Back to Journals » Infection and Drug Resistance » Volume 14
Expression of Serum miR-155 in Children with Mycoplasma pneumoniae Pneumonia and Its Role in Immunity to Mycoplasma pneumoniae
Authors Jin Y, Xue J, Ruan M, Lu J, Xu Q, Shi Y , Yu F
Received 17 August 2020
Accepted for publication 10 February 2021
Published 29 March 2021 Volume 2021:14 Pages 1273—1281
DOI https://doi.org/10.2147/IDR.S273423
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Yue Jin, Jie Xue, Mengran Ruan, Jinxing Lu, Qian Xu, Yuanyuan Shi, Fei Yu
Department of Pediatrics, Affiliated Shuyang Hospital of Xuzhou Medical University, Shuyang, 223600, Jiangsu, People’s Republic of China
Correspondence: Yue Jin
Department of Pediatrics, Affiliated Shuyang Hospital of Xuzhou Medical University, No. 9, Yingbin Road, Shucheng Town, Shuyang, 223600, Jiangsu, People’s Republic of China
Tel +86-14762621960
Fax +86-527-80817088
Email [email protected]
Objective: To investigate the expression of serum miR-155 in children with Mycoplasma pneumoniae pneumonia (MPP).
Methods: A total of 100 children at our hospital with pneumonia caused by Mycoplasma pneumoniae infection were enrolled as a study group, including 45 cases in the acute phase (acute phase group) and 55 in the recovery phase (recovery phase group). An additional 30 healthy children were enrolled during the same period as the control group. The expression levels of miR-155, tumor necrosis factor-α (TNF-α), interleukin-8 (IL-8), IL-10, IL-13, immunoglobulin (Ig) G, IgA, complements (C3 and CH50), and T lymphocyte subsets (CD3+, CD4+, CD8+, and CD4+/CD8+) were determined. Multivariate logistic regression analysis was performed to identify risk factors affecting MPP in children.
Results: miR-155, IL-10, IgG, IgA, CD3+, CD4+, and CD4+/CD8+ were poorly expressed in children with MPP, and their expression in the acute phase group was significantly lower than that in the recovery phase group. TNF-α, IL-13, C3, and CH50 were highly expressed in the children, and their expression was significantly higher in the acute phase group than in the recovery phase group. In the acute phase group, the expression of IL-8 was significantly higher than that in the control and recovery phase groups but without any significant differences between the recovery phase and control groups. Age, season, low complement state, epidemiological contact history, and antibiotic use time were independent risk factors affecting MPP in children.
Conclusion: Serum miR-155 is poorly expressed in children with MPP, and it can regulate inflammatory disorders and immune responses.
Keywords: miR-155, Mycoplasma pneumoniae, immunity, inflammation, risk factors
Introduction
Mycoplasma pneumoniae is a common pathogen encountered during childhood, and it is a major cause of pneumonia, namely, Mycoplasma pneumoniae pneumonia (MPP), in this population.1 According to epidemiological studies, the incidence of MPP is high during the epidemic period.2 MPP has intrapulmonary manifestations, such as viral symptoms and obstructive airway symptoms.3 It is difficult to diagnose MPP because some of its clinical symptoms are similar to those of pneumonia.4 Currently, MPP in children is primarily treated with antibiotic therapy. Nevertheless, the widespread application of antibiotics will increase drug-resistant Mycoplasma pneumoniae strains, thereby reducing the therapeutic effect of antibiotics to varying degrees.5,6 Therefore, for the treatment and prevention of MPP, it is essential to explore novel therapeutic targets and related risk factors.
Micro RNA (miRNA) has various biological functions; it plays the role of a gene expression regulator by binding to complementary target mRNA and inhibiting its expression. miRNA is involved in the development and differentiation of cells, by which it contributes to the regulation of human diseases, particularly those related to the pathogenesis of pulmonary diseases.7,8 miR-155 is a key regulator of inflammatory responses induced by proinflammatory stimuli involved in the regulation of innate and adaptive immune responses.9,10 Studies have shown that the upregulation of miR-155 expression can stabilize the overexpression of TNF-α mRNA, thereby blocking the inflammatory responses initiated by the TNF-α-NF-κB axis, while low expression of miR-155 results in a state of weak immune function.11,12
At present, research on the effects of serum miR-155 on MPP in children and on inflammatory and immune responses is scarce. Therefore, we aimed to study the expression of miR-155 and related inflammatory and immune indices in children with MPP to conduct a preliminary analysis of the role of miR-155 in the inflammation and immunity of MPP and suggest new strategies for the prevention and treatment of MPP.
