Back to Journals » OncoTargets and Therapy » Volume 12
Expression of NR1H3 in endometrial carcinoma and its effect on the proliferation of Ishikawa cells in vitro
Authors Fang F , Li D , Zhao L , Li Y, Zhang T, Cui B
Received 17 July 2018
Accepted for publication 5 November 2018
Published 18 January 2019 Volume 2019:12 Pages 685—697
DOI https://doi.org/10.2147/OTT.S180534
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sanjeev K. Srivastava
Fang Fang,1,2 Dawei Li,1,2 Lu Zhao,1 Yue Li,1,2 Teng Zhang,1 Baoxia Cui1
1Department of Obstetrics and Gynecology, Qilu Hospital, Shandong University, Jinan, Shandong, People’s Republic of China; 2Department of Obstetrics and Gynecology, Weihai Municipal Hospital, Weihai, Shandong, People’s Republic of China
Purpose: Our study aimed to investigate the expression of NR1H3 in endometrial carcinoma, its effect on the proliferation of endometrial carcinoma cells in vitro, and the underlying mechanism of this effect.
Materials and methods: Immunohistochemistry of paraffin-embedded, sectioned specimens and of a tissue microarray was conducted to estimate the expression of NR1H3 (liver X receptors α: LXRα) and NR1H2 (liver X receptors β: LXRβ) in endometrial carcinoma tissues. The subcellular localization of NR1H3 in the endometrial carcinoma cell line Ishikawa was determined by immunofluorescence. An agonist of NR1H3, TO901317, was then administered to activate the expression of NR1H3, and cell viability and cell-cycle progression were investigated through MTT and flow cytometric assays, respectively. The gene and protein expression levels of NR1H3, cyclin D1 (CCND1), and cyclin E (CCNE) in cells pretreated with different concentrations of TO901317 for different periods of time were also detected by real-time RT-PCR and Western blot, respectively.
Results: The results showed that, in contrast to NR1H2, which was expressed at low levels in endometrial tissues, NR1H3 was upregulated in endometrial adenocarcinoma tissues compared to levels in normal endometrial tissues and endometrial polyps. Moreover, NR1H3 was mainly expressed in the cytoplasm of Ishikawa cells. TO901317 significantly decreased cell viability and arrested the cell cycle in Ishikawa cells in a dose- and time-dependent manner. Furthermore, the administration of TO901317 not only promoted the expression of NR1H3 but also inhibited the expression of CCND1 and CCNE in Ishikawa cells.
Conclusion: We demonstrated that NR1H3 is upregulated in endometrial adenocarcinoma and that it inhibits cell viability by inhibiting the expression of CCND1 and CCNE in endometrial carcinoma cells. Our study indicates that NR1H3 may play a role in the development of endometrial cancer and may emerge as a promising therapeutic target.
Keywords: liver X receptor, CCND1, endometrial carcinoma, cell proliferation, cell cycle
Introduction
Liver X receptors (LXRs) are ligand-activated transcription factors belonging to the nuclear receptor superfamily. Nuclear receptor subfamily 1 group H member 3 (NR1H3), also called liver X receptors α (LXRα), and nuclear receptor subfamily 1 group H member 2 (NR1H2), also called liver X receptors β (LXRβ), are two isoforms LXRs, which are activated by oxysterols (oxidized cholesterol derivatives), such as 22®-hydroxycholesterol (HC), 20(S)-HC, 20,22-dihydroxycholesterol, and 24-HC.1–5 TO901317 was the first reported synthetic LXR agonist and is most commonly used to study the mechanistic behaviors of LXRs in breast cancer therapy.6,7 LXRs bind to nuclear retinoid X receptors (RXRs) and form obligate LXR/RXR heterodimers, which can be activated either by LXR agonists, such as oxysterols or synthetic ligands, like TO901317 (TO) and GW3965 (GW), or by RXR ligands, such as 9-cis retinoic acid.8–11 NR1H3 and NR1H2 are expressed in many tissues and cells, such as the liver, intestines, adipocytes, and macrophages.12 NR1H3 is highly expressed in the breast, colon, pancreas, esophagus, and liver, whereas NR1H2 is ubiquitously expressed.13–18 NR1H3 and NR1H2 are associated with various metabolic functions, such as cholesterol homeostasis, fatty acid homeostasis, steroidogenesis, glucose homeostasis, inflammation, and immunity.19–24 Recent studies have shown that LXR agonists also exert antiproliferative effects in multiple cancer cell lines, including prostate, breast, liver, lung, cervical, and skin cancer cell lines.25
Metabolic syndrome (MS), also called X syndrome or insulin resistance (IR) syndrome, is primarily associated with diabetes or abnormal glucose tolerance, hypertension, dyslipidemia, and obesity.26 A large number of epidemiological studies have shown that IR is closely related to many kinds of cancer, including colon cancer, pancreatic cancer, breast cancer, and endometrial cancer (EC).27–29 NR1H3 and NR1H2 are known to be associated with various metabolic functions, such as the homeostasis of cholesterol, fatty acids, and glucose, as well as steroidogenesis.30 At high cholesterol levels, NR1H3 and NR1H2 upregulate the expression of sterol regulatory element binding proteins (SREBP-1C), which contain LXR binding sites and are major transcriptional regulators involved in the synthesis of fat metabolism genes.31,32 SREBP has been confirmed to be upregulated in a variety of malignant tumors, such as colon, breast, prostate, and liver cancer, and is closely related to cancer progression and metastasis. Therefore, the abnormal functioning of these genes can cause MS symptoms and induce the occurrence of malignant tumors. The metabolic targets of LXR are also involved in the antitumor effects of LXR ligands. Previous experiments in breast cancer cell lines showed that treatment with LXR ligands led to a disruption in cell proliferation and increased apoptosis.33
EC is one of the most common malignant tumors of the female genital tract and is the tenth most common cause of cancer-related deaths in developed countries.34 Although the incidence of EC in developing countries is lower than that in developed countries, its mortality/morbidity rate is higher in developing countries.35 Several prospective and retrospective case–control studies have indicated that MS is an important risk factor for EC.36,37 We hypothesized that as NR1H3 and NR1H2 play an important role in the development of MS, it is also a risk factor for EC. Therefore, we speculated that NR1H3 and NR1H2 affect the incidence and prognosis of EC. Recently, the role of NR1H3 and NR1H2 in the occurrence, development, and metastasis of many malignancies has been studied, including breast cancer, and it has been widely exploited as a drug target for the treatment of breast cancer. The various mechanisms underlying the effects of NR1H3 and NR1H2 in this area are well studied. However, research regarding the role of NR1H3 and NR1H2 in EC remains limited.
