Back to Journals » OncoTargets and Therapy » Volume 11
Expression of Nemo-like kinase in cervical squamous cell carcinoma: a clinicopathological study
Authors Yang W, Gu L, Yang C, Liu T
Received 15 October 2017
Accepted for publication 13 December 2017
Published 8 February 2018 Volume 2018:11 Pages 743—749
DOI https://doi.org/10.2147/OTT.S154188
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Tohru Yamada
Weina Yang,1 Lina Gu,2 Chang Yang,3 Tianbo Liu3
1Department of Gynecology, the Second Hospital of Tianjin Medical University, Tianjin, China; 2Department of Radiation Oncology, Harbin Medical University Cancer Hospital, Harbin, China; 3Department of Gynecology, Harbin Medical University Cancer Hospital, Harbin, China
Objective: Nemo-like kinase (NLK) has been reported to play different roles in tumors. However, the role of NLK in cervical squamous cell carcinoma (CSCC) remains unknown. In this study, we explored the clinical significance including survival of NLK protein expression in CSCCs.
Patients and methods: Immunohistochemical method was performed using tissues from 130 patients with CSCC. The associations between NLK expression and the clinicopathological factors and prognosis of CSCCs were evaluated. Statistical analyses were performed using the chi-square test, the multivariate Cox proportional hazard model, and the Kaplan–Meier method.
Results: Immunohistochemical staining analysis showed that NLK was localized predominately in the nucleus of the tumor cells, and increased NLK expression was detected in 71 (54.6%) of 130 patients. NLK overexpression significantly correlated with higher histological grade (P=0.001), vascular/lymphatic invasion (P=0.010), lymph node metastasis (P=0.012), and recurrence (P=0.022). Patients with elevated NLK expression had poorer overall survival (OS) and disease-free survival (DFS) (P=0.006 and P=0.004, respectively) compared with patients with decreased NLK expression. Multivariate Cox analysis demonstrated that NLK overexpression was an independent factor for OS and DFS (P=0.034 and P=0.025, respectively).
Conclusion: NLK may be a valuable biomarker for predicting the prognosis of CSCC patients and may serve as a potential target for cancer therapy.
Keywords: Nemo-like kinase, cervical squamous cell carcinoma, prognosis, progression, lymph node metastasis, survival, biomarker
Introduction
Almost 90% of cervical cancer-related deaths occurred in the developing countries, and the highest cervical cancer death rates are in Asia, with an estimated 144,400 deaths occurring in 2012.1 Invasive cancer accounts for >70% of patients diagnosed with cervical cancer, among which squamous cell carcinoma is the most frequent type.2,3 Early-stage cervical squamous cell carcinoma (CSCC) cases have a greater possibility of being cured and are associated with excellent survival rates.4,5 Since early detection results in a better prognosis, there is an urgent need to identify more efficient early diagnostic biomarkers and therapeutic targets for CSCC.
The Nemo-like kinase (NLK), originally identified as a vertebrate ortholog of Drosophila nemo,6 is an evolutionarily conserved serine/threonine protein kinase and belongs to the extracellular signal-regulated kinases/microtubule-associated protein kinases family.7,8 NLK was proved to be important for tumor cell proliferation, migration, and apoptosis.9–13 Increased NLK expression has been reported in several cancers,14–17 and variation in NLK may be associated with the risk of invasive epithelial ovarian cancer.18 However, the status of NLK protein expression in CSCCs remains unknown. This study was performed to explore NLK expression in CSCCs and to analyze its relationship with various clinicopathological features, including patient outcome.
Patients and methods
Patients and surgical specimens
This study was approved by the ethical committee of the Harbin Medical University, and all tissue samples were obtained from patients (n=130) at the Department of Gynecology, Harbin Medical University Cancer Hospital, China, between January 2010 and December 2011. All patients with CSCC underwent radical hysterectomy and pelvic lymphadenectomy. None of the patients received chemotherapy, immunotherapy, or radiotherapy before surgery. Patients with high-risk factors underwent postoperative radiotherapy. The tumor stages were assessed according to the International Federation of Gynecology and Obstetrics staging system.19 The histological grades were classified according to the World Health Organization criteria.