Patients and Methods Study Population and Design
Children with pneumonia caused by Mycoplasma pneumoniae infection (MPI) admitted to our hospital between January 2018 and March 2019 were enrolled as the study group (SG), including 45 cases in the acute phase (acute phase group, APG) and 55 in the recovery phase (recovery phase group, RPG). Additionally, 30 healthy children were enrolled as the control group (CG) during the same period. The SG comprised 58 boys and 42 girls aged 3–12 years (mean age, 5.35 ± 2.26 years). The CG comprised 20 boys and 10 girls aged 3–11 years (mean age, 4.20 ± 2.15 years). This study was approved by the ethics committee of the Affiliated Shuyang Hospital of Xuzhou Medical University on September 27, 2017 (No. 20,160,312) and conducted in accordance with the Declaration of Helsinki. The participants and their families were informed, and all legal guardians signed a fully informed consent form. The inclusion criteria were as follows: children with MPI-induced pneumonia that was confirmed by clinical data and laboratory indices;13 children without other infections; children with complete clinical data; children in the acute phase presenting clinical symptoms such as fever, intractable cough, and abnormal lung auscultation;14 and children in the recovery phase, i.e., disappearing acute phase clinical symptoms and signs. The total number of white blood cells is normal or slightly increased, mainly neutrophils. The mycoplasma pneumonia antigen in blood and respiratory tract samples can be detected to confirm the diagnosis. X-ray examination showed various forms of infiltrates in the lungs, which were distributed in segments. It is self-limiting, and most cases can heal spontaneously without treatment. The exclusion criteria were as follows: children with other infectious diseases; children who had taken other drugs that affected immune function or anti-inflammatory drugs; and children who had recently received surgery. The inclusion criteria were applicable to children in the SG, and healthy children were included in the CG.
Experimental Methods
Fasting venous blood (3 mL) was drawn from each subject after admission and then placed in EDTA-K2 anticoagulation tubes and coagulation-promoting tubes. The total RNA was extracted from the serum according to the instruction of the mirVanaTM miRNA isolation kit (Shanghai Xinle Biotechnology Co., Ltd., China, RMI050). The concentration of the extracted RNA was measured using a NanoDrop 1000 UV-Vis spectrophotometer (Shanghai Chenlian Biotechnology Development Co., Ltd., China, 757). PCR amplification was performed with cDNA considered as a template, and U6 was used as an internal reference gene. The upstream and downstream primers of miR-155 were 5′-GCGGTTAATGCTAATCGTGAT-3′ and 5′-GTGCAGGGTCCGAGGT-3′. Those of U6 were 5′- CCTGGATCTTATCAGGCTC-3′ and 5′- GCCATCTCCCCGGACAAAG-3′. Primer sequences were designed by Shanghai Wcgene Biotechnology Co., Ltd., China. miR-155 was quantitatively detected using a real-time fluorescence quantitative PCR system (Shanghai AOLU Biological Technology Co., Ltd., China,7500) according to the instructions of the miRNA RT-qPCR detection kit (Genetimes Technology, Inc., Shanghai, China, 110001S). All samples were repeatedly analyzed three times, and the results were expressed by 2−ΔCT.
The expression of serum immunoglobulins (IgG and IgA) and complement C3 was determined using a fully automated biochemical analysis system (Shanghai Dibosi Biological Technology Co., Ltd., China, PUZS-300) and matching reagents. The levels of serum complement CH50, tumor necrosis factor-α (TNF-α), interleukin-8 (IL-8), IL-10, and IL-13 were detected using enzyme-linked immunosorbent assay (ELISA),15 with the procedures conducted in strict accordance with the instructions of human CH50, TNF-α, IL-8, IL-10, IL-13 ELISA kits (Shanghai Hengfei Biotechnology Co., Ltd., China). Sample wells, standard wells, and blank wells were set up. Horseradish peroxidase (HRP)-labeled detection antibodies (100 μL) were added to all wells except for the blank wells, sealed, and incubated at 37°C for 60 min. After the liquid was discarded, the ELISA plate was dried and then repeatedly washed five times. After the substrates A and B were fully mixed at a ratio of 1:1, the mixed solution (100 μL) was added to each well, sealed, and incubated at 37°C for 15 min. Finally, stop solution (50 μL) was added to each well. A microplate reader (Shanghai Qiming Biotechnology Co., Ltd., China, QM-SW-0918) was used to measure the optical density values of each well at 450 nm to calculate the expression levels of CH50, TNF-α, IL-8, IL-10, and IL-13.