Cyclins are important factors in the molecular mechanisms of cell-cycle progression. There are eight types of cyclins, cyclins A–H. Among these cyclins, cyclin D1 (CCND1) is the most important for the G1/S transition and is currently recognized as a cancer gene. Several studies have shown that CCND1 is closely related to the occurrence and development of EC.38 The expression of CCND1 is mainly regulated by transcription of activator protein 1 (Ap-1). Ap-1 combines with a variety of activated proteins (such as c-fos, etc.) and coregulatory factors (such as p300, etc.) to promote the transcription and expression of CCND1. However, p300 also plays a key role in regulating transcription during LXR transcription. When LXR was activated, the activation function of LXR ligand binding domain was combined with p300 and p160. Therefore, LXR expression is correlated with CCND1 expression. Studies have speculated that TO901317 may inhibit the expression of CCND1 by promoting the binding of LXR molecules to p300 and reducing the binding of p300 to c-fos. In addition, cyclin E (CCNE) is a key factor in the G1/S transition. Thus, the overexpression of CCNE shortens G1 phase and induces advancement into S phase, resulting in cell proliferation and tumor formation. Accordingly, CCND1 and CCNE were chosen by us as research object.
In this study, we aimed to reveal the differences in NR1H3 expression between EC tissues and normal endometrial tissues and to explore the effects on the proliferation of Ishikawa EC cells in vitro. We also assessed the expression levels of CCNE and CCND1 via real-time RT-PCR and Western blot analysis to determine the effect of NR1H3 on the cell cycle of Ishikawa EC cells.
Materials and methods
Immunohistochemistry
Paraffin-embedded sectioned specimens
Sectioned tissue specimens were obtained from patients at the Department of Pathology of Wei Hai Municipal Hospital, Shan Dong Province, PR China, from 2014 to 2015. The use of specimens was approved by the hospital’s ethics committee and the written informed consent was obtained from each patient. Patients were 21–77 years old and had not received preoperative chemotherapy or radiotherapy. The pathology of each section was reviewed and confirmed by H&E staining under a light microscope. A total of 90 specimens were collected, including 30 cases with normal endometrial tissues, 20 cases of endometrial polyps, and 40 cases of endometrial adenocarcinoma in different stages. The normal endometrial tissues were collected from patients with uterine fibroids or ovarian benign tumors who chose to undergo total hysterectomy, and the pathological assessments confirmed that there were no endometrial impairments in these cases, which included 22 cases in the proliferation phase and 8 cases in the secretory phase. Sections were fixed with 10% formalin and were embedded in paraffin.
Tissue microarray
To avoid discrepancies caused by differences in temperature, antibody concentration, and reaction time when repeating the immunohistochemistry experiments, we developed a tissue microarray of endometrial tissues for immunohistochemical analysis. The tissue microarrays were purchased from Alina Biological Science and Technology Co., Ltd. (Xi An, PR China) and numbered UT961, UTN801, UT243a, and UT501a. The array contained 93 endometrial adenocarcinoma tissues in different phases of progression and 84 normal endometrial tissues in different phases of progression (including 12 paracancerous tissues: 6 adjacent to endometrial adenocarcinoma, 2 adjacent to serous adenocarcinoma, 2 adjacent to glassy cell carcinoma, 1 adjacent to carcinosarcoma, and 1 adjacent to a malignant Mullerian mixed tumor).
Immunohistochemical procedures
The typical immunohistochemical methods used were consistent with the following protocol. Sections were typically dewaxed and dehydrated, followed by microwave repair. Sections were incubated in 3% H2O2 (Sigma-Aldrich Co., St Louis, MO, USA) at 37°C for 15 minutes to inactivate the endogenous peroxidase, and then washed with PBS three times for 3 minutes each time. After blocking with 5% BSA (Beyotime, Shanghai, PR China) for 1 hour, anti-NR1H3 (1:200 dilution; ab106464, Abcam, Cambridge, UK) or anti-NR1H2 (1:200 dilution; ab56237, Abcam) was added and incubated at 4°C overnight. Then, the cells were washed with PBS three times for 3 minutes each. Then, horseradish peroxidase (HRP)-labeled goat antirabbit IgG (Abcam) was added and incubated at 37°C for 20 minutes. Next, streptavidin–biotin complex (Boster, Biological Technology, Pleasanton, CA, USA) was added, and the sections were stained with a diaminobenzidine chromogenic reagent kit (Thermo Fisher Scientific, Waltham, MA, USA) at ~20°C for 5 minutes and then washed with distilled water. Sections were counterstained with hematoxylin for 1 minute, dehydrated, and coated with resin. A positive signal was identified by the appearance of a tan or brown color in the cytoplasm and nucleus. At least five high-powered fields were used to observe each sample, and the numbers of positive cells were counted. No staining and/or <30% positive cells indicated a negative sample, while samples with >30% positive cells were considered positive.
Cells and culture
The endometrial carcinoma cell line Ishikawa was purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA). The cells were maintained in RPMI-1640 in a humidified atmosphere (5% CO2) at 37°C, supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. When the cells reached 90% confluence, they were enzymatically digested with 0.25% trypsin. Malignant cells presented varying sizes, irregular shapes, high karyoplasmic ratios, and more than two visible nucleoli. Under an inverted microscope, monolayer cells were grown, and their density increased.