Written informed consent was obtained from the patients for the purpose of research. The patients with CSCC were followed up for clinical evaluation either in the hospital or by telephone. All patients with CSCC were followed up periodically for survival analysis until death or until the study closing date (December 2016). The median follow-up time was 64 months, with a range of 13–88 months. Overall survival (OS) was defined as the period from the date of surgery until death or to the time of the most recent follow-up, and disease-free survival (DFS) was defined as the time interval in months between completion of therapy and recurrence, respectively. Recurrence was either radiologically or histologically confirmed.
Western blot analysis
Proteins from 15 frozen CSCC and 5 frozen normal cervical tissue samples were extracted by suspending them in RIPA buffer consisting of 1% protease inhibitor mixture. The mixture was centrifuged at 12,000× g for 15 minutes at 4°C, and the supernatant was collected. Protein concentrations were quantified by the bicinchoninic acid protein assay kit, and 30 μg of protein extracts was separated by sodium dodecyl sulfate polyacrylamide gel and transferred to methanol-activated nitrocellulose filter membrane (Bio-Rad Laboratories Inc., Hercules, CA, USA). Membranes were blocked with 5% non-fat dry milk before immunodetection. Primary antibodies, NLK (1:1,000 dilution; Abcam, Cambridge, MA, USA), and β-actin were diluted in the buffer and incubated at 4°C overnight. After standard washing, membranes were incubated with a secondary antibody (horseradish peroxidase conjugated) for 1 hour at room temperature. The experiment was accomplished in triplicate.
Immunohistochemistry
Paraffin-embedded CSCC specimens were cut into 4 μm sections, and then the slides were blocked with 0.3% hydrogen peroxide at 37°C for 30 minutes. To increase specificity and sensitivity, samples were placed in 10 mmol/L citrate buffer (pH 6.0) in a pressure cooker for 2 minutes. The mouse monoclonal antibody against NLK antibodies (1:100 dilution; Abcam) was added to the sections for overnight at 4°C, followed by incubation with mouse secondary antibody (Zhong Shan Golden Bridge Biological Technology Beijing, China) for 20 minutes at room temperature. The sections were immersed in a solution of 3,3-diaminobenzidine tetrahydrochloride (Dako Denmark A/S, Glostrup, Denmark) and then counterstained with hematoxylin. The negative ones were stained with phosphate-buffered saline instead of primary antibodies.
Assessment of immunohistochemical staining
The NLK immunoreactivity was semiquantitatively estimated by combining intensity and percentage of positive tumor cells under the microscope as described earlier.17 The immunostaining results were optimized and judged by 2 independent senior pathologists according to the following standards. The percentage was classified as follows: 0 (0%), 1 (<5%), 2 (5%–50%), and 3 (>50%). The intensity was also scored as follows: 0 (negative staining), 1 (weak staining), 2 (moderate staining), and 3 (intense staining). The final score of NLK expression, ranging from 0 to 6, was the sum of the percentage of positive cells and the intensity score. Patients with a final score of <4 were classified as the low expression group and vice versa.
Statistical analysis
Student’s t-test was used to compare continuous variables, and chi-square test was performed to assess differences in clinicopathological variables. OS and DFS were evaluated using the Kaplan–Meier method, and the differences among the levels of possible prognostic factors were compared by the log-rank test in the univariate analyses. The Cox proportional hazards model was used for the multivariate analysis of the independent prognostic factors for OS and DFS. All analyses were performed using statistical software SPSS 13.0 (SPSS Inc., Chicago, IL, USA), and the findings were considered statistically significant at P<0.05.
Results
Patients’ characteristics and NLK protein expression in CSCC tissues
The expression of NLK was analyzed using specimens from 130 untreated CSCC patients. The median age of the patients was 43 years (range: 27–70). Of 130 patients, 72 patients (55.4%) had CSCC classified as the Federation of Gynecology and Obstetrics (FIGO) Stage I and 58 (44.6%) had FIGO Stage II. On the basis of the analysis of histological grade, 21 cases (16.2%) were well differentiated (G1), 75 (57.1%) were moderately differentiated (G2), and 34 (26.7%) were poorly differentiated (G3). Of these patients, lymph node metastases were present in 31 patients (23.8%) and absent in 99 patients (76.2%). A total of 96 patients (73.8%) were shown to have tumors <4 cm in size and 34 patients (26.2%) with tumors at least 4 cm in size. Parametrial involvement and vascular/lymphatic invasion occurred in 20 (15.4%) and 39 (30.0%) patients, respectively. Deep stromal involvement occurred in 53 (40.8%) of 130 patients, and recurrence was observed in 16 (12.3%) patients (Table 1).