Serum T lymphocyte subsets were determined using a flow cytometer (Morey Biosciences, Inc., Shanghai, China, DxFLEX). Fluorescein isothiocyanate (FITC)-labeled fluorescent monoclonal antibody (CD4-FITC/CD8-ECD) (10 μL) (Shanghai Haoran Biotechnology Co., Ltd., China, RM25013, 737,659) was added to each EDTA-K2 anticoagulation tube to mix well with the venous blood (100 μL) and then kept at room temperature for 20 min in the dark. Next, red blood cell lysis buffer (500 μL) was added to the mixture to lyse the red blood cells, which were then kept at room temperature for 15 min in the dark. Finally, the mixture was mixed well with phosphate buffer solution (PBS, 500 μL) and then kept at room temperature for 10 min in the dark. The samples were analyzed using flow cytometry (BD FACSAria) and CD4+, CD8+, and CD4+/CD8+ data were read using Cell Quest (Becton Dickinson, Franklin Lakes, NJ, USA).
Statistical Analysis
GraphPad Prism 6 was used for statistical analysis. Count data were expressed by the number of cases/percentages [n (%)], and the comparison of data between groups was performed using the chi-square test. Measurement data were expressed as mean ± SD. For group comparisons, an independent samples paired t-test was used, and to compare between three groups, an F-test was used. Multivariate Logistic regression analysis was conducted to identify the independent risk factors affecting MPP in children. P < 0.05 indicated a statistically significant difference.
Results
General Information and Clinicopathological Features
There was a significant difference between CG, APG and RPG in age (P < 0.05) but not in sex or place of residence (P > 0.05). There were significant differences between APG and RPG in season, low complement state, epidemiological contact history, and treatment duration with antibiotic drugs (P < 0.05) but not in the mean course of fever, results of routine blood tests, C-reactive protein, CK-MB, and chest radiographic images (P > 0.05) (Table 1).
 |
Table 1 General Information and Clinicopathological Features [n (%), Mean ± SD] |
Expression of Serum miR-155
The relative expression of serum miR-155 was 0.85 ± 0.14 in APG, 1.04 ± 0.15 in RPG, and 1.39 ± 0.19 in CG. The expression of serum miR-155 in APG and RPG was lower than that in CG, and that in the APG was lower than that in the RPG (P < 0.001; Figure 1).
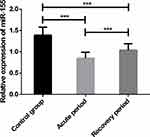 |
Figure 1 Expression levels of serum miR-155. Note: ***Indicates P < 0.001. |
Expression of Proinflammatory Cytokines TNF-α and IL-8
The expression of TNF-α in APG and RPG was higher than that in CG (40.98 ± 2.31 pg/mL), and that in the APG (75.29 ± 4.72 pg/mL) was higher than that in RPG (51.64 ± 5.87 pg/mL) (P < 0.001). The expression of IL-8 in the APG (110.49±20.83 pg/mL) was higher than that in CG (35.37 ± 3.08 pg/mL) and RPG (32.25 ± 2.13 pg/mL) (P < 0.001, Figure 2).
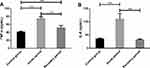 |
Figure 2 Expression levels of proinflammatory cytokines TNF-α and IL-8. (A) Expression of TNF-α in children with MPP. (B) Expression of IL-8 in children with MPP. Note: ***Indicates P < 0.001. |
Expression of Anti-Inflammatory Cytokines IL-10 and IL-13
The expression of IL-10 in APG and RPG was lower than that in CG (22.87 ± 3.65 pg/mL), and that in the APG (12.02 ± 4.28 pg/mL) was lower than that in RPG (16.31 ± 5.54 pg/mL) (P < 0.001). The expression of IL-13 in APG and RPG was higher than that in CG (82.14 ± 9.03 pg/mL), but that in APG (205.37 ± 16.97 pg/mL) was higher than that in RPG (140.38 ± 5.27 pg/mL) (P < 0.001; Figure 3).
