Back to Journals » OncoTargets and Therapy » Volume 8
Expression of MMP-1/PAR-1 and patterns of invasion in oral squamous cell carcinoma as potential prognostic markers
Authors Fan H, Chen Y, Ni B, Wang S, Sun M, Chen D, Zheng J
Received 13 March 2015
Accepted for publication 29 April 2015
Published 3 July 2015 Volume 2015:8 Pages 1619—1626
DOI https://doi.org/10.2147/OTT.S84561
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Daniele Santini
Hai-Xia Fan,1 Yan Chen,1 Bo-Xiong Ni,1 Shan Wang,1 Miao Sun,2 Dong Chen,2 Jin-Hua Zheng1
1Department of Anatomy, Basic Medical Science College, 2Department of Oral and Maxillofacial Surgery, Stomatological Hospital, Harbin Medical University, Harbin, People’s Republic of China
Background: Matrix metalloproteinase (MMP)-1 degrades type I collagen of the extracellular matrix and also activates protease activated receptor (PAR)-1 to induce angiogenesis. The aims of this study were to evaluate microvessel density (MVD) and the expression of PAR-1 and MMP-1 in oral squamous cell carcinoma (SCC) specimens with different patterns of invasion (POI) and to evaluate their association with clinical outcomes.
Methods: Seventy-four surgically obtained oral SCC samples were classified by POI according to hematoxylin-eosin staining. MVD and the localization and intensity of PAR-1 and MMP-1 expression were detected by immunohistochemistry.
Results: Of the 74 oral SCC samples, 18, 5, 34, and 17 showed type I, II, III, and IV POI, respectively. MVD and expression levels of MMP-1 and PAR-1 differed between POI types I–II and POI types III–IV. Patients with low tumor expression of MMP-1 and PAR-1 and low MVD had a longer survival time than those with high tumor expression of MMP-1 and PAR-1. Moreover, the survival time of patients with POI types III–IV was shorter than that of patients with POI types I–II.
Conclusion: POI combined with expression levels of MMP-1 and PAR-1 may be a valuable tool for assessing the clinical prognosis of patients with oral SCC.
Keywords: oral squamous cell carcinoma, pattern of invasion, immunohistochemistry, clinical outcomes
Introduction
Oral squamous cell carcinoma (SCC), a common malignancy occurring in the oral and maxillofacial region, has high potential for invasiveness and is thus associated with a high mortality rate.1 Distant organ metastasis and regional lymph node metastasis are the major causes of mortality due to oral SCC. For many years, the TNM staging system has been used to estimate responses to therapy and clinical outcomes. However, many patients with stage I/II disease, who are treated accordingly, continue to die from oral SCC.2 Therefore, more precise assessment of patterns of invasion (POI) and markers of invasion is needed.
A study by Bryne et al demonstrated that the molecular and morphological characteristics at the invasive front of various SCCs are a better reflection of tumor behavior than the molecular and morphological characteristics in other areas of the tumor.3 They also created an invasive front grading system, which is a valuable supplement to clinical staging. The process of oral mucosa carcinoma in situ invading the basement membrane, resulting in invasive and subsequently metastatic carcinoma, depends on the interactions between cancer cells and the mesenchyma.4–6 Collagenous fiber, the main component of the extracellular matrix, plays key roles in cell growth, proliferation, and migration.5 Matrix metalloproteinase-1 (MMP-1) promotes tumor cell invasion by degrading the mesenchyma and vascular endothelium.7,8 Moreover, protease-activated receptor-1 (PAR-1), which is activated by MMP-1, also plays a key role in vascular remodeling and tumorigenesis.9,10
PAR-1 was first identified as an oncogene by Whitehead et al in 199511 and has been shown to be involved in the invasive and metastatic processes of many malignancies.12,13 Furthermore, MMP-1 functions as a protease agonist of PAR-1 by cleaving the receptor at the distinct site.14 Thus, MMP-1 activation of PAR-1 may link extracellular matrix remodeling, cell migration, and invasion signaling. A previous study demonstrated the presence of activated MMP-1 in fiber cells; moreover, it is possible to change the behavior of cancer cells by manipulating PAR-1 expression, thereby promoting cell migration, invasion, growth, and angiogenesis and thus changing the behavior of cancer cells.15,16
In this study, we investigated the relationship between MMP-1 expression, PAR-1 expression, and POI. To better understand the underlying mechanisms of oral SCC tumor invasion and metastasis and their association with patient prognosis, the aims of this study were to classify the POI of oral SCC and to evaluate the relationships between MMP-1 expression, PAR-1 expression, and POI with metastasis.
