Back to Journals » Cancer Management and Research » Volume 10
Expression of GP88 (progranulin) in serum of prostate cancer patients is associated with Gleason scores and overall survival
Authors Greither T , Fischer K, Theil G, Marcou M, Holzhausen HJ, Weigelt K, Serrero G, Hicks D, Yue BB, Fornara P , Wullich B , Taubert H , Wach S, Lieb V
Received 24 April 2018
Accepted for publication 11 June 2018
Published 5 October 2018 Volume 2018:10 Pages 4173—4180
DOI https://doi.org/10.2147/CMAR.S172069
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Kenan Onel
Thomas Greither,1,* Kersten Fischer,2,* Gerit Theil,2 Marios Marcou,1,2 Hans-Juergen Holzhausen,3 Katrin Weigelt,4 Ginette Serrero,5,6 David Hicks,5 Binbin Yue,5 Paolo Fornara,2 Bernd Wullich,4 Helge Taubert,4 Sven Wach,4 Verena Lieb4
1Center for Reproductive Medicine and Andrology, Martin Luther University Halle-Wittenberg, Halle, Germany; 2Department of Urology, Martin Luther University Halle-Wittenberg, Halle, Germany; 3Department of Pathology, Martin Luther University Halle-Wittenberg, Halle, Germany; 4Department of Urology and Pediatric Urology, Universitätsklinikum Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany; 5A&G Pharmaceutical Inc., Columbia, Maryland, USA; 6Program in Oncology, University of Maryland Greenebaum Comprehensive Cancer Center, Baltimore, Maryland, USA
*These authors contributed equally to this work
Background: GP88/Progranulin is a well-recognized cell growth promoter in different cancers, and elevated serum GP88 levels have been described as negative prognostic factor in breast cancer. However, serum levels in prostate cancer (PCa) patients have not yet been studied.
Material and Methods: We analyzed serum GP88 levels by enzyme immunosorbent assay and correlated them with clinicopathological parameters in PCa patients. PCa patients were separated into two groups based on the serum GP88 median level (low ≤44.56 ng/mL or high >44.56 ng/mL) and according to their median age (younger ≤66 years or elder patients >66 years).
Results: Low serum GP88 levels were more often detected in younger patients and high levels in elder patients (P=0.018; Fisher’s exact test). PCa patients were separated into three groups, Gleason score (GS) ≤6; GS=7; and GS≥8. In receiver operating characteristic analyses, we could distinguish GS≤6 from GS=7 [area under the curve (AUC): 0.646; P=0.018] and GS≤6 from GS≥8 (AUC: 0.629; P=0.048) but not GS=7 from GS≥8. For survival analysis, GP88 levels were separated into two groups by an optimized cutoff value of 36.92 ng/mL. Using this GP88 stratification, all PCa patients and younger patients with a low serum GP88 level had a significantly better overall survival compared with patients with higher serum GP88 levels (log-rank test P=0.010 and P=0.024).
Conclusion: Serum GP88 levels are significantly different depending on age and GS, and they are associated with the prognosis of PCa patients.
Keywords: GP88, progranulin, prostate cancer, Gleason score
Introduction
GP88/Progranulin (GRN/PGRN), also known as teratoma PC cell-derived growth factor/PCDGF, acrogranin, granulin/epithelin precursor, is an 88-kD glycoprotein reported as an autocrine proliferation and survival factor for several cancer types.1 The PGRN gene was first cloned from human bone marrow and revealed 71/2 tandem double cysteine-rich granulin domains.2 GP88/PGRN stimulates proliferation in mesenchymal and epithelial cells via activation of different kinase pathways, such as mitogen-activated protein kinase (Erk1/2), phosphatidylinositol 3′-kinase, and focal adhesion kinase pathways.3,4 Its overexpression is associated with several drug resistance mechanisms in breast cancer cells, ie, it confers trastuzumab resistance to Her2-overexpressing cells, letrozole resistance to aromatase overexpressing cells, tamoxifen resistance in MCF7 cells, doxorubicin resistance in MCF7 cells, and also resistance to dexamethasone in human multiple myeloma.4–9 Elevated serum GP88 levels have been reported in patients with rheumatoid arthritis, breast cancer, lung cancer, malignant lymphoma, and ovarian cancer.10–14 A recently published study carried out with a cohort of Korean patients indicated that serum GP88 levels were clinically significant for predicting recurrence in patients with hormone receptor-positive breast cancer during adjuvant tamoxifen therapy.15
Concerning prostate cancer (PCa), published in vitro biological studies have reported that GP88/PGRN promotes cell growth, migration, and anchorage-independent growth.16 In addition, pathological studies on GP88 expression have indicated that while GP88 expression is negative in normal prostate epithelium prostatic intraepithelial neoplasia (PIN) lesions, GP88 expression was significantly increased in PCa lesions.17 However, serum GP88 levels have not been investigated in PCa patients. The present study investigated whether there were differences in the level of circulating GP88/PGRN levels using a GP88-specific enzyme immunosorbent assay (EIA).