Immunofluorescence
For immunofluorescent staining, Ishikawa cells were grown on coverslips in a 6-well plate (5×105 cells per well) for 24 hours to 70% confluence. After washing with PBS twice, the cells were fixed with 4% paraformaldehyde and washed again. Then, the cells were permeabilized with 0.1% Triton X-100 (Sigma-Aldrich) and washed with PBS three times for 5 minutes each. Cells were then blocked with 5% BSA at room temperature for 30 minutes. Anti-NR1H3 primary antibody (1:500 dilution) in blocking solution was applied for 16 hours at 4°C, after which the samples were washed with PBS three times for 5 minutes each and incubated with tetramethylrhodamineisothiocyanate-conjugated goat antirabbit secondary antibodies (excitation/emission =550/570±10 nm; 1:500 dilution; Abcam) for 1 hour at 37°C. The nuclei were then stained with 2 μg/mL Hoechst 33342 at room temperature for 15 minutes, and the slides were visualized by confocal fluorescence microscopy.
Cell viability assay
The viability of Ishikawa cells was detected by MTT (Sigma-Aldrich) method. Typically, the cells (5×103 cells per well) were maintained in RPMI-1640 with 10% FBS and grown in 96-well microtiter plates at 37°C in a 5% CO2 humidified chamber for 24 hours. Then, the cells were incubated with different concentrations (0, 5, 10, or 20 μM) of TO901317 for 24 hours. Next, 20 μL MTT solution was added to the cells and incubated at 37°C for 4 hours. After that, the supernatant was removed, and 150 μL dimethyl sulfoxide (DMSO) was added, followed by shaking at room temperature for 10 minutes to sufficiently dissolve the crystal. The OD value at 490 nm was then determined. The percent inhibition of cell viability was calculated using the following equation: (1 − (ODexperimental group/ODcontrol))×100. To assess the effect of an NR1H3 agonist on Ishikawa cells after different incubation periods, the procedure of it was similar to that for analyzing the effect of the agonist at different concentrations, with the exception of fixed concentration of TO901317 at 10 μM and exposure times of 0, 12, 24, and 48 hours. The control group was treated with isovolumetric DMSO.
Flow cytometric analysis of the cell cycle
Ishikawa cells were grown in a 6-well plate (3×105 cells per well), and pretreated with 20 μM TO901317 or isovolumetric DMSO for 48 hours. The treated cells were harvested by 0.25% trypsin, washed with PBS, and fixed with 70% ethanol at 20°C for 5 minutes. The cells were then incubated with propidium iodide (Dojindo, Tokyo, Japan) and 0.5 mL of RNase A at 37°C for 30 minutes. The cell cycle was determined by flow cytometry, and the proliferation index (PI) was used to measure the multiplicative division. PI was calculated by the following equation: PI=(S+G2)/(G1+S+G2). The higher value of PI demonstrated more significant cell proliferation.
Real-time RT-PCR
Total RNA was extracted using TRIzol reagent (TaKaRa Bio Inc, Dalian, China), and the white precipitate was dissolved in 20 μL DEPC water. The concentration of RNA was detected by determining the OD260/OD280 ratio using an ultraviolet spectrophotometer. cDNA was synthesized through the reverse transcription method using a cDNA synthesis kit. The primers for amplifying NR1H3 (F: CATATGTGGAAGCCCTGCAT; R: GGAGGCTCACCAGTTTCATTAG), CCND1 (F: GCGGAGGAGAACAAACAGAT; R: GAGGGCGGATTGGAAATGA), and CCNE (F: GTACTGAGCTGGGCAAATAGAG; R: GAAGAGGGTGTTGCTCAAGAA) genes were synthesized by Sangon Biotech Co., Ltd. (Shanghai, PR China). Then, real-time RT-PCR was performed with a thermal cycler and a master-cycler gradient (95°C for 10 minutes, followed by 95°C for 5 seconds and 60°C for 1 minute each for 40 cycles, and followed by 95°C for 15 seconds, and 60°C for 1 minute). The data were analyzed using StepOne software.
Western blot
Ishikawa cells were seeded in Petri dishes and incubated with different concentrations of TO901317 for different times. Then, extracts were prepared using a whole-cell extraction kit (Beyotime). Protein concentrations were determined using the bicinchoninic acid protein assay (Beyotime). Aliquots of protein were separated by electrophoresis for 80 minutes at 120 V and transferred to nitrocellulose membranes (Beyotime). Next, the membranes were blocked with 4% nonfat dry milk in Tris-buffered saline (pH 7.4; Beyotime) containing 0.01% Tween 20 (Sigma-Aldrich) and incubated with the following primary antibody: anti-NR1H3 (1:1,000 dilution; ab106464), anti-CCND1 (1:500 dilution; ab134175, Abcam), anti-CCNE (1:1,000 dilution; ab33911, Abcam), or anti-glyceraldehyde-3-phosphate dehydrogenase (anti-GAPDH; 1:5,000 dilution; ab8245, Abcam). The blots were then washed in PBS-Tween 20 and incubated with HRP-labeled goat antirabbit secondary antibodies (1:500) at room temperature for 1 hour. The protein of interest was visualized using an enhanced chemiluminescence system (ChemiDocMP; Bio-Rad Laboratories Inc., Hercules, CA, USA), and images were processed using ImageJ software (Alpha Imager2200).
Statistical analysis
Data shown in the study were obtained from at least three independent experiments. All data for different experimental groups are expressed as the mean ± SEM and analyzed by one-way ANOVA. Statistical analysis was performed using SPSS 15.0 software (SPSS, Inc., Chicago, IL, USA). A P-value <0.05 is considered statistically significant.
