Back to Journals » International Journal of Nanomedicine » Volume 13
Extended pain relief achieved by analgesic-eluting biodegradable nanofibers in the Nuss procedure: in vitro and in vivo studies
Authors Liu K , Chen WH, Lee C , Su Y, Liu SJ
Received 2 October 2018
Accepted for publication 22 November 2018
Published 7 December 2018 Volume 2018:13 Pages 8355—8364
DOI https://doi.org/10.2147/IJN.S189505
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Linlin Sun
Kuo-Sheng Liu,1 Wei-Hsun Chen,1 Cheng-Hung Lee,2 Yong-Fong Su,3 Shih-Jung Liu3,4
1Department of Thoracic and Cardiovascular Surgery, Chang Gung Memorial Hospital, Linkou, Taoyuan, Taiwan, Republic of China; 2Division of Cardiology, Department of Internal Medicine, Chang Gung Memorial Hospital, Linkou, Taoyuan, Taiwan, Republic of China; 3Department of Mechanical Engineering, Chang Gung University, Taoyuan, Taiwan, Republic of China; 4Department of Orthopedic Surgery, Chang Gung Memorial Hospital, Linkou, Taoyuan, Taiwan, Republic of China
Background: The most common complaint after the Nuss procedure is severe postoperative chest pain. The aim of this study was to evaluate the effectiveness of analgesic-eluting biodegradable nanofibers in pain relief after the Nuss procedure.
Materials and methods: Poly(D,L)-lactide-co-glycolide, lidocaine, and ketorolac were dissolved in 1,1,1,3,3,3-hexafluoro-2-propanol. This solution was electrospun into a nanofibrous membrane. The elution method and high-performance chromatography were used to characterize the in vitro drug release. Stainless steel bars with and without coating of the analgesic-eluting nanofibrous membrane were implanted underneath the sternums of New Zealand white rabbits. The in vivo characteristics were further investigated.
Results: The in vitro study showed that the biodegradable nanofibers released high doses of lidocaine and ketorolac within 10 days. The in vivo study demonstrated high local and systemic concentrations of lidocaine and ketorolac. The serum creatinine level was unaffected. Animals that received implants of the analgesic-eluting nanofiber-coated stainless steel bar exhibited significantly greater food and water ingestion and physical activity than the control group did, indicating effective pain relief.
Conclusion: The proposed analgesic-eluting biodegradable nanofibers contribute to the achievement of extended pain relief after the Nuss procedure, without obvious adverse effects, in an animal model.
Keywords: analgesic-eluting, nanofibers, biodegradable, pectus excavatum, Nuss procedure
Introduction
The minimally invasive repair of pectus excavatum, also known as the Nuss procedure, has become the procedure of choice for children, adolescents, and even adults who require surgical correction for pectus excavatum.1,2 Severe postoperative pain is the most common complaint after the Nuss procedure, especially in adult patients whose chest wall is more rigid than younger patients.3–5 Prolonged hospitalization for severe pain could further lead to increased medical expenses. Postoperative pain is usually managed with epidural analgesia or intravenous patient-controlled analgesia in the immediate postoperative period and with narcotics or NSAIDs afterward. A continuous infusion of local anesthetic for intercostal nerve blockade through a catheter placed in an extrapleural pocket during thoracotomy or thoracoscopic procedures also provides efficient pain relief.6,7 During the Nuss procedure, using cryoanalgesia to freeze the intercostal nerves has been reported to offer sustained pain control for 2 months.8 In the present study, we developed a novel biodegradable, analgesic-eluting, nanofibrous membrane for local delivery of sustainable high concentrations of analgesics. membrane was coated onto the stainless steel pectus bar, and the drug release behavior was evaluated both in vitro and in an animal model.
Materials and methods
This study was approved by the Institutional Review Board of Chang Gung Medical Foundation (approval no CGU105-052).
Fabrication of the biodegradable, analgesic-eluting, nanofibrous membrane
The primary polymer used in this study was poly(d,l)-lactide-co-glycolide (PLGA) (Resomer RG 503; Boehringer Ingelheim, Ingelheim, Germany), with a lactide/glycolide ratio of 50:50 and a molecular weight of 33,000 Da. The analgesics used in the study were lidocaine and ketorolac. Both drugs were purchased from Sigma-Aldrich Co. (St Louis, MO, USA).