Materials and methods
Patients
This retrospective study included a cohort of 76 patients with oral SCC who underwent primary tumor resections and radical or selective ipsilateral or bilateral neck dissection between January 2006 and December 2011 at the Harbin Medical University Stomatological Hospital, Harbin, Heilongjiang, People’s Republic of China. All patients gave informed consent, and the study was approved by the research ethics committee of Harbin Medical University. As some tissues were recently lost, we studied the remaining tissue samples from the 74 patients. Formalin-fixed paraffin-embedded samples were sectioned at 5 μm thickness and stained with hematoxylin and eosin for diagnostic confirmation. All tumors were staged according to the 1997 Union for International Cancer Control TNM Classification of Malignant Tumors.17
Immunohistochemical staining
The MMP-1 monoclonal antibody (BM1270) was purchased from Wuhan Boster Bioengineering Company Ltd (Wuhan, People’s Republic of China); PAR-1 (bs-0828R) and CD105 (bs-0579R) antibodies were purchased from Beijing Biosynthesis Biotechnology Co., LTD (Beijing, People’s Republic of China). All primary antibodies were diluted with an antibody diluent at 1:100 for MMP-1, 1:200 for PAR-1, and 1:100 for CD105. Immunohistochemical staining and analysis were performed as previously described.18
Computer-aided immunohistochemical staining analysis
Image-Pro Plus 6.0 (Media Cybernetics Inc, Rockville, MD, USA) was used to calculate immunohistochemical staining intensity. Three microscopic fields (original magnification ×400) were randomly selected and the integral optical densities of MMP-1, PAR-1, and CD105 were calculated. The greatest integral optical density value represents the highest level of antigen expression, and the smallest value represents the lowest level. The median values for all proteins were regarded as the cutoff values for low versus high expression.
Evaluation of microvessel density (MVD)
MVD was quantified using sections stained with anti-CD105 antibody. Under a microscope, three optical fields with the highest number of microvessels were identified in each sample at low magnification. Microvessels were then counted at ×200 magnification. MVD was defined as the number of microvessels per optical field.
POI classification
A POI classification system was originally introduced by Jakobsson et al19 and further defined by Bryne et al3 as follows: POI type 1, tumor invasion in a broad, pushing manner with a smooth border; POI type 2, tumor invasion with broad, pushing “fingers” and a less-defined border; POI type 3, groups of cells with no distinct border; and POI type 4, diffuse growth.
Statistical analysis
Statistical Package for the Social Sciences version 18.0 software (SPSS Inc, Chicago, IL, USA) was used for the statistical analysis. The expression levels of MMP-1 and PAR-1 in oral SCC tissues were expressed as the mean ± standard deviation. Associations between PAR-1 and MMP-1 expression, MVD, and clinicopathological characteristics were evaluated using a chi-square test. Survival analysis was performed using the Kaplan–Meier method and log-rank test. Spearman’s rank correlation was used to analyze interactions between PAR-1 expression, MMP-1 expression, and MVD. P-values <0.05 were considered to be statistically significant.
Results
Patient characteristics and oral SCC POI
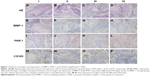
The clinical characteristics of the 74 patients included in this study are detailed in Table 1. Hematoxylin and eosin-stained samples representative of each POI type are depicted in Figure 1A–D. The expression patterns of MMP-1, PAR-1, and CD105 differed by POI type (Figure 1E–P).
  | Table 1 Summary of patient characteristics (n=74) |
MMP-1 expression
MMP-1 expression as determined by immunostaining was localized to the cytoplasm and membrane of the tumor cells and stromal cells (Figure 1E–H). In addition, the intensity of expression differed according to POI type: higher grades of invasiveness were associated with stronger expression levels (Table 2, r=0.273, P=0.019).
PAR-1 expression
Expression of PAR-1 in oral tissue specimens was also evaluated by immunohistochemical analysis. As shown in Figure 1I–L, PAR-1 staining intensity was stronger in surrounding stromal tissues than in oral SCC cell nests. Strong PAR-1 staining was also observed in the cytoplasm of both tumor and inflammatory cells. In addition, the stromal expression of PAR-1 in POI types III and IV was stronger than that in types I and II (Table 2, r=0.233, P=0.045).