Results
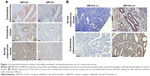
NR1H3 was overexpressed in endometrial adenocarcinoma tissues
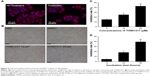
To study the expression of NR1H3 proteins in endometrial tissues, we first performed immunohistochemistry on paraffin-embedded, sectioned specimens. The resulting images indicated that the percentages of cells positive for NR1H3 expression in normal endometrial tissues, endometrial polyps, and endometrial adenocarcinoma tissues were 13%, 50%, and 85%, respectively (Figure 1A, Table 1), with a significant difference in the percentage of positive cells in endometrial adenocarcinoma tissues compared with those in the other two groups (P<0.01). For NR1H2, the percentages of positive cells in normal endometrial tissues, endometrial polyps, and endometrial adenocarcinoma tissues were 10%, 10%, and 12%, respectively, with no significant differences among groups (Figure 1A, Table 1). Therefore, NR1H3 was selected as the main target of follow-up experiments. Consistent with the paraffin-embedded section results, immunohistochemistry using the tissue microarray also indicated a higher percentage of cells expressing NR1H3 in endometrial adenocarcinoma tissues (88%) than in normal endometrial tissues (30%; Figure 1B, Table 2). In addition, the expression of NR1H3 in Ishikawa cells was studied by immunofluorescent staining. As shown in Figure 2A, red fluorescence, indicating the presence of NR1H3, was mainly distributed in the cytoplasm. Meanwhile, after treatment with TO901317, the result showed that there was no localization change of the intracellular localization of NR1H3.
TO901317 significantly decreased the viability of Ishikawa cells
To study the effect of NR1H3 on the viability of endometrial carcinoma cells, we first observed the growth of Ishikawa cells pretreated with different concentrations of TO901317, an agonist of NR1H3. As shown in Figure 2B, after 48 hours of incubation, the cells pretreated with PBS or DMSO were monoptychial and adherent and exhibited favorable growth, growing to ~90% confluence. However, growth conditions were poor for cells pretreated with TO901317, with considerable apoptosis observed. Furthermore, a higher dose of TO901317 resulted in a reduction in cellular proliferation: cells reached 60% confluence when pretreated with 10 μM TO901317 but only 20% confluence when treated with 20 μM TO901317.
Next, we assessed cell viability using the MTT method. Again, cellular proliferation was inhibited more dramatically with an increase in the concentration of TO901317, with inhibition rates of 15%±0.97%, 24.8%±2.18%, and 42.5%±2.87% in cells pretreated with 5, 10, and 20 μM TO901317, respectively at 48 hours (P<0.001; Figure 2C). Moreover, inhibition rates increased with increasing incubation time, resulting in rates of 4.7%±0.69%, 18.1%±1.87%, and 39.2%±2.67% after incubation periods of 12, 24, and 48 hours, respectively, in cells pretreated with 10 μM TO901317 (P<0.001; Figure 2D). These results indicate that increasing either the concentration or incubation time of the NR1H3 agonist inhibits the viability of Ishikawa cells.
TO901317 significantly arrested the cell cycle in Ishikawa cells
The cell-cycle phases of Ishikawa cells pretreated with TO901317 or DMSO were detected by flow cytometric analysis. As shown in Figure 3, compared to cells pretreated with DMSO, cells pretreated with TO901317 exhibited an increased percentage of G1 phase cells (40.10%±1.12% vs 63.20%±2.34%, respectively) and G2 phase cells (8.32%±1.12% vs 15.12%±1.14%, respectively) but a decreased percentage of S-phase cells (50.28%±2.03% vs 14.59%±1.18%, respectively). The PI was then calculated and found to be significantly lower in cells pretreated with TO901317 (0.37±0.02) than in those pretreated with DMSO (0.6±0.03) (P<0.001, n=5). These results indicate that the activation of NR1H3 inhibits the proliferation of endometrial carcinoma cells and the G1/S transition.
TO901317 inhibited the expression of CCND1 and CCNE in Ishikawa cells
To investigate the mechanism by which NR1H3 affects cell viability and the cell cycle, the gene and protein expression levels of NR1H3, CCND1, and CCNE in Ishikawa cells pretreated with different concentrations of TO901317 for different lengths of time were assessed using real-time RT-PCR and Western blot. The results showed that the mRNA and protein expression levels of NR1H3 increased along with increases in the concentration or incubation time of TO901317. Compared to the levels of the control (GAPDH), the relative mRNA expression levels of NR1H3 in cells pretreated with 0, 5, 10, and 20 μM TO901317 for 24 hours were 1.0±0.12, 1.46±0.09, 1.70±0.22, and 2.75±0.13, respectively (P<0.05, Figure 4A). Western blots also indicated that the protein expression of NR1H3 was increased along with increases in the concentration of TO901317; the corresponding protein expression levels for cells pretreated with 0, 5, 10, and 20 μM TO901317 were 15.8%±1.67%, 26.3%±2.2%, 47.6%±1.76%, and 57.8%±2.6% (100% of GAPDH, respectively) (P<0.05, Figure 5A and C). Similarly, the relative mRNA expression levels of NR1H3 in cells pretreated with 10 μM TO901317 for 0, 12, 24, and 48 hours were 1.10±0.12, 1.30±0.04, 2.06±0.09, and 2.49±0.07, respectively (P<0.001, Figure 4B). Western blots also indicated reductions in protein expression as a result of increases in the incubation time, with corresponding protein expression levels of 14.5%±1.73%, 29.4%±1.4%, 49.6%±2.12%, and 58.3±1.35, respectively (P<0.05, Figure 5B and D).
