Back to Journals » OncoTargets and Therapy » Volume 10
Expression of phosphatase of regenerating liver-3 is associated with prognosis of Wilms’ tumor
Authors Sun F, Li W, Wang L, Jiao C
Received 24 February 2016
Accepted for publication 8 April 2016
Published 10 January 2017 Volume 2017:10 Pages 311—317
DOI https://doi.org/10.2147/OTT.S107076
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Min Li
Fengyin Sun,1 Wenyi Li,2,3 Lie Wang,2 Changfeng Jiao3
1Department of Pediatric Surgery, Qilu Hospital, Shandong University, Jinan, Shandong Province, 2Department of General Surgery, Fuzhou General Hospital of Nanjing Command, PLA, Fuzhou, Fujian Province, 3Department of Vascular Surgery, Xinzhou City People’s Hospital, Xinzhou, Shanxi Province, People’s Republic of China
Objective: The current study was undertaken to explore the clinical and prognostic value of phosphatase of regenerating liver-3 (PRL-3) expression in Wilms’ tumor.
Methods: Seventy-six patients with Wilms’ tumor in Qilu Hospital from January 2003 to July 2009 were enrolled in the study. Protein expression level of PRL-3 was examined by immunohistochemical staining, and the correlation between PRL-3 expression and histopathological parameters, clinical variables, and outcome of patients with Wilms’ tumor were analyzed.
Results: We found that 19% of patients with unfavorable histology had tumor recurrence and 16% of patients died following the operation. PRL-3 was expressed in 15 out of 76 tumors (19%) and expressed highly in unfavorable histology Wilms’ tumor (P=0.04). PRL-3 protein expression level was correlated to 2.5-fold increase in recurrence rate of Wilms’ tumor (P=0.06) without any statistically significant difference. However, in favorable histology Wilms’ tumor, PRL-3 expression was correlated to an increase of 3.4-fold in recurrence rate (P=0.03).
Conclusion: The expression of PRL-3 protein was correlated with an increased recurrence rate of favorable histology Wilms’ tumor. PRL-3 may serve as a promising biomarker for predicting patients with high risk of Wilms’ tumor. Further investigations are warranted to investigate the clinical function of PRL-3 in Wilms’ tumor.
Keywords: Wilms’ tumor, prognosis, tumorigenesis, recurrence
Introduction
Wilms’ tumor, also known as nephroblastoma, is the most common type of pediatric malignant solid kidney tumor with an incidence rate of approximately 1 to 2 per 1,000,000 during childhood. Its peak incidence age was 3 years, and in 80% of cases, it was seen in children aged <5 years.1 Currently, combined treatment with surgery, chemotherapy, and radiotherapy has greatly improved the prognosis of patients with Wilms’ tumor, with a 5-year disease-free survival rate increase of 75% to 85%.2 However, because of the lack of specific clinical symptoms, ~39.2% of patients reach clinical stage III or above at the time of diagnosis, which leads to relatively low therapeutic efficacy.3 Additionally, due to its high invasion, metastasis, and recurrence rate, Wilms’ tumor remains a nodus for the treatment. So far, mechanisms underlying Wilms’ tumorigenesis remain largely unknown. Therefore, exploring the biological characteristics and mechanisms to reduce the incidence, improve the early diagnosis, and seek more effective treatment strategies is of great importance.
Phosphatase of regenerating liver-3 (PRL-3) was initially found in 1994. Xing et al cloned a protein phosphatase gene in the early stages of regeneration of liver cells, which was named PRL-1,4 and its related members PRL-2 and PRL-3 were found later.5 Recent studies have shown that, in addition to promoting liver regeneration, PRL-1 has also participated in mitosis of tumor cells.6 PRL-2 can promote tumor cell proliferation,7 but studies on its expression in tumor tissues are rare. It has been illustrated that PRL-3 has promotive effects on cell proliferation, migration, and invasion.8 Upregulation of PRL-3 expression occurs in various human cancers, including colorectal,9 gastric,10 ovarian,11 breast, and prostate cancers.12,13 However, the expression of PRL-3 in Wilms’ tumor and its association with the clinical parameters are unclear. In this study, we investigated the expression of PRL-3 and evaluated its clinical significance in predicting the prognosis of Wilms’ tumor.