The biodegradable drug-eluting nanofibers were fabricated in our laboratory by using the electrospinning method.9 The electrospinning setup has been described previously;10,11 it consists of a 10 mL syringe and needle (internal diameter of 0.42 mm), a ground electrode, a collection plate, and a high-voltage supply. The needle was connected to the high-voltage supply, which generated positive direct current voltages. Two solutions were prepared. Solution 1 was prepared by dissolving PLGA (672 mg) and lidocaine (168 mg) in 3 mL of 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) (Sigma-Aldrich Co.); solution 2 was prepared by dissolving PLGA (672 mg) and ketorolac (168 mg) in another 3 mL of HFIP. The two solutions were sequentially delivered and electrospun using the syringe pump with a volumetric flow rate of 0.01 mL/min to obtain a bilayered, drug-loaded, nanofibrous membrane on the collection plate. The distance between the needle tip and the collecting plate was 15 cm, and the positive voltage applied to the polymer solution was 17,000 V. The electrospinning process was performed at room temperature. The nanofibrous membrane was placed in a fume hood at room temperature for 12 hours for solvent evaporation before being used in the experiment.
Characterization of the analgesic-eluting nanofibers
The morphology of the electrospun PLGA/lidocaine/ketorolac nanofibers was observed on a field emission scanning electron microscope (JSM-7500F; JEOL, Tokyo, Japan) after gold coating. The average diameter and diameter distribution were obtained by measuring the diameters of 50 randomly selected fibers.
The water contact angle of the nanofibrous PLGA/lidocaine/ketorolac membrane was measured using a water contact angle analyzer (First Ten Angstroms, Portsmouth, VA, USA). Samples of dimensions 1 × 1 cm were cut out from the membrane and placed on the testing plate. Distilled water was carefully dropped on their surfaces. The contact angle of the nanofibrous membrane was measured using a video monitor. Contact angles from 0 to 30° were defined as highly hydrophilic, 30 to 90° as hydrophilic, 90 to 150° as hydrophobic, and 150 to 180° as highly hydrophobic.
The spectra of electrospun nanofibrous scaffolds were characterized using Fourier transform infrared (FTIR) spectrometry. FTIR analysis was performed on a Nicolet iS5 spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) at a resolution of 4 cm−1 for 32 scans. Samples of the nanofibrous membranes were pressed as KBr discs, and spectra were analyzed over the 400–4,000 cm−1 range.
In vitro drug elution
The release pattern of lidocaine and ketorolac from the drug-loaded nanofibrous membrane was determined using an in vitro elution method and an HPLC assay. Phosphate buffer (0.15 mol/L, pH 7.4) was used as the dissolution medium. The nanofibrous membrane (1 × 1 cm) was soaked in 1 mL of phosphate buffer in a glass test tube. The test tubes were incubated at 37°C. The dissolution medium was collected daily to measure lidocaine and ketorolac release. One milliliter of fresh phosphate buffer was subsequently added for the next 24-hour period, and this procedure was repeated for 30 days. The lidocaine concentration analyses in the buffer were determined using a Hitachi L-2200R multisolvent delivery system (Hitachi Ltd, Tokyo, Japan) with an Atlantis dC18 4.6 cm × 150 mm HPLC column (Waters Corp., Milford, MA, USA). The mobile phase included 0.01 mol ammonium formate (Sigma-Aldrich Co.) and methanol (20:80 vol/vol; Sigma-Aldrich Co.). The absorbency was monitored using an ultraviolet light detector (L-2400R; Hitachi, Tokyo, Japan) at a wavelength of 254 nm, with the flow rate set at 1.0 mL/min. To characterize ketorolac, a Discovery C18, 5 μm, 4.6 cm × 250 mm column was employed. The mobile phase contained acetonitrile (Mallinckrodt, Milford, CT, USA) and 0.1% acetic acid (Sigma-Aldrich Co.) in a volume ratio of 70:30. The absorbency was monitored at 220 nm, and the flow rate was 1.0 mL/min.
Surgical procedure, animal activity, and in vivo drug release
A total of six adult New Zealand white rabbits (Animal Health Research Institute, Panchiao, Taiwan, Republic of China) with a mean weight of 3.3±0.7 kg were used in the experimental Nuss procedure. Under the supervision of a licensed veterinarian, all rabbits involved in this study were cared for in a manner that was consistent with the regulations of the National Health Research Institutes of Taiwan.