The expression of CCND1 was also assessed in Ishikawa cells. The relative mRNA expression levels of CCND1 in cells pretreated with 0, 5, 10, and 20 μM TO901317 for 24 hours were 1.0±0.11, 0.84±0.06, 0.72±0.07, and 0.38±0.09, respectively (P<0.05, Figure 4C). Western blots also indicated that the protein expression of CCND1 was reduced along with increases in the concentration of TO901317, and the corresponding protein expression levels were 60.3%±1.67%, 45.6%±2.8%, 36.0%±2.2%, and 24.3%±1.1%, respectively (P<0.05, Figure 5A and C). Similarly, the relative mRNA expression levels of CCND1 in cells pretreated with 10 μM TO901317 for 0, 12, 24, and 48 hours were 1.0±0.16, 0.81±0.12, 0.70±0.08, and 0.59±0.17, respectively (P<0.05, Figure 4D). Western blots also indicated reductions in CCND1 protein expression as a result of increases in the incubation time, with corresponding protein expression levels of 46.3%±1.7%, 34.2%±2.19%, 26.7%±2.14%, and 16.3%±1.43%, respectively (P<0.05, Figure 5B and D).
Finally, determination of the expression of CCNE indicated that the gene and protein expression levels of CCNE decreased with increases in the concentration or incubation time of TO901317. The relative mRNA expression levels of CCNE in cells pretreated with 0, 5, 10, and 20 μM TO901317 for 24 hours were 1.0±0.08, 0.8±0.01, 0.64±0.09, and 0.35±0.08, respectively (Figure 4E), and the corresponding protein expression levels were 44.2%±2.1%, 38.6%±1.4%, 34.2%±2.7%, and 14.4%±0.9%, respectively (P<0.05, except for 5 μM treatment, Figure 5A and B). The relative mRNA expression levels of CCNE in cells pretreated with 10 μM TO901317 for 0, 12, 24, and 48 hours were 1.12±0.19, 0.87±0.13, 0.80±0.04, and 0.76±0.03, respectively (P<0.05, Figure 4F), and the corresponding protein levels were 48.5%±2.1%, 45.6%±1.54%, 39.8%±2.04%, and 32.3%±1.83%, respectively (P<0.05, except for 5 μM treatment, Figure 5B and D). These results indicate that TO901317 not only promotes the expression of NR1H3 but also inhibits the expression of CCND1 and CCNE in Ishikawa cells.
Discussion
NR1H3 and NR1H2 are nuclear transcription factors that are considered important cholesterol receptors in the body and that play important roles in various metabolic diseases.39 The activation of NR1H3 can significantly inhibit the progression of atherosclerosis, diabetes, and other pathological changes.40 However, the role of NR1H3 and NR1H2 in malignant tumors should be further explored.
The pathogenesis of malignant tumors is highly complex. In recent years, the relationship between cholesterol and the development of malignant tumors has been a major concern.41 It has been reported that the generation and secretion of cholesterol are significantly increased in tumor tissues, suggesting that endogenous cholesterol plays an important role in the pathological changes in tumors.42 Some studies have discovered that the expression levels of SREBP-1c, FAS, ABCA1, and ABCG1, the downstream target genes of NR1H3 and NR1H2, are significantly increased in breast cancer, EC, and other pathological changes, suggesting that NR1H3 and NR1H2 may regulate the lipid metabolic network, which is related to the development of malignant tumors.43,44
To this end, we selected normal endometrial tissues, endometrial polyps, and endometrial carcinoma tissues for detecting NR1H3 and NR1H2 expression. We found that the expression of NR1H3 was significantly higher in endometrial carcinoma tissues than in normal endometrial tissues and endometrial polyps. This finding indicates that NR1H3 is upregulated in endometrial carcinoma tissues and might be an important factor regulating the occurrence and development of endometrial carcinoma. However, there were no significant differences in the expression of NR1H2 among the normal endometrial, endometrial polyps, and endometrial carcinoma groups, and the expression of NR1H2 was clearly lower than that of NR1H3 in the endometrial carcinoma group. Therefore, NR1H2 might not be involved in regulating the proliferation of endometrial cells, thus, NR1H3 was selected as the main focus of follow-up experiments.
It has been reported that NR1H3 promotes the expression of ABCA1 and ABCG1, genes associated with lipid transport, reducing cholesterol in cells, and transforming the biological functions of tumor cells.45,46 Moreover, NR1H3 plays an important role in the immune inflammatory response.47 Recently, Russo et al determined that NR1H3 plays a key role in tumor cell immunity and immune avoidance.48 Therefore, the expression of NR1H3 in cancer tissues may be a potential mechanism to protect the body from tumors.
A number of studies have reported that NR1H3 is expressed in the nucleus in various tissues, such as breast cancer, oral cancer, and prostate cancer.49–51 However, our research found that NR1H3 was primarily expressed in the cytoplasm in endometrial tissues and in Ishikawa cells, which is in disagreement with most of the literature. This difference may be attributed to nuclear receptor nucleoplasm shuttle transport, as recent studies have suggested that some nuclear receptors, such as GR and PR, can bind to the heat shock protein Hsp70 or Hsp90 and steadily persist in the cytoplasm where there are no appropriate ligands for these receptors. In addition, some nuclear receptors, such as estrogen receptor,52 androgen receptor,53 and glucocorticoid receptors,54 can bind with their ligands and shuttle between the nucleus and cytoplasm, while thyroid hormone receptors,55 progesterone receptor,56 and vitamin D receptor57 can complete this movement without ligands. Several studies have suggested that nuclear receptors may be capable of rapidly moving into the nucleus and shuttling back and forth between the nucleoplasm. Activated NR1H3 combines with RXR to form dimers, resulting in transcription factor activity. LXR/RXR heterodimers then regulate the transcription of target genes by binding to the LXR response element; the reaction component is specific to the nucleotide sequences of LXR.
Cell proliferation is an important factor in the development of malignant tumors and is one of its main pathological features. Cholesterol is the most important isoprenoid substrate for DNA replication and regulates signal transduction associated with tumor cell proliferation.58 A variety of cholesterol inhibitors (statins) have been shown to inhibit cell proliferation in several tumors.59,60 Studies have reported that the artificially synthesized LXR agonists TO901317 and GW3965 significantly inhibit the proliferation of prostate cancer cells in vitro.61 TO901317 also significantly inhibited tumor growth in a prostate cancer xenograft mouse model. To study the biological effects of NR1H3 on endometrial carcinoma, we activated NR1H3 using TO901317 and observed its effects on the proliferation of Ishikawa cells. Cell viability analysis showed that TO901317 significantly inhibited the proliferation of Ishikawa cells and arrested the cell cycle in S phase, as indicated by flow cytometry. However, the effects of TO901317 on the cholesterol metabolism pathway in Ishikawa cells should be further explored.