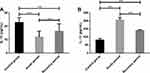 |
Figure 3 Expression levels of anti-inflammatory cytokines IL-10 and IL-13. (A) Expression of IL-10 in children with MPP. (B) Expression of IL-13 in children with MPP. Note: ***Indicates P < 0.001. |
Humoral Immune Function
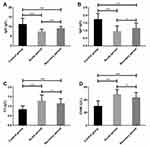
The concentration of the humoral immune index IgG in APG and RPG was lower than that in CG (11.21 ± 3.16 g/L) (P < 0.001), and that in the APG (7.36 ± 1.32 g/L) was lower than that in RPG (9.05 ± 1.10 g/L) (P < 0.001). The concentration of IgA in APG and RPG was lower than that in CG (1.73 ± 0.39 g/L) (P < 0.001), and that in the APG (0.95 ± 0.36 g/L) was lower than that in RPG (1.15 ± 0.34 g/L) (P < 0.05). The concentrations of C3 and CH50 in APG and RPG were higher than those in CG (0.83 ± 0.19 g/L, 30.18 ± 8.53 U/L) (P < 0.001), and those in the APG (1.28 ± 0.31 g/L, 48.37 ± 8.05 U/L) were higher than those in RPG (1.14 ± 0.26 g/L, 43.74 ± 7.96 U/L) (P < 0.05; Figure 4).
Cellular Immune Function
The concentration of the cellular immune index CD3+ in APG and RPG was lower than that in CG (49.25 ± 5.96%) (P < 0.001), and that in APG (37.18 ± 6.58%) was lower than that in RPG (40.34 ± 6.67%) (P < 0.05). The concentration of CD4+ in APG and RPG was lower than that in CG (34.76 ± 5.23%) (P < 0.001), and that in APG (23.68 ± 5.14%) was lower than that in RPG (28.87 ± 6.72%) (P < 0.001). There was no significant difference in the concentration of CD8+ between APG (27.36 ± 5.21%), RPG (27.62 ± 4.25%) and CG (28.29 ± 6.24%) (P > 0.05). The concentration of CD4+/CD8+ in APG and RPG was lower than that in CG (1.26 ± 0.20) (P < 0.01), and that in APG (0.87 ± 0.25) was lower than that in RPG (1.09 ± 0.26) (P < 0.001; Figure 5).
Multivariate Logistic Regression Analysis
Multivariate logistic regression analysis was conducted on the factors with significant differences. The results showed that age (P = 0.003), season (P = 0.017), low complement state (P = 0.021), epidemiological contact history (P = 0.013), and antibiotic use time (P = 0.001) were independent risk factors affecting MPP in children. Older age, autumn and winter, presence of a low complement state, presence of epidemiological contact history, and prolonged antibiotic treatment duration could increase the risk of MPP (Tables 2 and 3).