Patients and methods
Patients
One hundred forty-two prostate carcinoma patients were recruited to this study, which was positively evaluated by the local ethics committee of the Medical Faculty of the Martin Luther University and is in accordance with the precepts established by the Declaration of Helsinki. All patients gave written informed consent. Twenty-five patients developed distant metastases and two patients already had metastases when the blood sampling was performed. The patients are part of the cohort that has been previously described18 (Tables 1 and S1).
Preanalytical sampling
Ten microliters of venous blood was obtained during patient follow-up and immediately processed by centrifugation at 400×g. Serum was transferred in a separate reaction tube and stored at –80°C. For about 70% of the patients, blood sampling occurred before surgery or treatment. Further, blood sampling details are given in Table S2. Serum GP88 level was not different between the blood sampling groups (Kruskal–Wallis test: P=0.181; data not shown).
GP88 EIA
Serum GP88 levels were determined by a quantitative GP88 sandwich EIA developed and manufactured by A&G Pharmaceutical Inc., Columbia, MD, USA, as described previously,10 using the antihuman GP88 6B3 monoclonal antibody as coating antibody (10 µg/mL) and rabbit polyclonal 37 k antibody as detection antibody. Standard samples (consisting of human GP88 at concentrations from 0 to 20 ng/mL) were measured in duplicates and patients and control samples in triplicates. EIA reaction was measured by absorbance readout at 620 nm on a GENios Microplate Reader (Tecan, Männedorf, Switzerland), and serum GP88 levels were quantified against the human GP88 standard curve.
Statistics
Statistics were performed with SPSS 20.0 (IBM, Ehningen, Germany). Distribution of serum GP88 levels between different groups [Gleason score (GS)] was compared with nonparametric tests (Mann–Whitney U-test; Kruskal–Wallis test). Serum GP88 concentrations and patients’ age were divided according to the median and groups were compared by chi-squared tests (Fisher’s exact test). Diagnostic applicability was analyzed by receiver operating characteristics (ROCs). Survival analyses were performed with Kaplan–Meier analyses and univariate/multivariate Cox’s regression analyses. Overall survival (OS) was considered from the date of serum collection (that was applied for the analysis of GP88 levels) to the last contact (death or last follow-up date).
Results
Expression of GP88 in serum of PCa patients
Serum of 142 PCa was analyzed for GP88 levels by EIA. The PCa patients showed a mean level of 48.67 ng/mL (median: 44.56 ng/mL; range 0–208.48 ng/mL).
Correlation of GP88 levels with clinicopathological parameters
Serum GP88 level was not different in PSA level groups (<4 ng vs ≥4 ng) or tumor stage groups (T1/2 vs T3/4) but was different with borderline significance in age groups (≤66 years vs >66 years; P=0.068) in nonparametric tests.
Next, PCa patients were separated by their median of serum GP88 level in two groups (low: ≤44.56 ng/mL vs high levels: >44.56 ng/mL). Low serum GP88 levels were more often detected in younger patients (≤66 years) and high levels in elder patients (>66 years; P=0.018; Fisher’s exact test).
