Signal Strengthened the Anticancer Effects of Chemotherapy
Given the crucial role of integrin α2β1/JAK1/2-STAT3 signaling pathway in osteosarcoma development, it might be feasible to suppress integrin α2β1 for an improved anticancer effect. Thus, we used α2β1 integrin ligand peptide combined with chemotherapy/radiotherapy for osteosarcoma treatment. As a result, α2β1 integrin ligand peptide treatment significantly increased the cytotoxicity of IFO (Figure 4A) or radiotherapy (Figure 4B) to Saos-2 and MG-63. Next, we further established subcutaneous Saos-2-bearing mice and treated mice with PBS, α2β1 integrin ligand peptide, IFO/radiotherapy or α2β1 integrin ligand peptide combined with IFO/radiotherapy. As anticipated, suppression of integrin α2β1 signal remarkably strengthened the tumor suppression effects of IFO (Figure 4C) and prolonged the overall survival time (Figure 4D). Meanwhile, α2β1 integrin ligand peptide treatment also improved the outcome of radiotherapy in our subcutaneous tumor-bearing mice (Figure 4E and F). Those results reminded us that blockade of integrin α2β1 signal could efficiently strengthen the anticancer effects of traditional clinical intervention, which provides a novel sight in clinical osteosarcoma treatment.
Discussion
In our study, we further described the crucial role of type I collagen in osteosarcoma progression. The osteosarcoma tissues from patients provided evidence that enrichment of collagen could mediate the osteosarcoma development, leading to the carcinoma malignancy. Importantly, in vitro 2D collagen culture significantly strengthened the capability of proliferation and tumorigenesis of osteosarcoma cells. Our results also further demonstrated that collagen culture is capable of mediating the activation of JAK/STAT3 signals in osteosarcoma cells through integrin α2β1, eventually resulting in the tumor stemness up-regulation. More importantly, application of α2β1 integrin ligand peptide to suppress the integrin-associated signals efficiently improved the outcome of radiotherapy/chemotherapy, providing a novel strategy for osteosarcoma treatment.
Increasing evidence has implicated that elevated expression of COL1A1 gene, which encodes type I collagen protein submits, is tightly correlated to the poor outcome of osteosarcoma.17–19 Also, compelling reports demonstrated that type I could facilitate the tumor stemness up-regulation in several tumor types, such as breast cancer and colorectal cancer.20,21 In addition, the enrichment of collagen in tumor tissues might mediate the EMT process of tumor cell,22 resulting in the anabatic tumor invasion and distant metastasis. However, the correlation of type I collagen and osteosarcoma development still remains controversial. More importantly, the underlying mechanisms of type I collagen-induced tumor progression in osteosarcoma remain unclear. Indeed, our results suggested that addition of soluble collagen had no obvious influence on osteosarcoma cells proliferation, suggesting that collagen could not directly mediate the pro-survival pathways activation through chemical signals. Further, we seeded osteosarcoma cells on 2D collagen gel, in which osteosarcoma cells revealed enhanced stem-like phenotypes and proliferative characteristics. Our data provided evidence to suggest that type I collagen might participate in the osteosarcoma progression regulation through the physico-mechanical force associated signaling pathways. Intriguingly, previous reports have confirmed that the expression of biodynamic signal receptor integrin α2β1 are positively correlated to the outcome and tumor progression in osteosarcoma patients.23 Integrin α2β1 also has been demonstrated to promote cells migration and cancer metastasis in osteosarcoma.24 On the basis of these findings, we further determined the role of collagen in osteosarcoma stemness regulation, which is dependent on the integrin α2β1 involved JAK/STAT3 signaling pathway.
Given the essential role of integrin α2β1 in collagen-induced osteosarcoma progression, integrin α2β1 might represent a feasible therapeutic target in clinical osteosarcoma therapy. Indeed, small molecule inhibitors or neutralizing integrin binding with antibodies have been proved to efficiently suppress tumor progression and disrupt angiogenesis in immunodeficient mice models.25,26 In addition to tumor growth suppression, integrin α2β1 inhibition could also block tumor cells distant metastasis or peripheral tissue invasion.27,28 Also, the application of integrin α2β1 inhibitor could efficiently improve the outcome of cancer patients.29
Our study further identified the tumor suppression effects of α2β1 integrin ligand peptide in tumor-bearing mice when combined with chemotherapy/radiotherapy. Collectively, these data suggested that integrin α2β1 associated signal molecular, including integrin α2β1 and JAK/STAT3, might serve as markers of osteosarcoma diagnosis and malignancy analysis.