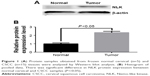
Western blot analysis was used to assess the different levels of NLK expression in CSCC and normal cervical tissues. In Figure 1, it is shown that NLK protein expression is significantly elevated in CSCC tissues compared with that in normal cervical tissues (P<0.05).
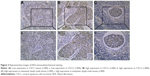
As shown in Figure 2, NLK was localized mainly in the nucleus of the tumor cells. Of the CSCC specimens examined, low NLK expression was detected in 59 (45.4%) of 130 patients, whereas high NLK expression was detected in 71 (54.6%) of 130 patients. Elevated NLK expression was observed in 28 (90.3%) CSCC patients with lymph node metastasis (Figure 2C).
Association of NLK protein expression and clinicopathological features
We analyzed the associations between NLK expression status and a series of clinicopathological characteristics, including age, FIGO stage, histological grade, tumor size, parametrial involvement, depth of stromal involvement, vascular/lymphatic invasion, lymph node metastasis, and recurrence (Table 1). Elevated NLK expression was significantly associated with higher histological grade (P=0.001), vascular/lymphatic invasion (P=0.010), lymph node metastasis (P=0.012), and recurrence (P=0.022). However, no strong association was observed between NLK and age, FIGO stage, tumor size, parametrial involvement, and deep stromal involvement (P>0.05).
Univariate and multivariate analyses for the prognosis of patients with CSCC
The Kaplan–Meier 5-year survival curves stratified for NLK expression are shown in Figure 3. Both univariate and multivariate survival analyses were used to evaluate the effects of NLK expression and clinicopathological characteristics on prognosis. For OS (Table 2), univariate analyses indicated NLK expression (P=0.006), FIGO stage (P=0.011), tumor size (P=0.043), parametrial involvement (P=0.045), vascular/lymphatic invasion (P=0.003), and lymph node metastasis (P<0.001) as significant prognostic predictors. Other features had no prognostic value. Using multivariate analysis, we found that NLK overexpression (HR: 2.760; 95% CI: 1.079–7.058; P=0.034), FIGO stage (HR: 2.339; 95% CI: 1.027–5.327; P=0.043), and lymph node metastasis (HR: 3.102; 95% CI: 1.394–6.901; P=0.006) were independently prognostic predictors in CSCC. For DFS (Table 3), univariate analyses showed that NLK expression (P=0.004), FIGO stage (P=0.014), vascular/lymphatic invasion (P=0.003), and lymph node metastasis (P<0.001) were all associated with prognosis. In multivariate Cx regression analysis, the increased expression of NLK (HR: 2.920; 95% CI: 1.143–7.458; P=0.025) and lymph node metastasis (HR: 3.375; 95% CI: 1.512–7.532; P=0.003) were considered as independent prognostic markers.
Discussion
In this paper, we found that increased NLK expression was significantly associated with higher histological grade, vascular/lymphatic invasion, lymph node metastasis, and recurrence in CSCCs. In multivariate Cox regression analysis, elevated NLK expression was an independent prognostic factor for both OS and DFS. These findings reveal that NLK may serve a potential function in CSCC progression and may thus be used as a valuable biomarker in predicting prognosis in CSCCs.
Subcellular fractionation from COS7 cells and HEK293 cells verifies that ~60%–70% of NLK is localized in the nucleus and 30%–40% is localized in the cytoplasm.20 NLK was predominantly located in the nucleus of tumor cells in nasopharyngeal carcinoma, hepatocellular carcinoma, glioma, and gallbladder cancer;10,14,15,17 however, NLK was expressed mainly in the cytoplasm of tumor cells in colorectal cancer.21 NLK-positive staining was confined mainly to the nucleus and cytoplasm in non-small-cell lung cancer (NSCLC).22 In our study, we found that NLK was localized mainly in the nucleus of the tumor cells. The abovementioned findings suggested that different cytological localizations of NLK in different tumor types might contribute to different biological mechanisms by various signal pathways.