The Gleason scores (GS) of the PCa patients were separated into three groups; GS≤6, GS=7, and GS≥8. In PCa patients with GS≤6, serum GP88 levels were lower (mean 41.8 ng/mL; median: 40.5 ng/mL) than in patients with GS=7 (mean: 52.2 ng/mL; median 46.6 ng/mL). The serum GP88 levels for patients with GS=7 were comparable with levels observed in GS≥8 patients (mean: 51.5 ng/mL; median: 45.2 ng/mL). The GP88 levels appeared as not equally distributed between the three GS groups (P=0.043; Kruskal–Wallis test). Interestingly, in ROC analyses using serum GP88 levels, it was possible to distinguish GS≤6 from GS=7 [area under the curve (AUC): 0.646; P=0.018; Figure 1] and GS≤6 from GS≥8 (AUC: 0.629; P=0.048; Figure 1) but not GS=7 from GS≥8.
Association of GP88 levels with OS in PCa patients
An optimal serum GP88 cutoff level of 36.92 ng/mL was determined by ROC analysis for all PCa patients and the younger PCa patients. Kaplan–Meier analysis revealed that for all PCa patients the group with a lower GP88 levels (≤36.92 ng/mL) had a significantly longer OS of 111.9 months (95% CI: 102.8–121.2 months) than the group with higher levels (>36.92 ng/mL) with an OS of 88.8 months (95% CI: 77.6–100.1 months; P=0.010; log-rank test, Kaplan–Meier analysis; Table 2). Univariate Cox’s regression analysis showed that the group with higher GP88 levels possessed a 3.3-fold increased risk of death (P=0.015) compared with the low-level GP88 group (Table 2). In a multivariate Cox’s regression backward analysis (adjusted for Gleason grade and tumor stage), the tumor stage [relative risk (RR) = 2.7; P=0.018] and GP88 level (RR=3.0; P=0.032) remained as independent prognostic factors (Table 2).
  | Table 2 Association of serum GP88 levels with overall survival Note: Significant values are given in bold face. Abbreviations: PCa, prostate cancer; RR, relative risk. |
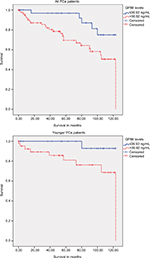
After separating the PCa patients into two groups according to the median age, the younger patients with a low serum GP88 level (≤36.92 ng/mL) had a significantly better prognosis with a mean OS of 119.9 months (95% CI: 114.1–125.7 months) compared with the younger patients with higher GP88 levels (>36.92 ng/mL) who had a mean OS of 100.7 months (95% CI: 86.0–115.5 months; P=0.024; log-rank test; Kaplan–Meier analysis; Figure 2; Table 2).
Univariate Cox’s regression analysis revealed that higher GP88 level in younger patients was associated with a 7.75-fold increased risk of death, although only with a border line significance (P=0.054) probably due to the limited number of patients in the study (Table 2). However, there was no association between serum GP88 levels and OS in elder patients (P=0.337, log-rank test; Table 2) and no association between GP88 levels and tumor-specific survival for all patients or all age patient groups (data not shown).
Discussion
GP88/PGRN has been shown to be a critical driver of tumorigenesis in several cancer types.19 In particular, biological studies have established that GP88 plays a major role in stimulating survival, angiogenesis, drug resistance, migration, and invasion, all hallmarks of tumor aggressiveness and poor prognosis.20,21 Pathological studies have also shown that for several types of cancer, GP88 was overexpressed in tumor biopsies, whereas it was not expressed in normal tissues and/or benign lesions.22 Additionally, elevated serum or plasma levels have been reported in breast, lung, lymphomas, and ovarian cancers.10,11,13,14 Both biological and pathological studies have underscored the role of GP88 expression in PCa.16,17,19 However, up to now, no investigation of GP88 levels in serum of PCa patients had been undertaken. In the present study, we examined serum GP88 levels in PCa patients. The detected mean GP88 levels (48.67 ng/mL) were comparable with the levels detected in patients with breast cancer (45.3 ng/mL), lung cancer (49.9 ng/mL), rheumatoid arthritis (50.2 ng/mL), and osteoarthritis (45.4 ng/mL) but slightly increased compared with published serum GP88 levels in healthy subjects (28.7±5.8 ng/mL).11,12,14 Interestingly, we detected for the first time that serum GP88 levels were age associated, ie, increased in elder PCa patients. However, previous studies have not shown an age association with serum GP88 levels in healthy male volunteers (N=260; mean age 50 years) or in breast cancer patients (N=189, median age 51 years).11,12 GP88 levels in GS=7 and GS≥8 PCa patients were significantly increased compared with GS≤6 PCa patients. This finding would suggest that serum GP88 determination could provide complementary information to the GS evaluation. However, other diseases such as rheumatoid arthritis and osteoarthritis, found preferentially in an older population, also show elevated serum GP88 levels. Therefore, further studies are required to evaluate the relationship between age/GS and GP88 levels in PCa patients, particularly in older populations where these diseases maybe more prevalent.