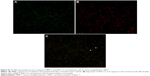
To further investigate the relationship between PAR-1 and MMP-1 expression, we performed double immunofluorescence using the two antibodies. Double staining of oral SCC specimens indicated colocalization of PAR-1 and MMP-1 (Figure S1); correlation analysis showed PAR-1 expression to be positively correlated with MMP-1 expression (r=0.390, P=0.003).
MVD as assessed by CD105 expression
Angiogenesis in tissue specimens was visualized using an anti-CD105 antibody. New blood vessels were detected in all specimens. MVD ranged from 2 to 15, and the mean MVD value was 7.58±3.46. Furthermore, differences in MVD were evident between different POI types (Figure 1M–P, Table 2, r=0.271, P=0.019).
Associations between clinicopathological characteristics and PAR-1 expression, MMP-1 expression, and MVD
As shown in Table 3, the expression levels of MMP-1 and PAR-1 were correlated with clinical stage, POI, and lymph node metastasis (P<0.05). MVD was associated with POI (P<0.05), but not with other clinicopathological factors, such as patient age, patient sex, lymph node metastasis, tumor differentiation, clinical stage, or recurrence.
Associations between PAR-1 expression, MMP-1 expression, and MVD
To evaluate the relationship between MVD and invasion-related factors, Spearman’s correlation analysis was performed to quantify the degree of association between the two variables. MMP-1 and PAR-1 levels were significantly correlated to MVD (Table 4).
Associations between patient survival and PAR-1 expression, MMP-1 expression, MVD, and POI
To determine the prognostic significance of PAR-1 expression, MMP-1 expression, and MVD, we assessed the correlation of these variables with overall survival. Survival curves demonstrated that patients with high PAR-1 and MMP-1 protein expression had worse prognosis than those with the low levels of PAR-1 and MMP-1 expression (Figure 2A, B). Those with high MVD in tumors had a lower 5-year survival rate than those with low levels of MVD (Figure 2C). Moreover, our results also indicated that patients with POI types III and IV had shorter survival times than those with POI types I and II (Figure 2D).
Discussion
Oral SCC has a high potential for invasiveness and is thus associated with a high mortality rate. Accordingly, overexpression of MMP-1 and PAR-1 is associated with invasion and metastasis, as these proteins affect matrix remodeling, cell adhesion, angiogenesis, and tumor cell survival.10,12,16,20,21 Some studies have shown that cancer-associated MMP-1, which cleaves and activates PAR-1, is not produced by cancer cells themselves but is instead secreted by mesenchymal cells.22,23 Our results indicate that PAR-1 and MMP-1 are not expressed in tumor cells alone; they are also expressed in the mesenchymal cells surrounding the nests. In addition, both the localization and intensity of PAR-1 and MMP-1 expression are associated with the carcinomatous POI. The expression levels of MMP-1 and PAR-1 in POI types III and IV are significantly higher than those in POI types I and II. Correlation analyses also demonstrated a strong correlation between MMP-1 expression, PAR-1 expression, and POI. As a new example of tumor-host interdependence, our results confirm the hypothesis that MMP-1 derived from cancer or mesenchymal cells may help degrade the extracellular matrix at the invasion front; MMP-1/PAR-1 signaling may then result in invasion of cancer cells into the surrounding stromal tissues. Similarly, Bryne et al proposed this mechanism of invasion was related to the POI.3
Paracrine MMP-1/PAR-1 signaling between the tumor and stromal cells is known to promote vascular intravasation and metastatic dissemination of cancer.24,25 In this study, we observed colocalization of MMP-1 and PAR-1 on the same tumor cell surface by immunofluorescence. Moreover, the expression of these proteins was closely related to MVD in the correlation analysis, and we also found an association between PAR-1 expression and angiogenesis. Thus, our data demonstrate that an MMP-1/PAR-1 signaling axis does indeed exist in oral SCC, and is closely related to tumor angiogenesis. This association with angiogenesis not only provides necessary nutrients to the tumor cells, but also facilitates tumor invasion and migration. Furthermore, these findings are in accordance with those of previous studies.5,8 Our results demonstrate that MMP-1 expression is correlated with lymph node metastasis, and MMP-1-activated PAR-1 can induce angiogenesis and promote metastasis.