Materials and methods
Patients
A total of 106 consecutive patients with Wilms’ tumor were identified in Qilu Hospital from 2003 to 2009 according to the Children’s Oncology Group criteria. Exclusion criteria were as follows 1) chemotherapy or neoadjuvant radiation was received before biopsy and 2) tumor specimen and medical history data were insufficient. Finally, 76 patients were included for further analysis. All participants enrolled in this study underwent primary surgical therapy according to the National Wilms’ Tumor Study protocols active. Written informed consent of patients were acquired. The study was approved by the Research Ethics Committee of Qilu Hospital, Shandong University.
Clinical variables and pathological parameters
Clinical variables were measured and histopathologic review was conducted by expert pathologists. Clinical variables include patient age at surgery; gender; clinical stage stratified by I, II, III, or IV; tumor necrosis; outcome; extent of cell infiltration; tumor histology (favorable histology [FH] or unfavorable histology [UH]). Cell infiltration was indicated as negative (absent) or positive (exist). For positive, cell infiltration was classified according to severity as “+”, “++”, or “+++”. “+” refers to inflammatory cell clusters ≤2 at ×100 magnification, “++” to inflammatory cell clusters >2, and “+++” to inflammatory cell clusters >10.
Immunohistochemical analysis of PRL-3 expression
For immunohistochemical analysis, 5 μm thick specimens were obtained from 76 patients. Specimens were fixed and embedded in formalin and paraffin at 55°C for no <2 hours and then deparaffinized. Endogenous peroxidase activity was blocked by incubation with 3% H2O2 for 20 minutes. After antigen retrieval, the tissue specimens were blocked with 5% normal serum, followed by incubation with anti-PRL-3 mouse monoclonal antibody (sc-130355; Santa Cruz Biotechnology Inc., Dallas, TX, USA ) diluted (1:100) in phosphate-buffered saline overnight at 4°C. Phosphate-buffered saline was used as a negative control. Immunoreactivity both in cytoplasm and cytoplasmic membrane was evaluated. Positive staining of PRL-3 in tumor tissues was examined by expert pathologists. The staining intensity of PRL-3 was graded and recorded in two groups, the negative (absent, “−”) and the positive (exist) group. If positive, it was further classified into three groups: weak “+”, moderate “++”, and strong “+++”.
Western blot analysis of PRL-3 expression
Tumor tissue was lysed in radioimmunoprecipitation assay buffer supplemented with a protease/phosphatase inhibitor cocktail (Cell Signaling Technology, Danvers, MA, USA) with sonication. Lysates were cleared by centrifugation and protein concentrations were determined by bicinchoninic acid assay method. Equal amounts of total cellular proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel and transferred to polyvinylidene difluoride membranes. After blocking, the membranes were incubated with primary, followed by secondary antibodies. Protein bands were visualized by the electrochemiluminescence system (Amersham Biosciences, Piscataway, NJ, USA).
Statistical methods
Data were analyzed using the statistical software package R-3.0.0 (SPSS17.0, SPSS Inc., Chicago, IL, USA). The overall survival and recurrence-free survival (RFS) curves were estimated by the Kaplan–Meier method. The associations between the PRL-3 expression and the clinicopathological features were analyzed using the Pearson’s chi-square test. The relationship between the PRL-3 expression and outcome was assessed by Cox proportional hazards model with clinicopathological features. Statistical significance was considered as P-value <0.05.