CCND1 has been implicated as a proto-oncogene in recent years. It regulates the G1/S transition and promotes the cell cycle, thereby affecting tissue cell proliferation.62 CCNE is another cycle protein important for G1 phase in cells, and it can combine with cyclin-dependent kinase 2 to promote the phosphorylation of the Rb protein. CCNE also combines with proliferating cell nuclear antigen and cyclin-dependent kinase inhibitor and promotes the cell cycle transition from G1 to S phase.63 Recently, CCND1 and CCNE were found to be upregulated in EC tissues and were associated with patient prognosis.64,65 There are several papers reporting transcriptional suppression of CCND1 by LXR.66–68 Therefore, we further assessed the effect of LXR agonist TO901317 on the expression of CCND1 and CCNE. TO901317 was shown to reduce the expression of both CCND1 and CCNE in a dose- and time-dependent manner, suggesting that the activation of NR1H3 inhibits the proliferation of endometrial carcinoma cells by inhibiting the expression of CCND1 and CCNE. Some studies have shown that the expression of CCND1 is mainly activated by the transcriptional regulation of Ap-1.69 When Ap-1 is combined with this sequence, various activated proteins, such as c-Fos or auxiliary regulators, such as p300, are rapidly recruited, leading to the transcription and expression of CCND1.70 We hypothesize that there is a competitive inhibition relationship between NR1H3- and Ap-1-mediated transcriptional activation, and further molecular biological experiments should be explored to confirm this mechanism.
Conclusion
In summary, this study confirmed the expression of NR1H3 in endometrial carcinoma. In vitro, an agonist of NR1H3, TO901317, was confirmed to significantly inhibit the proliferation of EC cells. The mechanism of this inhibition likely involves the cyclin cell pathway, although further studies are needed to confirm this hypothesis. Additional research on NR1H3 activation will provide experimental evidence for the prevention of endometrial carcinoma; thus, NR1H3 may represent a potential therapeutic target for endometrial carcinoma.
Acknowledgments
This study was supported by grants from the National Key R&D Program of China (2016YFC1302900), the Fundamental Research Fund of Shandong University (2014QLKY23), and the Key Research and Development Project of Shandong Province (2015GSF118097).
Author contributions
Baoxia Cui conceived, designed the study, and reviewed the manuscript. Fang Fang performed the majority of experiments and wrote the manuscript. Dawei Li contributed to experimental and clinical studies. Lu Zhao performed study concepts and Yue Li performed the statistical analysis. Teng Zhang performed manuscript editing. All authors contributed toward data analysis, drafting and revising the paper, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Janowski BA, Grogan MJ, Jones SA, et al. Structural requirements of ligands for the oxysterol liver X receptors LXRalpha and LXRbeta. Proc Natl Acad Sci U S A. 1999;96(1):266–271. | ||
Soodgupta D, Kaul D, Kanwar AJ, Parsad D. Modulation of LXR-α and the effector genes by ascorbic acid and statins in psoriatic keratinocytes. Mol Cell Biochem. 2014;397(1–2):1–6. | ||
Kim WK, Meliton V, Tetradis S, et al. Osteogenic oxysterol, 20(S)-hydroxycholesterol, induces notch target gene expression in bone marrow stromal cells. J Bone Miner Res. 2010;25(4):782–795. | ||
Ruan B, Wilson WK, Schroepfer GJ Jr. An improved synthesis of (20R,22R)-cholest-5-ene-3beta,20,22-triol, an intermediate in steroid hormone formation and an activator of nuclear orphan receptor LXR alpha. Steroids. 1999;64(6):385–395. | ||
Cook IT, Duniec-Dmuchowski Z, Kocarek TA, Runge-Morris M, Falany CN. 24-hydroxycholesterol sulfation by human cytosolic sulfotransferases: formation of monosulfates and disulfates, molecular modeling, sulfatase sensitivity, and inhibition of liver x receptor activation. Drug Metab Dispos. 2009;37(10):2069–2078. | ||
Collins JL, Fivush AM, Watson MA, et al. Identification of a nonsteroidal liver X receptor agonist through parallel array synthesis of tertiary amines. J Med Chem. 2002;45(10):1963–1966. | ||
Gong H, Guo P, Zhai Y, et al. Estrogen deprivation and inhibition of breast cancer growth in vivo through activation of the orphan nuclear receptor liver X receptor. Mol Endocrinol. 2007;21(8):1781–1790. | ||
Willy PJ, Umesono K, Ong ES, Evans RM, Heyman RA, Mangelsdorf DJ. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. 1995;9(9):1033–1045. | ||
Jakobsson T, Treuter E, Gustafsson JÅ, Steffensen KR. Liver X receptor biology and pharmacology: new pathways, challenges and opportunities. Trends Pharmacol Sci. 2012;33(7):394–404. | ||
Houck KA, Borchert KM, Hepler CD, et al. T0901317 is a dual LXR/FXR agonist. Mol Genet Metab. 2004;83(1–2):184–187. | ||
Stafslien DK, Vedvik KL, De Rosier T, Ozers MS. Analysis of ligand-dependent recruitment of coactivator peptides to RXRbeta in a time-resolved fluorescence resonance energy transfer assay. Mol Cell Endocrinol. 2007;264(1–2):82–89. | ||