When applying an optimized GP88 cutoff level of 36.92 ng/mL based on ROC analysis, it was found that PCa patients could be stratified into two groups with better or worse OS. We could show for the first time that a low GP88 level was significantly associated with better OS in PCa patients. PCa patients with high(er) GP88 levels had a significantly increased risk of death (RR=3.0). Multivariate Cox’s regression analysis showed that serum GP88 level was an independent prognostic factor for OS in PCa patients. It is interesting to note that in younger patients (N=71), low GP88 levels were significantly associated with a better prognosis when compared with patients with higher serum GP88. In fact, the low levels of GP88 in the younger PCa patients (19.61–36.92 ng/mL; Table 1) were rather comparable with those reported in healthy subjects (28.7±5.8 ng/mL). But the high levels of GP88 in the younger patients (>36.92–147.04 ng/mL) associated with shorter OS were in almost all cases above the levels previously reported in healthy subjects.11,14 It is surprising to note that there was no association between the GP88 levels and OS in the elder patient group. However, it is conceivable that this could be due to the fact that the OS of the older population is expectedly shorter than that of the younger population. It is also possible that GP88 levels may play different roles in prostate biology in younger and older patients.
Furthermore, in addition to its role as prognostic factor, GP88 shows potential as therapeutic target. In particular, inhibition of GP88 in urothelial cancer cells resulted in the inhibition of cell migration, invasion, and anchorage-independent growth in vitro, and it resensitized urothelial cancer cells to cisplatin. Moreover, urothelial cells stably transfected with GP88/PGRN shRNA displayed a reduction of tumor growth in xenograft and orthotopic mouse tumor models.23
Altogether, the analysis of GP88 levels in a liquid biopsy, ie, the serum of PCa patients, may provide additional information to better assess tumor differentiation and prognosis especially in younger PCa patients in a noninvasive way.
Acknowledgments
The authors thank the Rudolf und Irmgard Kleinknecht-Stiftung for supporting HT, the Johannes und Frieda Marohn-Stiftung for supporting HT and SW, and the ELAN Fonds for supporting VL (14-07-11-1-Huppert). They are thankful to Dr P Schmidt/Tumorzentrum Martin Luther University Halle-Wittenberg for his help with follow-up data. They also acknowledge the financial support of the Open Access Publication Fund of the Martin-Luther-University Halle-Wittenberg.
Disclosure
GS, DH, and BY are employees of A&G Pharmaceutical Inc., Columbia, Maryland, USA. The authors report no other conflicts of interest in this work.