 |
Table 2 Assignment for Multivariate Logistic Regression Analysis |
 |
Table 3 Multivariate Logistic Regression Analysis |
Discussion
As one of the miRNA members, miR-155 is widely present in human serum and other body fluids and plays a panbiological function, and extraction of miR-155 by RT-PCR can allow us to discover its abnormal disorders children with MPP.16 In this study, the expression level of serum miR-155 in APG and RPG was significantly lower than that in CG, and the expression in APG was lower than that in RPG, suggesting that serum miR-155 is involved in the development and progression of MPP in children and is negatively correlated with the severity of the disease. In a study by Yang et al, miR-155 was poorly expressed but the mRNA expression of TNF-α and IL-6 was upregulated in the bronchoalveolar lavage fluid (BALF) of patients with pneumonia,17 indicating that miR-155-mediated gene regulation promotes the repolarization of the human alveolar macrophage phenotype and realizes the early transformation of proinflammatory cytokines to anti-inflammatory cytokines. In a study by Fan et al, the levels of TNF-α and IL-8 were higher in the BALF than in plasma of children with MPP, and the levels were significantly higher in refractory MPP than in general MPP,18 indicating that the two cytokines may serve as markers to indicate the pathological changes and severity of pulmonary diseases. In this study, TNF-α and IL-8 were highly expressed in APG and RPG, and the expression was higher in APG than in RPG, suggesting that serum miR-155 is involved in inflammatory responses of children with MPP by upregulating the expression of these two cytokines. Chai et al found that the serum IL-10, IgG, and IgA levels were upregulated after treatment, whereas the IL-13 level was downregulated,19 which was similar to the findings of this study. In our study, IL-10, IgG, and IgA were highly expressed in APG and RPG, and their expression significantly decreased in RPG; in contrast, IL-13 expression significantly increased, indicating that serum miR-155 may mediate the stress regulation of tissue damage and humoral immunity in children by upregulating IL-10, IgG, and IgA, and downregulating IL-13. In a report by Wang et al, C3 and CH50 levels in children with MPP significantly increased,20 suggesting that the two indices indicate immunosuppression in children. In this study, the C3 and CH50 levels in APG and RPG were significantly higher than those in CG, and those in APG were higher than those in RPG, implying that serum miR-155 may intervene in humoral immunity by increasing C3 and CH50 levels. In a report by Li et al, CD3+, CD4+, and CD4+/CD8+ levels in the peripheral blood of children were lower than those in CG, whereas CD8+ levels were higher than those in CG.21 This was different from the results of this study. In our study, serum CD3+, CD4+, and CD4+/CD8+ were poorly expressed in APG and RPG, but their expression in APG was lower than that in RPG, without significant difference in CD8+ expression between the three groups. These findings show that serum miR-155 may mediate cellular immunity in children with MPP by downregulating CD3+, CD4+, and CD4+/CD8+, but it has no significant regulatory effect on CD8+.
According to the multivariate logistic regression analysis, older age, autumn and winter, presence of a low complement state, presence of epidemiological contact history, and long antibiotic treatment duration could increase the risk of MPP in children, especially long antibiotic use time, older age, and the presence of epidemiological contact history. Therefore, we should pay attention to the physical protection of children in autumn and winter, keep them away from possible places of epidemics, and avoid long-term use of antibiotics during their growth.
Although this study confirms that the downregulation of serum miR-155 expression has a regulatory effect on inflammatory disorders and immune responses in children with MPP, further improvements are possible. First, basic experiments can be increased and animal models can be established to explore the cellular biological function of serum miR-155 in children and supplement relevant regulatory mechanisms. Second, the pathogenic types of the disease can be assessed in detail, and the expression and related pathological mechanisms of serum miR-155 can be investigated in more disease phenotypes. Furthermore, the diagnostic value of miR-155 and related inflammatory and immune indices can be investigated in detail. Finally, further studies should assess larger sample sizes to improve the accuracy of the results. In addition, more manifestations of serum miR-155 in macrolide-resistance and sensitive MPP as well as refractory and non-refractory MPP can be analyzed, which is beneficial to further expand the potential value of serum miR-155 in MPP.
Conclusions
In summary, serum miR-155 expression is low in children with MPP, and there are also inflammation and immune disorders; therefore, it may become a therapeutic target for MPP in children.
Ethics Approval and Informed Consent
This study was approved by the ethics committee of the Affiliated Shuyang Hospital of Xuzhou Medical University on September 27, 2017 (No. 20,160,312). The participants and their families were informed, and all legal guardians signed a fully informed consent form.
Funding
No funding was received.
Disclosure
The authors declare that they have no competing interests.