Based on the limitations of preliminary findings, our study firstly demonstrated the role of type I collagen in osteosarcoma development. Firstly, we found the correlation of type I collagen/integrin α2β1 and osteosarcoma cells stemness, suggesting that type I collagen mediated osteosarcoma cells stemness up-regulation, leading to malignant progression. Secondly, we further clarified the underlying mechanism of the osteosarcoma stemness induced by collagen. Our results proved that collagen could facilitate osteosarcoma development through an integrin α2β1/JAK/STAT3 signaling pathway. Third, the application of α2β1 integrin ligand peptide was proved to efficiently improve the anticancer effects of chemotherapy/radiotherapy in osteosarcoma-bearing mice model, providing new support for clinical integrin α2β1 inhibitors application. Finally, the expression level of type I collagen and integrin α2β1 in tumor tissues might serve as potential biomarkers for osteosarcoma progression analysis and novel treatment guidelines.
In conclusion, we demonstrated that type I collagen could mediate osteosarcoma cells stemness up-regulation through an integrin α2β1/JAK/STAT3 signaling pathway, eventually leading to tumor-sustained growth and development. Suppression of integrin α2β1 signals efficiently strengthened the anticancer effects of traditional clinical interventions, which might serve as an innovative approach for clinical osteosarcoma treatment.
Abbreviations
CSCs, cancer stem cells; ECM, extracellular matrix; PBS, phosphate-buffered saline; HD, high degree group; LD, low degree group; PVDF, polyvinylidene fluoride; BSA, bovine serum albumin; SPF, specific pathogen-free; IFO, ifosfamide; EMT, epithelial–mesenchymal transition.
Ethics Statement
This study was approved by the Ethical Review Committee of the Affiliated Hospital of Southwest Medical University. All experiments followed the guidelines and regulations of the Ethical Review Committee of the Affiliated Hospital of Southwest Medical University.
Acknowledgment
This work was supported by the Research Project of Education Department of Sichuan Province (grant number: 18ZA0533)
Disclosure
The authors report no conflicts of interest for this work.
References
1. El-Naggar AM, Clarkson PW, Negri GL, et al. HACE1 is a potential tumor suppressor in osteosarcoma. Cell Death Dis. 2019;10(1):21. doi:10.1038/s41419-018-1276-4
2. Zhao G-S, Gao Z-R, Zhang Q, et al. TSSC3 promotes autophagy via inactivating the Src-mediated PI3K/Akt/mTOR pathway to suppress tumorigenesis and metastasis in osteosarcoma, and predicts a favorable prognosis. J Exp Clin Cancer Res. 2018;37(1):188. doi:10.1186/s13046-018-0856-6
3. Misaghi A, Goldin A, Awad M, Kulidjian AA. Osteosarcoma: a comprehensive review. SICOT J. 2018;4:12. doi:10.1051/sicotj/2017028
4. Mitra A, Mishra L, Li S. EMT, CTCs and CSCs in tumor relapse and drug-resistance. Oncotarget. 2015;6(13):10697–10711. doi:10.18632/oncotarget.4037
5. Harisi R, Dudas J, Timar F, et al. Invasive growth and topoisomerase-switch induced by tumorous extracellular matrix in osteosarcoma cell culture. Cell Biol Int. 2005;29(11):959–967. doi:10.1016/j.cellbi.2005.08.010
6. Garcia-Mendoza MG, Inman DR, Ponik SM, et al. Neutrophils drive accelerated tumor progression in the collagen-dense mammary tumor microenvironment. Breast Cancer Res. 2016;18(1):49. doi:10.1186/s13058-016-0703-7
7. Harisi R, Tímár F, Pogány G, et al. Antiproliferative and antimigratory effects of doxorubicin in human osteosarcoma cells exposed to extracellular matrix. Anticancer Res. 2005;25:805–814.
8. Eble JA, Niland S. The extracellular matrix in tumor progression and metastasis. Clin Exp Metastasis. 2019;36(3):171–198.
9. Erdogan B, Webb DJ. Cancer-associated fibroblasts modulate growth factor signaling and extracellular matrix remodeling to regulate tumor metastasis. Biochem Soc Trans. 2017;45(1):229–236. doi:10.1042/BST20160387
10. Kokenyesi R, Murray KP, Benshushan A, Huntley ED, Kao MS. Invasion of interstitial matrix by a novel cell line from primary peritoneal carcinosarcoma, and by established ovarian carcinoma cell lines: role of cell-matrix adhesion molecules, proteinases, and E-cadherin expression. Gynecol Oncol. 2003;89(1):60–72.