Approximately 50% of patients with oral SCC are diagnosed with neck lymphatic metastasis, which is closely associated with a poor prognosis.4 Our results clearly demonstrated that MMP-1 protein expression is correlated with lymphatic metastasis, and high MMP-1 expression is a poor prognostic indicator. Furthermore, we found that the survival time of patients with POI types III and IV was significantly shorter than that of patients with POI types I and II. This confirms that POI is a useful clinical assessment tool to better predict prognosis and thus determine appropriate clinical treatment. Patients with high tumor expression of PAR-1 or high MVD in tumor specimens had a poor prognosis; this may be because PAR-1 not only promotes invasion of oral SCC by inducing integrin αvβ6 expression,26 but also by promoting angiogenesis, thus resulting in oral SCC metastasis.
Thrombin, an activator of PAR-1, plays a role in promoting tumor growth and metastasis as a result of its involvement in regulating numerous critical cellular events, including cell proliferation, cell adhesion, angiogenesis, and invasion.27,28 Sullivan et al proposed that thrombin-activated PAR-1 can increase the expression of αvβ6 stimulated by transforming growth-β.2 Although we did not include thrombin in the current analysis, our previous study demonstrated that epithelial expression of integrin αvβ6 was closely associated with not only the invasion ability of cancer cells, but also tumorigenesis and patient prognosis.29 In addition, αvβ6 expression was associated with PAR-1 expression by Spearman’s relevance analysis (data not shown). Thus, we hypothesize that either MMP-1 or thrombin activates PAR-1, which further stimulates the invasion of oral SCC cells. Whether the two proteinases cooperate in the tissue microenvironment to promote both tumor invasion and metastasis is still uncertain.
Conclusion
Our data demonstrate the existence of an MMP-1/PAR-1 signaling axis in oral SCC, and this axis promotes angiogenesis and accelerates oral SCC tumorigenesis and invasion. POI in combination with MMP-1 and PAR-1 expression levels may thus be utilized as a clinical assessment tool and prognostic marker in patients with oral SCC.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (grant 305400083).
Disclosure
The authors report no conflicts of interest in this work.
References
Byers RM, El-Naggar AK, Lee YY, et al. Can we detect or predict the presence of occult nodal metastases in patients with squamous carcinoma of the oral tongue? Head Neck. 1998;20(2):138–144. | ||
Chang YC, Nieh S, Chen SF, Jao SW, Lin YL, Fu E. Invasive pattern grading score designed as an independent prognostic indicator in oral squamous cell carcinoma. Histopathology. 2010;57(2):295–303. | ||
Bryne M, Koppang HS, Lilleng R, Stene T, Bang G, Dabelsteen E. New malignancy grading is a better prognostic indicator than Broders’ grading in oral squamous cell carcinomas. J Oral Pathol Med. 1989; 18(8):432–437. | ||
Zhang Z, Pan J, Li L, Wang Z, Xiao W, Li N. Survey of risk factors contributed to lymphatic metastasis in patients with oral tongue cancer by immunohistochemistry. J Oral Pathol Med. 2011;40(2):127–134. | ||
Eck SM, Blackburn JS, Schmucker AC, Burrage PS, Brinckerhoff CE. Matrix metalloproteinase and G protein coupled receptors: co-conspirators in the pathogenesis of autoimmune disease and cancer. J Autoimmun. 2009;33(3–4):214–221. | ||
Thomas GT, Lewis MP, Speight PM. Matrix metalloproteinases and oral cancer. Oral Oncol. 1999;35(3):227–233. | ||
Meneses-Garcia A, Betancourt AM, Abarca JH, Montes AB, Roa LS, Ruiz-Godoy L. Expression of the metalloproteases MMP-1, MMP-2, MMP-3, MMP-9, MMP-11, TIMP-1 and TIMP-2 in angiocentric midfacial lymphomas. World J Surg Oncol. 2008;6:114. | ||
Blackburn JS, Rhodes CH, Coon CI, Brinckerhoff CE. RNA interference inhibition of matrix metalloproteinase-1 prevents melanoma metastasis by reducing tumor collagenase activity and angiogenesis. Cancer Res. 2007;67(22):10849–10858. | ||