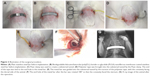
A curved stainless steel bar was designed and fabricated specifically for this study (Figure 1). During the surgical procedure, the rabbits received general anesthesia through inhalation of isoflurane (Aesica Queenborough Limited, Queenborough, UK) in an anesthesia chamber that comprised a 40 × 20 × 28 cm transparent acrylic box. The anesthetized rabbit was then placed in supine position, with isoflurane inhalation maintained via a facial mask. The anterior chest wall was shaved, disinfected, and draped in aseptic fashion. Two 1.5 cm longitudinal skin incisions were made in the bilateral chest at the anterior axillary line at the level about 2 cm above the xiphoid process. The wound was deepened to the subpectoral muscle plane on each side. A Pean clamp was used to penetrate the intercostal muscle, advancing in the substernal plane, then piercing out of the intercostal muscle in the contralateral side. Care was taken not to enter the pleural space, which would cause pneumothorax. A polyester tape was brought into the substernal tunnel by using the Pean clamp. The tape was attached to the end hole in the stainless steel pectus bar. The pectus bar was guided through the substernal tunnel with the convexity facing the dorsal side by applying gentle traction on the polyester tape. Once the bar was in place with an equal length of the bar protruding on each side, it was rotated 180° so that the convexity faced the sternum. The end holes of the stainless steel bar were fixed to the adjacent tissue with absorbable sutures on both sides. The wounds were then closed in layers. Three rabbits received stainless steel bars that were coated with the analgesic-eluting nanofibrous membrane and served as study group 1. The drug-loaded nanofibrous membrane (200 mg in weight) was immersed in 70% ethanol for 30 minutes for sterilization and then left to dry. The membrane was wetted with distilled water and then wrapped around the stainless steel pectus bar immediately before the surgical procedure. The other three rabbits received plain stainless steel bars and served as study group 2. The steps of the surgical procedure are depicted in Figure 2.
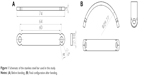  | Figure 1 Schematic of the stainless steel bar used in this study. |
After the experimental Nuss procedure, the rabbits were individually housed in an animal behavior cage, which was specifically designed by our laboratory for this study, for the first 6 days for the evaluation of animal activity. As shown in Figure 3, the animal behavior cage has a dimension of 120 × 120 × 60 cm and is equipped with nine diffusion scan-type photoelectric switch sensors on top that possess self-contained amplifiers (HP100-A1; Azbil Corp., Tokyo, Japan). The sensors were employed to monitor the movements of the rabbit within the cage. As the rabbit moved from one area of the cage to another, the sensor in the “approached” area was triggered. A microprocessor with an acquisition interface was employed to record the total number of triggers. The activity of each animal was observed and monitored for 6 days. Food and water were placed under sensor number 7. The amount of food and water ingested was also measured each day. Three normal adult rabbits that did not undergo any surgical procedure were also separately housed in the cage for 6 days and served as the control group. After 6 days, the experimental rabbits were transferred to an ordinary cage for routine care according to the regulations of the animal center at Chang Gung University.
  | Figure 3 (A) Picture of the animal behavior cage. (B) Map of the sensor placement over the animal behavior cage. Food and water were placed under sensor number 7. |
Specimens (blood and tissue) were collected at days 7, 14, 21, and 28 after the experimental Nuss procedure in study group 1. The animals received the same general anesthesia as described above at each time point. Blood samples were withdrawn from the marginal ear vein, and the concentrations of lidocaine and ketorolac were determined using an HPLC assay. Serum creatinine levels were also measured to evaluate kidney function. For the evaluation of analgesic drug release in the local tissue, a skin incision was made just above the stainless steel pectus bar. The wound was deepened until the pectus bar was visible. A piece of soft tissue that was in direct contact with the pectus bar was excised, weighed, mixed with 1 mL of phosphate buffer solution, homogenized using a bead mill homogenizer, and then centrifuged.12 The supernatant was filtrated using a filter with a mesh size of 0.22 mm. The concentrations of lidocaine and ketorolac in the filtered supernatant were analyzed using HPLC.
Statistical analysis
The collected data were analyzed using one-way ANOVA. Differences were considered statistically significant at P<0.05.
Results
In vitro characteristics of the PLGA/lidocaine/ketorolac biodegradable nanofibers
The analgesic-eluting nanofibrous membrane was successfully prepared through the electrospinning method. Figure 4 shows scanning electron microscope photomicrographs of the nanofibers and the distributions of fiber diameters. The calculated diameters of electrospun nanofibers were 560±272, 207±63, and 158±67 nm for plain PLGA, lidocaine-loaded, and ketorolac-loaded nanofibers, respectively. The measured water contact angles were 131.08°, 102.13°, and 71.31° for PLGA, lidocaine-loaded, and ketorolac-loaded nanofibers, respectively. The contact angles decreased with the addition of analgesics, and the ketorolac-loaded PLGA nanofibers exhibited the highest hydrophilicity.