Results
Correlation between PRL-3 expression and clinicopathological features in patients with FH and UH Wilms’ tumor
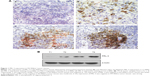
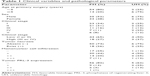
The clinicopathological characteristics in 76 patients are shown in Table 1. The mean age of patients at surgery was 4.7 years. Of all patients, 60 out of 76 (79%) underwent radical nephrectomy, 14 out of 76 (18%) underwent open kidney biopsy, and two out of 76 (3%) underwent renal biopsy. The protein expression of PRL-3 was examined using immunohistochemistry (Table 2). Positive PRL-3 expression was found in 15 out of 76 tumors (19%). The positive expression rate of PRL-3 was significantly higher in FH compared with UH Wilms’ tumor (38% vs 7.6%, P=0.028). In both FH and UH Wilms’ tumors, PRL-3 expression was found primarily among the components of blastemal tissue (Figure 1A). The PRL-3 expression in Wilms’ tumors was further confirmed by Western blot (Figure 1B).
  | Table 1 Clinical variables and pathological parameters |
PRL-3 expression, an independent prognostic factor in patients with FH and UH Wilms’ tumors
The mean recurrence time postoperatively was 2.4 years in the 16 patients seen in follow-up, with a median time period of 1.2 years. Overall survival of patients with positive PRL-3 expression was significantly lower than that of patients with negative PRL-3 expression (P=0.04, Figure 2A). Also, the 3-year RFS rate of patients with positive PRL-3 expression was significantly shorter than that of patients with negative PRL-3 expression (P=0.009, Figure 2B). The association between PRL-3 expression and clinicopathological characteristics in patients with tumor recurrence is summarized in Table 3. Multivariate analysis showed that the recurrence rate in patients with stage III or IV disease was dramatically increased compared with those with stage I or II tumors (P=0.004). PRL-3 protein expression level was correlated to 2.5-fold increased recurrence rate in patients with Wilms’ tumor, although there was no significant difference (P=0.067).
Twelve of 76 patients died postoperatively at a mean age of 3.6 years. Among the remaining 64 patients, the average follow-up duration was 5.1 years. The postoperative 1-, 3-, and 5-year estimated RFS rates (95% confidence interval, number of at-risk patients) of patients were 95.1% (92–96, 73), 91.3% (93–99, 67), and 87.1% (88–93, 58), respectively. The death rate of patients aged ≥5 years at the time of surgery was 5.3-fold higher than that of patients aged <5 years at the time of surgery(P=0.007). The death rate increased by 6% when the age at surgery increased by 1 year (P=0.001). Additionally, the death rate of patients having stage III or IV disease was dramatically higher compared to those having stage I or II disease (P=0.006). Notably, the death rate of patients with UH Wilms’ tumor was higher than those having FH Wilms’ tumor (P=0.008). However, PRL-3 expression showed no relationship with the death rate of patients with Wilms’ tumor (P=0.093).
Correlation between PRL-3 expression and clinicopathological index in patients with FH Wilms’ tumor
Clinicopathological index and PRL-3 expression of 68 patients with FH Wilms’ tumor are summarized in Table 1. The mean age at surgery was 4.3 years. Among this subset, PRL-3 expression was positive in 15 out of 68 patients (19%). However, none of the clinicopathological index (surgery, age, or gender) in patients with FH Wilms’ tumor had statistically significant association with PRL-3 protein expression (P>0.05).
PRL-3 expression is a potential prognostic index in patients with FH Wilms’ tumor
Nine patients in follow-up showed a mean recurrence time postoperatively of 3.0 years, with a median recurrence time of 2.6 years. The postoperative 1-, 3-, and 5-year estimated RFS rates of patients with FH Wilms’ tumor were 87.3%, 80.6%, and 79.5%, respectively. The association between clinicopathological characteristics and PRL-3 expression in patients with tumor recurrence is summarized in Table 3. The recurrence rate in patients having stage III or IV disease was dramatically higher compared to those with stage I or II disease (P=0.034). Among 14 patients with recurrent FH Wilms’ tumor, three showed positive expression of PRL-3. Moreover, in this subset of patients, PRL-3 expression was positively related to the recurrence rate, which was 3.4-fold higher (P=0.029).