Sedda S, Marafini I, Caruso R, Pallone F, Monteleone G. Proteinase activated-receptors-associated signaling in the control of gastric cancer. World J Gastroenterol. 2014;20(34):11977–11984. | ||
Yang R, Xu Y, Li P, et al. Combined upregulation of matrix metalloproteinase-1 and proteinase-activated receptor-1 predicts unfavorable prognosis in human nasopharyngeal carcinoma. Onco Targets Ther. 2013;6:1139–1146. | ||
Whitehead I, Kirk H, Kay R. Expression cloning of oncogenes by retroviral transfer of cDNA libraries. Mol Cell Biol. 1995;15(2):704–710. | ||
Wang J, Liu D, Zhou W, Wang M, Xia W, Tang Q. Prognostic value of matrix metalloprotease-1/protease-activated receptor-1 axis in patients with prostate cancer. Med Oncol. 2014;31(6):968. | ||
Reiner O, Sapir T. Mark/Par-1 marking the polarity of migrating neurons. Adv Exp Med Biol. 2014;800:97–111. | ||
Austin KM, Covic L, Kuliopulos A. Matrix metalloproteases and PAR1 activation. Blood. 2013;121(3):431–439. | ||
Arora P, Cuevas BD, Russo A, Johnson GL, Trejo J. Persistent transactivation of EGFR and ErbB2/HER2 by protease-activated receptor-1 promotes breast carcinoma cell invasion. Oncogene. 2008;27(32):4434–4445. | ||
Villares GJ, Zigler M, Bar-Eli M. The emerging role of the thrombin receptor (PAR-1) in melanoma metastasis – a possible therapeutic target. Oncotarget. 2011;2(1–2):8–17. | ||
Sobin LH, Fleming ID. TNM classification of malignant tumors, fifth edition (1997). Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer. 1997;80(9):1803–1804. | ||
Fan HX, Li HX, Chen D, Gao ZX, Zheng JH. Changes in the expression of MMP2, MMP9, and ColIV in stromal cells in oral squamous tongue cell carcinoma: relationships and prognostic implications. J Exp Clin Cancer Res. 2012;31:90. | ||
Jakobsson PA, Eneroth CM, Killander D, Moberger G, Martensson B. Histological classification grading of malignancy in carcinoma of larynx. Acta Radiol Ther Phys Biol. 1973;12(1):1–8. | ||
Kim GE, Lee JS, Choi YD, et al. Expression of matrix metalloproteinases and their inhibitors in different immunohistochemical-based molecular subtypes of breast cancer. BMC Cancer. 2014;14(1):959. | ||
Zhu Q, Luo J, Wang T, Ren J, Hu K, Wu G. The activation of protease-activated receptor 1 mediates proliferation and invasion of nasopharyngeal carcinoma cells. Oncol Rep. 2012;28(1):255–261. | ||
Boire A, Covic L, Agarwal A, Jacques S, Sherifi S, Kuliopulos A. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell. 2005;120(3):303–313. | ||
Malaquin N, Vercamer C, Bouali F, et al. Senescent fibroblasts enhance early skin carcinogenic events via a paracrine MMP-PAR-1 axis. PLoS One. 2013;8(5):e63607. | ||
Juncker-Jensen A, Deryugina EI, Rimann I, et al. Tumor MMP-1 activates endothelial PAR1 to facilitate vascular intravasation and metastatic dissemination. Cancer Res. 2013;73(14):4196–4211. | ||
Hu L, Ibrahim S, Liu C, Skaar J, Pagano M, Karpatkin S. Thrombin induces tumor cell cycle activation and spontaneous growth by down-regulation of p27Kip1, in association with the up-regulation of Skp2 and MiR-222. Cancer Res. 2009;69(8):3374–3381. | ||
Sullivan BP, Weinreb PH, Violette SM, Luyendyk JP. The coagulation system contributes to alphaVbeta6 integrin expression and liver fibrosis induced by cholestasis. Am J Pathol. 2010;177(6):2837–2849. | ||
Hu L, Roth JM, Brooks P, Luty J, Karpatkin S. Thrombin up-regulates cathepsin D which enhances angiogenesis, growth, and metastasis. Cancer Res. 2008;68(12):4666–4673. | ||
Green D, Karpatkin S. Role of thrombin as a tumor growth factor. Cell Cycle. 2010;9(4):656–661. | ||
Li HX, Zheng JH, Fan HX, Li HP, Gao ZX, Chen D. Expression of alphavbeta6 integrin and collagen fibre in oral squamous cell carcinoma: association with clinical outcomes and prognostic implications. J Oral Pathol Med. 2013;42(7):547–556. |
Supplementary material
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.