References
Zhang H, Pan CX, Cheng L. GRN (granulin). Atlas Genet Cytogenet Oncol Haematol. 2008;12:208–212. | ||
Bhandari V, Palfree RG, Bateman A. Isolation and sequence of the granulin precursor cDNA from human bone marrow reveals tandem cysteine-rich granulin domains. Proc Natl Acad Sci U S A. 1992;89(5):1715–1719. | ||
Bateman A, Bennett HP. The granulin gene family: from cancer to dementia. Bioessays. 2009;31(11):1245–1254. | ||
Serrero G. Autocrine growth factor revisited: PC-cell-derived growth factor (progranulin), a critical player in breast cancer tumorigenesis. Biochem Biophys Res Commun. 2003;308(3):409–413. | ||
Tangkeangsirisin W, Hayashi J, Serrero G. PC cell-derived growth factor mediates tamoxifen resistance and promotes tumor growth of human breast cancer cells. Cancer Res. 2004;64(5):1737–1743. | ||
Kim WE, Serrero G. PC cell-derived growth factor stimulates proliferation and confers Trastuzumab resistance to Her-2-overexpressing breast cancer cells. Clin Cancer Res. 2006;12(14 Pt 1):4192–4199. | ||
Wang W, Hayashi J, Serrero G. PC cell-derived growth factor confers resistance to dexamethasone and promotes tumorigenesis in human multiple myeloma. Clin Cancer Res. 2006;12(1):49–56. | ||
Abrhale T, Brodie A, Sabnis G, et al. GP88 (PC-Cell Derived Growth Factor, progranulin) stimulates proliferation and confers letrozole resistance to aromatase overexpressing breast cancer cells. BMC Cancer. 2011;11:231. | ||
Kudoh K, Ramanna M, Ravatn R, et al. Monitoring the expression profiles of doxorubicin-induced and doxorubicin-resistant cancer cells by cDNA microarray. Cancer Res. 2000;60(15):4161–4166. | ||
Han JJ, Yu M, Houston N, Steinberg SM, Kohn EC. Progranulin is a potential prognostic biomarker in advanced epithelial ovarian cancers. Gynecol Oncol. 2011;120(1):5–10. | ||
Tkaczuk KR, Yue B, Zhan M, et al. Increased circulating level of the survival factor GP88 (Progranulin) in the serum of breast cancer patients when compared to healthy subjects. Breast Cancer. 2011;5:155–162. | ||
Yamamoto Y, Takemura M, Serrero G, et al. Increased serum GP88 (Progranulin) concentrations in rheumatoid arthritis. Inflammation. 2014;37(5):1806–1813. | ||
Yamamoto Y, Goto N, Takemura M, et al. Association between increased serum GP88 (progranulin) concentrations and prognosis in patients with malignant lymphomas. Clin Chim Acta. 2017;473:139–146. | ||
Edelman MJ, Feliciano J, Yue B, et al. GP88 (progranulin): a novel tissue and circulating biomarker for non-small cell lung carcinoma. Hum Pathol. 2014;45(9):1893–1899. | ||
Koo DH, Park CY, Lee ES, Ro J, Oh SW. Progranulin as a prognostic biomarker for breast cancer recurrence in patients who had hormone receptor-positive tumors: a cohort study. PLoS One. 2012;7(6):e39880. | ||
Monami G, Emiliozzi V, Bitto A, et al. Proepithelin regulates prostate cancer cell biology by promoting cell growth, migration, and anchorage-independent growth. Am J Pathol. 2009;174(3):1037–1047. | ||
Pan CX, Kinch MS, Kiener PA, et al. PC cell-derived growth factor expression in prostatic intraepithelial neoplasia and prostatic adenocarcinoma. Clin Cancer Res. 2004;10(4):1333–1337. | ||
Wach S, Al-Janabi O, Weigelt K, et al. The combined serum levels of miR-375 and urokinase plasminogen activator receptor are suggested as diagnostic and prognostic biomarkers in prostate cancer. Int J Cancer. 2015;137(6):1406–1416. | ||
Tanimoto R, Lu KG, Xu S-Q, et al. Mechanisms of progranulin action and regulation in genitourinary cancers. Front Endocrinol. 2016;7:1–7. | ||
Abella V, Pino J, Scotece M, et al. Progranulin as a biomarker and potential therapeutic agent. Drug Discov Today. 2017;22(10):1557–1564. | ||
Arechavaleta-Velasco F, Perez-Juarez CE, Gerton GL, Diaz-Cueto L. Progranulin and its biological effects in cancer. Med Oncol. 2017;34(12):194. | ||
Serrero G. Potential of theranostic target mining in the development of novel diagnostic and therapeutic products in oncology: progranulin/GP88 as a therapeutic and diagnostic target for breast and lung cancers. Rinsho Byori. 2016;64:1296–1309. | ||
Buraschi S, Xu SQ, Stefanello M, et al. Suppression of progranulin expression inhibits bladder cancer growth and sensitizes cancer cells to cisplatin. Oncotarget. 2016;7(26):39980–39995. |
Supplementary materials
  | Table S1 Treatment options Abbreviations: TUR-P, transurethral resection of the prostate; HIFU, high-intensity focused ultrasound. |
  | Table S2 Blood sampling |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.