Figure 5A shows the FTIR spectra of pure PLGA and analgesic-loaded PLGA nanofibrous membranes. The increased absorption of the analgesic-loaded nanofibers at wavenumbers 3,400, 1,725, and 1,300 cm−1 corresponded to the amine N−H stretch, carbonyl C=O stretch, and ether C−O stretch, respectively. Because lidocaine molecules are rich in N−H and C=O bonds and ketorolac molecules are rich in C=O and C−O bonds (Figure 5B and C), the FTIR spectroscopy results indicated successful incorporation of both drugs to the PLGA polymer.
In vitro lidocaine and ketorolac elution from the nanofibrous membrane
Figure 6 plots the daily in vitro release curves of analgesics. The drug-eluting nanofibers continuously released lidocaine and ketorolac for 30 days. An initial burst drug release was seen on day 1, followed by a steady release on days 2–12 for lidocaine and on days 2–6 for ketorolac, after which the concentrations gradually decreased. Ninety percent of the drugs were released within the second week.
  | Figure 6 In vitro release of lidocaine and ketorolac. |
In vivo animal activity test
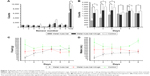
Figure 7A shows the counts of different sensors in the animal behavior cage. Animals of study group 1 had higher sensor counts than animals of study group 2 did, whereas animals of the control group had the highest sensor counts. The rabbits spent more time under sensor number 7, where food and water were supplied, than any other sensor.
Figure 7B shows the sensor counts on each day. Animals of study group 1 had statistically higher sensor counts on days 1, 2, 3, and 5 than animals of study group 2 did.
Figure 7C and D shows the food and water ingestion on each day. Compared with animals of study group 2, animals of study group 1 ate more food on days 1 and 2 and drank more water on days 1–3.
In vivo lidocaine and ketorolac elution
Figure 8A depicts the analgesic drug concentrations in the plasma and in the local tissue adjacent to the drug-eluting, nanofiber-coated stainless steel bar in animal study group 1. The drug release curve verified a sustained, steady release of high concentrations of lidocaine and ketorolac to the local tissue for at least 4 weeks. The plasma concentrations of both drugs were approximately one-tenth of the tissue concentrations. Although the plasma concentrations of lidocaine were above 10 μg/mL,13 none of the study animals developed any neurological or cardiac disturbance. The serum creatinine levels (Figure 8B) were within the normal range during the study period.
Discussion
Severe pain is still the most common complaint after the Nuss procedure, which is already a minimally invasive surgical approach for the correction of pectus excavatum. Contributors to the intense pain include an iatrogenic sternum fracture and injury to the intercostal nerves. Strategies to reduce postoperative pain are appealing to both patients and physicians and could further optimize the advantages of a minimally invasive procedure. In the present study, we developed analgesic-eluting, biodegradable PLGA nanofibrous membrane-coated pectus bars that provided sustained release of lidocaine and ketorolac to the surgically traumatized local tissue for effective pain relief in an animal study.
PLGA is a synthetic biodegradable copolymer. It triggers only a minimal inflammatory response in the human body. PLGA has been used to produce resorbable sutures, resorbable surgical clips, and controlled-release implants. It is primarily degraded via hydrolysis of the ester linkages to generate biocompatible lactic and glycolic acids. The time required for PLGA resorption can be manipulated by changing the monomer ratio.14 This unique feature is desirable for the development of a drug delivery system because PLGA of different monomer ratios can be selected according to the appropriate degradation time required in various clinical situations. For the relief of postoperative pain, which usually lasts a few weeks after the surgical procedure, we select PLGA of 50% glycolide and 50% lactide as the base polymer, which is completely resorbed in the human body within 1–2 months, the fastest among the PLGA family.15
Lidocaine is the prototypical amide local anesthetic. It interacts with the voltage-gated sodium channels and blocks nerve signal conduction by decreasing or preventing the large transient increase in sodium permeability of excitable cell membranes.16 When used in local anesthesia, the duration of action of lidocaine is ~2 hours. Ketorolac is an NSAID. NSAIDs exert their anti-inflammatory reaction via inhibition of cyclooxygenase-mediated prostaglandin production. Ketorolac has a potent analgesic but only moderate anti-inflammatory effect compared with other NSAIDs, and it has been widely used in the postoperative setting as an alternative to opioids.17 With the method developed in the present study, high concentrations of lidocaine and ketorolac could be detected in the local tissue of animals for up to 1 month. Increased total sensor counts and increased food and water ingestion measured in the animal study in the study group 1 rabbits indicated an effective pain-reducing reaction caused by the analgesic-eluting biodegradable PLGA nanofibers.