Additionally, among patients with FH Wilms’ tumor, six patients died postoperatively at a mean age of 3.2 years. In the remaining 62 FH patients, the average follow-up duration was 5.3 years. The postoperative 1-, 3-, and 5-year estimated RFS rates of patients with FH Wilms’ tumor were 97.4%, 93.7%, and 91.1%, respectively. The death rate was increased 6% when the age at surgery was increased 1 year (P=0.012). Additionally, the death rate of patients with high stage tumor and tumor necrosis was dramatically higher compared with those with stage I or II disease (P<0.05), and the death rate was positively correlated to tumor necrosis (P>0.05).
Discussion
Studies on the correlation of PRL-3 expression and tumor recurrence are rare. Previous studies have reported that high level of PRL-3 is associated with high tumor recurrence in colorectal cancer.14,15 Our study demonstrated that PRL-3 expression may serve as a novel prognostic predictor of Wilms’ tumor. Since the cohort in our study had limited number of candidates, no significant relationship was found between PRL-3 expression and tumor-specific death.
Our study also indicated that PRL-3 protein was positively expressed by 19% Wilms’ tumor tissues and illustrated a significant correlation with aggressiveness of Wilms’ tumor, indicating that it may provide a novel target for the treatment. Currently, it has been found that knockdown of PRL-3 mediated by small interfering RNA significantly inhibited the proliferation of ovarian cancer cells.16–18 Some scholars have developed a PRL-3 monoclonal antibody, which serves as a specific detector of PRL-3 protein expression in colorectal cancer.19,20 However, whether PRL-3-targeted interference can achieve therapeutic purposes remains to be further elucidated.
Most notably, we found that high levels of PRL-3 expression was associated with poorer outcome in patients with FH Wilms’ tumor who had no prognostic features previously, indicating that PRL-3 expression, to some extent, can function as an potential independent marker predicting the aggressiveness of FH Wilms’ tumor. Therefore, PRL-3 may be an ideal indicator to distinguish patients with FH Wilms’ tumor from those with low risk of disease recurrence or death, thus exempting patients from unnecessary overtreatment. Our finding showed that PRL-3 expression correlated with 3.4-fold increase in recurrence rate of FH tumors, remarkably implying the necessity for management of Wilms’ tumor.
Limitations
Patients undergoing multiple therapeutic schedules, of which the variation in chemo- or radiotherapy, as well as surgical technique, could persuasively affect our experiment outcomes. Studies of a multicenter and more uniform cohort of patients were needed for sufficiently powerful identification of additional predictive features of PRL-3 expression in patients with Wilms’ tumor. Second, the small cohort size of our study did not allow in-depth analysis and validations in other populations of large study cohorts including Caucasians, Africans, or other Asians are warranted.
Conclusion
PRL-3 expression occurs in Wilms’ tumor and is associated with tumor recurrence in patients with FH Wilms’ tumor, indicating that PRL-3 might serve as an ideal prognostic biomarker for FH Wilms’ tumor.
Acknowledgments
We thank the Department of Vascular Surgery, Xinzhou City People’s Hospital for the statistical assistance and are indebted to Fuzhou General Hospital of Nanjing Command, PLA for the financial support.
Disclosure
The authors report no conflicts of interest in this work.