References
1. Hon KL, Leung AS, Cheung KL, et al. Typical or atypical pneumonia and severe acute respiratory symptoms in PICU. Clin Respir J. 2015;9(3):366–371. doi:10.1111/crj.12149
2. Kim EK, Youn YS, Rhim JW, Shin MS, Kang JH, Lee KY. Epidemiological comparison of three Mycoplasma pneumoniae pneumonia epidemics in a single hospital over 10 years. Korean J Pediatr. 2015;58(5):172–177. doi:10.3345/kjp.2015.58.5.172
3. Poddighe D. Extra-pulmonary diseases related to Mycoplasma pneumoniae in children: recent insights into the pathogenesis. Curr Opin Rheumatol. 2018;30(4):380–387. doi:10.1097/BOR.0000000000000494
4. Bajantri B, Venkatram S, Diaz-Fuentes G. Mycoplasma pneumoniae: a potentially severe infection. J Clin Med Res. 2018;10(7):535–544. doi:10.14740/jocmr3421w
5. Søndergaard MJ, Friis MB, Hansen DS, Jørgensen IM, Schildgen O. Clinical manifestations in infants and children with Mycoplasma pneumoniae infection. PLoS One. 2018;13(4):e0195288. doi:10.1371/journal.pone.0195288
6. Meyer Sauteur PM, Unger WW, Nadal D, Berger C, Vink C, van Rossum AM. Infection with and carriage of Mycoplasma pneumoniae in children. Front Microbiol. 2016;7:329. doi:10.3389/fmicb.2016.00329
7. Chaudhry R, Ghosh A, Chandolia A. Pathogenesis of Mycoplasma pneumoniae: an update. Indian J Med Microbiol. 2016;34(1):7–16. doi:10.4103/0255-0857.174112
8. Ebrahimi A, Sadroddiny E. MicroRNAs in lung diseases: recent findings and their pathophysiological implications. Pulm Pharmacol Ther. 2015;34:55–63. doi:10.1016/j.pupt.2015.08.007
9. Dumitriu I, Bullenkamp J, Mengoni V, Kaski J. Deep phenotyping of pro-inflammatory Cd4+Cd28null T lymphocytes in patients with atherosclerosis reveals distinct gene signatures and function. Atherosclerosis. 2019;287:e24.
10. Michaille JJ, Awad H, Fortman EC, Efanov AA, Tili E. miR-155 expression in antitumor immunity: the higher the better? Genes Chromosomes Cancer. 2019;58(4):208–218. doi:10.1002/gcc.22698
11. Diwakar BT, Yoast R, Nettleford S, et al. Crth2 receptor signaling down-regulates lipopolysaccharide-induced NF-κB activation in murine macrophages via changes in intracellular calcium. FASEB J. 2019;33(11):12838–12852. doi:10.1096/fj.201802608R
12. Mashima R. Physiological roles of miR-155. Immunology. 2015;145(3):323–333. doi:10.1111/imm.12468
13. Figallo C, Bayes L, Lanata M, Etinger V. A diagnostic dilemma: PCR or serology to detect Mycoplasma pneumoniae pneumonia in children. 2017.
14. Lin LJ, Chang FC, Chi H, et al. The diagnostic value of serological studies in pediatric patients with acute Mycoplasma pneumoniae infection. J Microbiol Immunol Infect. 2020;53(2):351–356. doi:10.1016/j.jmii.2018.09.001
15. Hornbeck PV. Enzyme-linked immunosorbent assays. Curr Protoc Immunol. 2015;110:
16. Li H, Qiu F, Tian F, et al. Changes of miR-155 expression in serum of uremic patients before and after treatment and risk factors analysis. Exp Ther Med. 2020;20(4):3352–3360. doi:10.3892/etm.2020.9067
17. Yang Y, Wu BQ, Wang YH, et al. Regulatory effects of miR-155 and miR-146a on repolarization and inflammatory cytokine secretion in human alveolar macrophages in vitro. Immunopharmacol Immunotoxicol. 2016;38(6):502–509. doi:10.1080/08923973.2016.1248845
18. Fan H, Lu B, Yang D, Zhang D, Shi T, Lu G. Distribution and expression of IL-17 and related cytokines in children with Mycoplasma pneumoniae pneumonia. Jpn J Infect Dis. 2019;72(6):387–393. doi:10.7883/yoken.JJID.2019.113
19. Chai Q-L. Effect of azithromycin combined with licorzinc therapy on inflammatory response and immune response in children with mycoplasma pneumonia. J Hainan Med Univ. 2017;23(14):62–65.
20. Wang T, Ding Y, Shi Y, Huang Z, Jia Z, Yang J. Changes on serum myocardial enzyme of mycoplasma pneumoniae pneumonia and clinical significance. Biomed Res. 2017;28:9593–9595.
21. Li S-Q. Evaluation of humoral immunity and peripheral blood T lymphocyte subset levels after Mycoplasma pneumoniae infection. J Hainan Med Univ. 2017;23(3):58–60.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.