Evidence shows that NLK can serve as an oncogene or tumor suppressor gene dependent on the organ/cell-type specificity. On one hand, NLK acts as an oncogene, and downregulation of this gene may prevent the progression of cancers. Jung et al9 showed that silencing of NLK decreases cell growth and positively regulates the expression of cyclinD1, a core component of cell cycle regulation in hepatocellular carcinoma cells: Hep3B and SNU-423. Furthermore, low NLK expression decreased significantly the proliferation and migration abilities of GBC-SD and SGC-996 cells in gallbladder carcinoma.10 On the other hand, NLK functions as a tumor suppressor and elevated expression of NLK may prevent the progression of cancers by inhibiting cell proliferation, migration, and invasion or inducing cell apoptosis. Upregulated NLK results in an obvious decrease in the number of cells and a sharp increase in apoptosis in prostate cancer cell PC-3 by inhibiting androgen receptor-mediated transcription.23 In human colon cancer, overexpression of NLK in DLD-1 human colon cancer cell line induced cell apoptosis and inhibited cell proliferation.24 Overexpression of NLK prevents proliferation and elicits apoptosis in breast cancer cell MCF-7.25 In glioblastoma cell U87MG, upregulated NLK increases cell apoptosis.16 In addition, suppression of NLK expression resulted in significant promotion of proliferation in NSCLC cells by facilitating S-phase and mitotic entry, and the activity of β-catenin/TCF in A549 cells was remarkably enhanced.26 Our results indicated that NLK played as oncogene; however, we still need to conduct cytological experiments to further verify that NLK gene functions as carcinogenic factor or suppressor in cervical cancer cells.
NLK has been demonstrated to correlate with histological grade, advanced stage, and lymph node metastasis.14,15,17,21 Similarly, our study suggests that tumors with high NLK expression had greater invasive potential including vascular/lymphatic invasion. NLK overexpression was also observed in high-invasive regions such as metastatic lymph node. As regards NSCLC, NLK has been found to be inversely correlated with NSCLC histological differentiation, clinical stage, lymph node status, and Ki-67.26 Besides, NLK expression was reported to be inversely correlated with glioma grade.16 These different results may attribute to different cytological functions.
Furthermore, high NLK expression can be a strong predictor of poor prognosis.14,15,17,21 To date, this report is the first to show in detail an association between clinicopathological variables and the prognostic significance of NLK in CSCCs by using clinical samples. Yet, Lv et al26 reported that low NLK expression was an independent prognostic factor for NSCLC patients’ low survival rate by multivariate analysis. However, Lv et al27 revealed that lentivirus-mediated knockdown of NLK inhibits small-cell lung cancer growth and metastasis. These suggest that the same gene NLK may function differently in the location of histology and cytology.
Conclusion
NLK is overexpressed in the nucleus of the tumor cells in CSCC, and high NLK expression can be correlated with progression, metastasis, and poor survival. These results suggest that NLK may be a potentially novel biomarker for prediction of CSCC. However, these findings remain to be confirmed by a larger future study.
Acknowledgments
This study was supported by the grants of National Natural Science Foundation of China (81502225), the Postdoctoral Foundation of China (2015M581480), the Postdoctoral Foundation of Heilongjiang Province of China (LBH-Z15123), the Haiyan Foundation of the Affiliated Tumor Hospital of Harbin Medical University (JJMS2016-01), the Fundamental Research Funds for the Provincial Universities (2016lczx77), and the Youth Elite Training Foundation of the Affiliated Tumor Hospital of Harbin Medical University (JY2016-03).