References
Kletzel M, Chou PM, Olszewski M, Rademaker AW, Khan S. Expression of Wilms tumor gene in high risk neuroblastoma: complementary marker to tyrosine hydroxylase for detection of minimal residual disease. Transl Pediatr. 2015;4(3):219–225. | ||
Sarin YK, Thakkar NC, Sinha S. Synchronous ipsilateral Wilms’ tumor and neuroblastoma in an infant. APSP J Case Rep. 2016;7(1):2. | ||
Yasri S, Wiwanitkit V. Wilms’ tumour and chemotherapeutic access. Afr J Paediatr Surg. 2015;12(3):208. | ||
Xing X, Lian S, Hu Y, et al. Phosphatase of regenerating liver-3 (PRL-3) is associated with metastasis and poor prognosis in gastric carcinoma. J Transl Med. 2013;11:309. | ||
Dumaual CM, Sandusky GE, Soo HW, Werner SR, Crowell PL, Randall SK. Tissue-specific alterations of PRL-1 and PRL-2 expression in cancer. Am J Transl Res. 2012;4(1):83–101. | ||
Wang Y, Lazo JS. Metastasis-associated phosphatase PRL-2 regulates tumor cell migration and invasion. Oncogene. 2012;31(7):818–827. | ||
Akiyama S, Dhavan D, Yi T. PRL-2 increases Epo and IL-3 responses in hematopoietic cells. Blood Cells Mol Dis. 2010;44(4):209–214. | ||
Lee SK, Han YM, Yun J, et al. Phosphatase of regenerating liver-3 promotes migration and invasion by upregulating matrix metalloproteinases-7 in human colorectal cancer cells. Int J Cancer. 2012;131(3):E190–E203. | ||
Saha S, Bardelli A, Buckhaults P, et al. A phosphatase associated with metastasis of colorectal cancer. Science. 2001;294(5545):1343–1346. | ||
Miskad UA, Aemba S, Kato H, et al. Expression of PRL-3 phosphatase in human gastric carcinomas: close correlation with invasion and metastasis. Pathobiology. 2004;71(4):176–184. | ||
Polato F, Codegoni A, Fruscio R, et al. PRL-3 phosphatase is implicated in ovarian cancer growth. Clin Cancer Res. 2005;11(19 Pt 1):6835–6839. | ||
Radke I, Gotte M, Kerating C, Mattsson B, Kiesel L, Wülfing P. Expression and prognostic impact of the protein tyrosine phosphatases PRL-1.PRL-2, and PRL-3 in breast cancer. Br J Cancer. 2006;95(3):347–354. | ||
Shin Y, Kim GD, Jeon JE, Shin J, Lee SK. Antimetastatic effect of halichondramide, a trisoxazole macrolide from the marine sponge Chondrosia corticata, on human prostate cancer cells via modulation of epithelial-to-mesenchymal transition. Mar Drugs. 2013;11(7):2472–2485. | ||
Molleví DG, Aytes A, Padullés L, et al. PRL-3 is essentially overexpressed in primary colorectal tumours and associates with tumour aggressiveness. Br J Cancer. 2008;99(10):1718–1725. | ||
Krndija D, Münzberg C, Maass U, et al. The phosphatase of regenerating liver 3 (PRL-3) promotes cell migration through Arf-activity-dependent stimulation of integrin α5 recycling. J Cell Sci. 2012;125(Pt 16):3883–3892. | ||
Ooki A, Yamashita K, Kikuchi S, et al. Therapeutic potential of PRL-3 targeting and clinical significance of PRL-3 genomic amplification in gastric cancer. BMC Cancer. 2011;11:122. | ||
Stephens B, Han H, Hostetter G, Demeure MJ, Von Hoff DD. Small interfering RNA-mediated knockdown of PRL phosphatases results in altered Akt phosphorylation and reduced clonogenicity of pancreatic cancer cells. Mol Cancer Ther. 2008;7(1):202–210. | ||
Al-Aidaroos AQ, Yuen HF, Guo K, et al. Metastasis-associated PRL-3 induces EGFR activation and addiction in cancer cells. J Clin Invest. 2013;123(8):3459–3471. | ||
Wang Y, Li ZF, He J, et al. Expression of the human phosphatases of regenerating liver (PRLs) in colonic adenocarcinoma and its correlation with lymph node metastasis. Int J Colorectal Dis. 2007;22(10):1179–1184. | ||
Guo K, Tang JP, Jie L, et al. Engineering the first chimeric antibody in targeting intracellular PRL-3 oncoprotein for cancer therapy in mice. Oncotarget. 2012;3(2):158–171. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.