Darimont C, Avanti O, Zbinden I, et al. Liver X receptor preferentially activates de novo lipogenesis in human preadipocytes. Biochimie. 2006;88(3–4):309–318. | ||
Swales KE, Korbonits M, Carpenter R, Walsh DT, Warner TD, Bishop-Bailey D. The farnesoid X receptor is expressed in breast cancer and regulates apoptosis and aromatase expression. Cancer Res. 2006;66(20):10120–10126. | ||
Maran RR, Thomas A, Roth M, et al. Farnesoid X receptor deficiency in mice leads to increased intestinal epithelial cell proliferation and tumor development. J Pharmacol Exp Ther. 2009;328(2):469–477. | ||
Lee JY, Lee KT, Lee JK, et al. Farnesoid X receptor, overexpressed in pancreatic cancer with lymph node metastasis promotes cell migration and invasion. Br J Cancer. 2011;104(6):1027–1037. | ||
Guan B, Li H, Yang Z, Hoque A, Xu X. Inhibition of farnesoid X receptor controls esophageal cancer cell growth in vitro and in nude mouse xenografts. Cancer. 2013;119(7):1321–1329. | ||
Su H, Ma C, Liu J, et al. Downregulation of nuclear receptor FXR is associated with multiple malignant clinicopathological characteristics in human hepatocellular carcinoma. Am J Physiol Gastrointest Liver Physiol. 2012;303(11):G1245–G1253. | ||
Caldas Y, Giral H, Sorribas V, Levi M. Nuclear receptor LXR: a new partner for sodium-dependent phosphate cotransporters. Contrib Nephrol. 2013;180:64–73. | ||
Peet DJ, Turley SD, Ma W, et al. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell. 1998;93(5):693–704. | ||
Laffitte BA, Chao LC, Li J, et al. Activation of liver X receptor improves glucose tolerance through coordinate regulation of glucose metabolism in liver and adipose tissue. Proc Natl Acad Sci U S A. 2003;100(9):5419–5424. | ||
Dalen KT, Ulven SM, Bamberg K, Gustafsson JA, Nebb HI. Expression of the insulin-responsive glucose transporter GLUT4 in adipocytes is dependent on liver X receptor alpha. J Biol Chem. 2003;278(48):48283–48291. | ||
Viennois E, Mouzat K, Dufour J, Morel L, Lobaccaro JM, Baron S. Selective liver X receptor modulators (SLiMs): what use in human health? Mol Cell Endocrinol. 2012;351(2):129–141. | ||
Beaven SW, Matveyenko A, Wroblewski K, et al. Reciprocal regulation of hepatic and adipose lipogenesis by liver X receptors in obesity and insulin resistance. Cell Metab. 2013;18(1):106–117. | ||
Castrillo A, Joseph SB, Vaidya SA, et al. Crosstalk between LXR and toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism. Mol Cell. 2003;12(4):805–816. | ||
Chuu CP, Lin HP. Antiproliferative effect of LXR agonists T0901317 and 22(R)-hydroxycholesterol on multiple human cancer cell lines. Anticancer Res. 2010;30(9):3643–3648. | ||
Komninou D, Ayonote A, Richie JP Jr, Rigas B. Insulin resistance and its contribution to colon carcinogenesis. Exp Biol Med (Maywood). 2003;228(4):396–405. | ||
Ishino K, Mutoh M, Totsuka Y, Nakagama H. Metabolic syndrome: a novel high-risk state for colorectal cancer. Cancer Lett. 2013;334(1):56–61. | ||
Xue F, Michels KB. Diabetes, metabolic syndrome, and breast cancer: a review of the current evidence. Am J Clin Nutr. 2007;86(3):s823–s835. | ||
Mitsuzuka K, Arai Y. Metabolic changes in patients with prostate cancer during androgen deprivation therapy. Int J Urol. 2018;25(1):45–53. | ||
Cummins CL, Volle DH, Zhang Y, et al. Liver X receptors regulate adrenal cholesterol balance. J Clin Invest. 2006;116(7):1902–1912. | ||
Repa JJ, Liang G, Ou J, et al. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 2000;14(22):2819–2830. | ||
Eberlé D, Hegarty B, Bossard P, Ferré P, Foufelle F. SREBP transcription factors: master regulators of lipid homeostasis. Biochimie. 2004;86(11):839–848. | ||
El Roz A, Bard JM, Huvelin JM, Nazih H. LXR agonists and ABCG1-dependent cholesterol efflux in MCF-7 breast cancer cells: relation to proliferation and apoptosis. Anticancer Res. 2012;32(7):3007–3013. | ||
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. | ||
Ali AT. Reproductive factors and the risk of endometrial cancer. Int J Gynecol Cancer. 2014;24(3):384–393. | ||
Ni J, Zhu T, Zhao L, et al. Metabolic syndrome is an independent prognostic factor for endometrial adenocarcinoma. Clin Transl Oncol. 2015;17(10):835–839. | ||
Zhang Y, Liu Z, Yu X, et al. The association between metabolic abnormality and endometrial cancer: a large case-control study in China. Gynecol Oncol. 2010;117(1):41–46. | ||
Shevra CR, Ghosh A, Kumar M. Cyclin D1 and Ki-67 expression in normal, hyperplastic and neoplastic endometrium. J Postgrad Med. 2015;61(1):15–. | ||
Schulman IG. Liver X receptors link lipid metabolism and inflammation. FEBS Lett. 2017;591(19):2978–2991. | ||
Steffensen KR, Gustafsson JA. Putative metabolic effects of the liver X receptor (LXR). Diabetes. 2004;53(Suppl 1):S36–S42. | ||
Drabkin HA, Gemmill RM. Obesity, cholesterol, and clear-cell renal cell carcinoma (RCC). Adv Cancer Res. 2010;107:39–56. | ||
Jiang JT, Xu N, Zhang XY, Wu CP. Lipids changes in liver cancer. J Zhejiang Univ Sci B. 2007;8(6):398–409. | ||