When absorbed into the systemic circulation, lidocaine has a dose-related adverse effect on the central nervous and cardiovascular systems. In the present study, the plasma lidocaine concentrations reached a toxic level seen in humans. However, neurological or cardiovascular disturbance was not observed in the animals in this study. Similarly, the animals’ plasma ketorolac concentrations were much higher than the values (1.45–3.82 mg/L) observed in healthy human volunteers after intramuscular injection of a regular dose of 30 mg of ketorolac.18 Ketorolac, as is true of other NSAIDs, carries a well-known risk of acute kidney injury,19 though the association of renal injury with the plasma ketorolac concentration was less clear. In the present study, the kidney function of experimental animals was not affected. These findings highlight the fact that the amount of systemic absorption of the locally eluted drugs cannot be ignored. The reason why high plasma concentrations of lidocaine and ketorolac did not result in adverse events in the rabbit was unknown, but clearly, the doses of lidocaine and ketorolac used in the manufacturing process of the analgesic-eluting PLGA nanofibers must be adjusted before future experiments in large animals or in humans.
An initial burst release of lidocaine and ketorolac was found in the in vitro study. After the electrospinning procedure, most drugs were scattered in the bulk of the PLGA matrix; however, some drugs could have been retained on the surface of the nanofibers, leading to the initial burst of drug release. Although the initial burst is undesirable for a zero-order drug release, the high concentrations of lidocaine and ketorolac in the immediate postoperative period is potentially beneficial because the intensity of the postoperative pain is highest during that period.
This study represents another example of using biodegradable PLGA nanofibers as a platform for creating a local drug delivery system. In conjunction with an implantable medical device, a sustainable amount of certain drugs could be delivered to the local tissue for a particular function for a prolonged period. For example, a cisplatin-PLGA nanofiber-coated tracheal stent could deliver the chemotherapeutic agent to the trachea for the treatment of malignant airway obstruction,20 and a vancomycin-PLGA nanofiber-coated vascular stent could carry the antibiotic to the aortic wall for the treatment of an infected aortic aneurysm.10,11 In the present study, an analgesic and a local anesthetic were released from the biodegradable PLGA nanofiber system for effective pain relief after surgical implantation of a stainless steel pectus bar. This approach has a great prospect in clinical use for a variety of orthopedic procedures and could potentially reduce the need of postoperative narcotic use, enhance patient satisfaction, and promote early return to normal life after surgery.
Despite successful pain reduction after the experimental Nuss procedure, there are limitations associated with this study. First, as mentioned above, the plasma concentrations of lidocaine and ketorolac in the rabbit were too high. The doses of lidocaine and ketorolac used in the manufacturing process need to be adjusted before further experimentation. Second, the duration of the animal study was not long enough for complete resorption of the PLGA nanofibers. Third, a nondiseased animal model was used. The extent and effect of pain reduction may differ in real pectus excavatum, in which the surgical trauma and pain intensity are higher. Further evaluation of the dose-modified, analgesic-eluting PLGA nanofibers in a diseased, large animal model of pectus excavatum for a prolonged period is required to address these study limitations.
Conclusion
In this study, we designed and fabricated biodegradable lidocaine- and ketorolac-eluting PLGA nanofibrous membrane-coated pectus bars by using the electrospinning technique for sustained drug delivery to the local tissue in an attempt to relieve pain in the immediate postoperative period after the Nuss procedure. Ninety percent of the drugs were released within 2 weeks in the in vitro study. The animal study demonstrated that the biodegradable nanofibers released high concentrations of lidocaine and ketorolac to the local tissue for 4 weeks and effectively reduced immediate postprocedural pain. The proposed biodegradable lidocaine-/ketorolac-eluting nanofibers significantly contribute to the achievement of local and sustainable delivery of analgesics to the local tissue adjacent to the Nuss bar and may reduce postoperative pain and increase patient satisfaction in clinical application.
Acknowledgments
The authors thank Ministry of Science and Technology of Taiwan (Project No 106-2314-B-182A-153-) for financially supporting this research. The Ministry of Science and Technology of Taiwan has no involvement in any of the stages from study design to submission of the paper for publication.