11. Chandrasekaran S, Guo NH, Rodrigues RG, Kaiser J, Roberts DD. Pro-adhesive and chemotactic activities of thrombospondin-1 for breast carcinoma cells are mediated by alpha3beta1 integrin and regulated by insulin-like growth factor-1 and CD98. J Biol Chem. 1999;274(16):11408–11416. doi:10.1074/jbc.274.16.11408
12. Cross VL, Won Choi N, Verbridge SS, et al. Dense type I collagen matrices that support cellular remodeling and microfabrication for studies of tumor angiogenesis and vasculogenesis in vitro. Biomaterials. 2010;31(33):8596–8607. doi:10.1016/j.biomaterials.2010.07.072
13. Krause SMM, Soto AM, Sonnenschein C. The microenvironment determines the breast cancer cells’ phenotype: organization of MCF7 cells in 3D cultures. BMC Cancer. 2010;10:263. doi:10.1186/1471-2407-10-263
14. Szot CS, Freeman JW, Rylander MN. 3D in vitro bioengineered tumors based on collagen I hydrogels. Biomaterials. 2011;32(31):7905–7912. doi:10.1016/j.biomaterials.2011.07.001
15. C L, Xiao Z, Meng Y, et al. The enhancement of cancer stem cell properties of MCF-7 cells in 3D collagen scaffolds for modeling of cancer and anti-cancer drugs. Biomaterials. 2012;33(5):1437–1444. doi:10.1016/j.biomaterials.2011.10.056
16. Guo Y-S, Zhao R, Ma J, et al. βig-h3 promotes human osteosarcoma cells metastasis by interacting with integrin α2β1 and activating PI3K signaling pathway. PLoS One. 2014;9(3):e90220–e90220. doi:10.1371/journal.pone.0090220
17. He M, Wang Z, Zhao J, Chen Y, Wu Y. COL1A1 polymorphism is associated with risks of osteosarcoma susceptibility and death. Tumour Biol. 2014;35(2):1297–1305. doi:10.1007/s13277-013-1172-6
18. Prockop DJ. Mutations that alter the primary structure of type I collagen. The perils of a system for generating large structures by the principle of nucleated growth. J Biol Chem. 1990;26:265.
19. Kostik MM, Smirnov AM, Demin GS, Mnuskina MM, Scheplyagina LA, Larionova VI. Genetic polymorphisms of collagen type I α1 chain (COL1A1) gene increase the frequency of low bone mineral density in the subgroup of children with juvenile idiopathic arthritis. EPMA J. 2013;4(1):15. doi:10.1186/1878-5085-4-15
20. Shea MP, O’Leary KA, Wegner KA, Vezina CM, Schuler LA. High collagen density augments mTOR-dependent cancer stem cells in ERα+ mammary carcinomas, and increases mTOR-independent lung metastases. Cancer Lett. 2018;433:1–9. doi:10.1016/j.canlet.2018.06.025
21. Kirkland SC. Type I collagen inhibits differentiation and promotes a stem cell-like phenotype in human colorectal carcinoma cells. Br J Cancer. 2009;101(2):320–326. doi:10.1038/sj.bjc.6605143
22. Procacci P, Moscheni C, Sartori P, Sommariva M, Gagliano N. Tumor-stroma cross-talk in human pancreatic ductal adenocarcinoma: a focus on the effect of the extracellular matrix on tumor cell phenotype and invasive potential. Cells. 2018;7(10):158. doi:10.3390/cells7100158
23. Nissinen LWJ, Koivisto L, Kähäri VM, Heino J. Transcription of alpha2 integrin gene in osteosarcoma cells is enhanced by tumor promoters. Exp Cell Res. 1998;243(1):1–10. doi:10.1006/excr.1998.4128
24. Kawashima A, Kawahara E, Tokuda R, Nakanishi I. Tumour necrosis factor-alpha provokes upregulation of alpha2beta1 and alpha5beta1 integrins, and cell migration in OST osteosarcoma cells. Cell Biol Int. 2001;25(4):319–329. doi:10.1006/cbir.2000.0652
25. Kimura HTY, Tome Y, Momiyama M, et al. Imaging the inhibition by anti-β1 integrin antibody of lung seeding of single osteosarcoma cells in live mice. Int j Cancer. 2012;131(9):2027–2033. doi:10.1002/ijc.27475
26. Grzesiak J, Cao T, Hop K, Sharmeela H, Robert B. Abstract 547: a function-blocking monoclonal antibody directed against the collagen-binding α 2 integrin subunit inhibits primary tumor growth and metastasis in a fluorescent orthotopic mouse model of pancreatic cancer. Cancer Res. 2011;71:547.
27. Benedicto AMJ, Herrero A, Olaso E, Kolaczkowska E. Decreased expression of the β integrin on tumor cells is associated with a reduction in liver metastasis of colorectal cancer in mice. BMC Cancer. 2017;17(1):827. doi:10.1186/s12885-017-3823-2
28. Yoshimasu T, Matsuura N, Ota I, et al. Integrins α2β1, α5β1 and αvβ5 are related to tumor growth and metastasis of non-small cell lung cancer. Japanese J Lung Cancer. 2001;41:111–115. doi:10.2482/haigan.41.111
29. HU X-H, SHI X, WU S-J. Chemotherapy and integrin α2β1 in the plasma of osteosarcoma patients. J Med Postgraduates. 2011.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.