Lu S, Archer MC. Sp1 coordinately regulates de novo lipogenesis and proliferation in cancer cells. Int J Cancer. 2010;126(2):416–425. | ||
Trasino SE, Kim YS, Wang TT. Ligand, receptor, and cell type-dependent regulation of ABCA1 and ABCG1 mRNA in prostate cancer epithelial cells. Mol Cancer Ther. 2009;8(7):1934–1945. | ||
Scoles DR, Xu X, Wang H, et al. Liver X receptor agonist inhibits proliferation of ovarian carcinoma cells stimulated by oxidized low density lipoprotein. Gynecol Oncol. 2010;116(1):109–116. | ||
Panagiotakos DB, Pitsavos C, Polychronopoulos E, et al. Total cholesterol and body mass index in relation to 40-year cancer mortality (the Corfu cohort of the seven countries study). Cancer Epidemiol Biomarkers Prev. 2005;14(7):1797–1801. | ||
Hong C, Tontonoz P. Coordination of inflammation and metabolism by PPAR and LXR nuclear receptors. Curr Opin Genet Dev. 2008;18(5):461–467. | ||
Russo V. Metabolism, LXR/LXR ligands, and tumor immune escape. J Leukoc Biol. 2011;90(4):673–679. | ||
Wu Y, Yu DD, Yan DL, et al. Liver X receptor as a drug target for the treatment of breast cancer. Anticancer Drugs. 2016;27(5):373–382. | ||
Kaneko T, Kanno C, Ichikawa-Tomikawa N, et al. Liver X receptor reduces proliferation of human oral cancer cells by promoting cholesterol efflux via up-regulation of ABCA1 expression. Oncotarget. 2015;6(32):33345–33357. | ||
Chuu CP, Kokontis JM, Hiipakka RA, Liao S. Modulation of liver X receptor signaling as novel therapy for prostate cancer. J Biomed Sci. 2007;14(5):543–553. | ||
Maruvada P, Baumann CT, Hager GL, Yen PM. Dynamic shuttling and intranuclear mobility of nuclear hormone receptors. J Biol Chem. 2003;278(14):12425–12432. | ||
Tyagi RK, Lavrovsky Y, Ahn SC, Song CS, Chatterjee B, Roy AK. Dynamics of intracellular movement and nucleocytoplasmic recycling of the ligand-activated androgen receptor in living cells. Mol Endocrinol. 2000;14(8):1162–1174. | ||
Haché RJ, Tse R, Reich T, Savory JG, Lefebvre YA. Nucleocytoplasmic trafficking of steroid-free glucocorticoid receptor. J Biol Chem. 1999;274(3):1432–1439. | ||
Bunn CF, Neidig JA, Freidinger KE, et al. Nucleocytoplasmic shuttling of the thyroid hormone receptor alpha. Mol Endocrinol. 2001;15(4):512–533. | ||
Guiochon-Mantel A, Lescop P, Christin-Maitre S, Loosfelt H, Perrot-Applanat M, Milgrom E. Nucleocytoplasmic shuttling of the progesterone receptor. EMBO J. 1991;10(12):3851–3859. | ||
Prüfer K, Barsony J. Retinoid X receptor dominates the nuclear import and export of the unliganded vitamin D receptor. Mol Endocrinol. 2002;16(8):1738–1751. | ||
Palmer CP, Mahen R, Schnell E, Djamgoz MB, Aydar E. Sigma-1 receptors bind cholesterol and remodel lipid rafts in breast cancer cell lines. Cancer Res. 2007;67(23):11166–11175. | ||
Yu O, Boudreau DM, Buist DS, Miglioretti DL. Statin use and female reproductive organ cancer risk in a large population-based setting. Cancer Causes Control. 2009;20(5):609–616. | ||
Sivaprasad U, Abbas T, Dutta A. Differential efficacy of 3-hydroxy-3-methylglutaryl CoA reductase inhibitors on the cell cycle of prostate cancer cells. Mol Cancer Ther. 2006;5(9):2310–2316. | ||
Chuu CP, Hiipakka RA, Kokontis JM, Fukuchi J, Chen RY, Liao S. Inhibition of tumor growth and progression of LNCaP prostate cancer cells in athymic mice by androgen and liver X receptor agonist. Cancer Res. 2006;66(13):6482–6486. | ||
Shimura T, Fukumoto M, Kunugita N. The role of cyclin D1 in response to long-term exposure to ionizing radiation. Cell Cycle. 2013;12(17):2738–2743. | ||
Xiangming C, Natsugoe S, Takao S, et al. The cooperative role of p27 with cyclin E in the prognosis of advanced gastric carcinoma. Cancer. 2000;89(6):1214–1219. | ||
Chappuis PO, Donato E, Goffin JR, et al. Cyclin E expression in breast cancer: predicting germline BRCA1 mutations, prognosis and response to treatment. Ann Oncol. 2005;16(5):735–742. | ||
Rudas M, Lehnert M, Huynh A, et al; Austrian Breast and Colorectal Cancer Study Group. Cyclin D1 expression in breast cancer patients receiving adjuvant tamoxifen-based therapy. Clin Cancer Res. 2008;14(6):1767–1774. | ||
Hu C, Liu D, Zhang Y, et al. LXRα-mediated downregulation of FOXM1 suppresses the proliferation of hepatocellular carcinoma cells. Oncogene. 2014;33(22):2888–2897. | ||
Vedin LL, Gustafsson JÅ, Steffensen KR. The oxysterol receptors LXRα and LXRβ suppress proliferation in the colon. Mol Carcinog. 2013;52(11):835–844. | ||
Lin CY, Gustafsson JÅ. Targeting liver X receptors in cancer therapeutics. Nat Rev Cancer. 2015;15(4):216–224. | ||
Albanese C, D’Amico M, Reutens AT, et al. Activation of the cyclin D1 gene by the E1A-associated protein p300 through AP-1 inhibits cellular apoptosis. J Biol Chem. 1999;274(48):34186–34195. | ||
Wang C, Fu M, D’Amico M, et al. Inhibition of cellular proliferation through IkappaB kinase-independent and peroxisome proliferator-activated receptor gamma-dependent repression of cyclin D1. Mol Cell Biol. 2001;21(9):3057–3070. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.