Disclosure
The authors report no conflicts of interest in this work.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. | ||
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. | ||
Pecorelli S, Odicino F. Cervical cancer staging. Cancer J. 2003;9:390–394. | ||
Cole L, Stoler MH. Issues and inconsistencies in the revised gynecologic staging systems. Semin Diagn Pathol. 2012;29(3):167–173. | ||
Denny L. Cervical cancer: prevention and treatment. Discov Med. 2012;14(75):125–131. | ||
Choi KW, Benzer S. Rotation of photoreceptor clusters in the developing Drosophila eye requires the nemo gene. Cell. 1994;78(1):125–136. | ||
Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. 2011;75(1):50–83. | ||
Obara Y, Nakahata N. The signaling pathway leading to extracellular signal-regulated kinase 5 (ERK5) activation via G-proteins and ERK5-dependent neurotrophic effects. Mol Pharmacol. 2010;77(1):10–16. | ||
Jung KH, Kim JK, Noh JH, et al. Targeted disruption of Nemo-like kinase inhibits tumor cell growth by simultaneous suppression of cyclin D1 and CDK2 in human hepatocellular carcinoma. J Cell Biochem. 2010;110(3):687–696. | ||
Tan Z, Li M, Wu W, et al. K is a key regulator of proliferation and migration in gallbladder carcinoma cells. Mol Cell Biochem. 2012;369:27–33. | ||
Mendes-Pereira AM, Lord CJ, Ashworth A. NLK is a novel therapeutic target for PTEN deficient tumour cells. PLoS One. 2012;7(10):e47249. | ||
Li Z, Cui G, Wang J, Yu Z, Zhao L, Lv Z. Nemo-like kinase (NLK) involves in neuronal apoptosis after traumatic brain injury. Cell Mol Neurobiol. 2012;32(3):381–389. | ||
Ota S, Ishitani S, Shimizu N, Matsumoto K, Itoh M, Ishitani T. NLK positively regulates Wnt/β-catenin signalling by phosphorylating LEF1 in neural progenitor cells. EMBO J. 2012;31(8):1904–1915. | ||
Chen S, Ma Z, Chen X, Zhang J. Prognostic significance of nemo-like kinase in nasopharyngeal carcinoma. Mol Med Rep. 2014;10(1):131–136. | ||
Chen HW, Qiao HY, Li HC, et al. Prognostic significance of Nemo-like kinase expression in patients with hepatocellular carcinoma. Tumour Biol. 2015;36(11):8447–8453. | ||
Cui G, Li Z, Shao B, et al. Clinical and biological significance of nemo-like kinase expression in glioma. J Clin Neurosci. 2011;18(2):271–275. | ||
Li M, Zhang S, Wang Z, et al. Prognostic significance of nemo-like kinase (NLK) expression in patients with gallbladder cancer. Tumour Biol. 2013;34(6):3995–4000. | ||
Stevens KN, Kelemen LE, Wang X, et al. Common variation in Nemo-like kinase is associated with risk of ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2012;21(3):523–528. | ||
Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103–104. | ||
Brott BK, Pinsky BA, Erikson RL. Nlk is a murine protein kinase related to Erk/MAP kinases and localized in the nucleus. Proc Natl Acad Sci U S A. 1998;95(3):963–968. | ||
Zhang W, He J, Du Y, et al. Upregulation of nemo-like kinase is an independent prognostic factor in colorectal cancer. World J Gastroenterol. 2015;21(29):8836–8847. | ||
Suwei D, Liang Z, Zhimin L, et al. NLK functions to maintain proliferation and stemness of NSCLC and is a target of metformin. J Hematol Oncol. 2015;8:120. | ||
Emami KH, Brown LG, Pitts TE, Sun X, Vessella RL, Corey E. Nemo-like kinase induces apoptosis and inhibits androgen receptor signaling in prostate cancer cells. Prostate. 2009;69(14):1481–1492. | ||
Yasuda J, Tsuchiya A, Yamada T, Sakamoto M, Sekiya T, Hirohashi S. Nemo-like kinase induces apoptosis in DLD-1 human colon cancer cells. Biochem Biophys Res Commun. 2003;308(2):227–233. | ||
Huang Y, Jiang Y, Lu W, Zhang Y. Nemo-like kinase associated with proliferation and apoptosis by c-Myb degradation in breast cancer. PLoS One. 2013;8(7):e69148. | ||
Lv L, Wan C, Chen B, et al. Nemo-like kinase (NLK) inhibits the progression of NSCLC via negatively modulating WNT signaling pathway. J Cell Biochem. 2014;115(1):81–92. | ||
Lv M, Li Y, Tian X, et al. Lentivirus-mediated knockdown of NLK inhibits small-cell lung cancer growth and metastasis. Drug Des Devel Ther. 2016;10:3737–3746. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.