Author contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Nuss D, Kelly RE Jr, Croitoru DP, Katz ME. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg. 1998;33(4):545–552. | ||
Pawlak K, Gąsiorowski Ł, Gabryel P, Gałęcki B, Zieliński P, Dyszkiewicz W. Early and late results of the Nuss procedure in surgical treatment of pectus excavatum in different age groups. Ann Thorac Surg. 2016;102(5):1711–1716. | ||
Kim DH, Hwang JJ, Lee MK, Lee DY, Paik HC. Analysis of the Nuss procedure for pectus excavatum in different age groups. Ann Thorac Surg. 2005;80(3):1073–1077. | ||
Jaroszewski DE, Temkit M, Ewais MM, et al. Randomized trial of epidural vs. subcutaneous catheters for managing pain after modified Nuss in adults. J Thorac Dis. 2016;8(8):2102–2110. | ||
Sacco Casamassima MG, Gause C, Goldstein SD, et al. Patient satisfaction after minimally invasive repair of pectus excavatum in adults: long-term results of Nuss procedure in adults. Ann Thorac Surg. 2016;101(4):1338–1345. | ||
Detterbeck FC. Efficacy of methods of intercostal nerve blockade for pain relief after thoracotomy. Ann Thorac Surg. 2005;80(4):1550–1559. | ||
Detterbeck FC. Subpleural catheter placement for pain relief after thoracoscopic resection. Ann Thorac Surg. 2006;81(4):1522–1523. | ||
Graves C, Idowu O, Lee S, Padilla B, Kim S. Intraoperative cryoanalgesia for managing pain after the Nuss procedure. J Pediatr Surg. 2017;52(6):920–924. | ||
Wang S, Hu F, Li J, et al. Design of electrospun nanofibrous mats for osteogenic differentiation of mesenchymal stem cells. Nanomedicine. 2018;14(7):2505–2520. | ||
Liu KS, Lee CH, Wang YC, Liu SJ. Sustained release of vancomycin from novel biodegradable nanofiber-loaded vascular prosthetic grafts: in vitro and in vivo study. Int J Nanomedicine. 2015;10:885–891. | ||
Liu KS, Lee CH, Lee D, Liu M, Tsai FC, Tseng YY. Sustained local delivery of high-concentration vancomycin from a hybrid biodegradable, antibiotic-eluting, nanofiber-loaded endovascular prosthesis for treatment of mycotic aortic aneurysms. J Vasc Surg. 2018;68(2):597–606. | ||
Volonté MG, Yuln G, Quiroga P, Consolini AE. Development of an HPLC method for determination of metabolic compounds in myocardial tissue. J Pharm Biomed Anal. 2004;35(3):647–653. | ||
Schwartz DR, Kaufman B. Local anesthetics. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, editors. 10th ed. Goldfrank’s Toxicologic Emergencies. New York: McGraw-Hill; 2015:925. | ||
Goupil D. Sutures. In: Ratner BD, Ho!man AS, Schoen FJ, Lemons JE, editors. Biomaterials Science. New York: Academic Press; 1996:356–360. | ||
Middleton JC, Tipton AJ. Synthetic biodegradable polymers as orthopedic devices. Biomaterials. 2000;21(23):2335–2346. | ||
Catterall WA, Mackie K. Chapter 14. Local anesthetics. In: Brunton LL, Lazo JS, Parker KL, editors. 11th ed. Goodman & Gilman’s the Pharmacological Basis of Therapeutics. New York: McGraw-Hill; 2006:369–386. | ||
Gillis JC, Brogden RN. Ketorolac. A reappraisal of its pharmacodynamic and pharmacokinetic properties and therapeutic use in pain management. Drugs. 1997;53(1):139–188. | ||
Jallad NS, Garg DC, Martinez JJ, Mroszczak EJ, Weidler DJ. Pharmacokinetics of single-dose oral and intramuscular ketorolac tromethamine in the young and elderly. J Clin Pharmacol. 1990;30(1):76–81. | ||
Pearce CJ, Gonzalez FM, Wallin JD. Renal failure and hyperkalemia associated with ketorolac tromethamine. Arch Intern Med. 1993;153(8):1000–1002. | ||
Chao YK, Liu KS, Wang YC, Huang YL, Liu SJ. Biodegradable cisplatin-eluting tracheal stent for malignant airway obstruction: in vivo and in vitro studies. Chest. 2013;144(1):193–199. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.