Back to Journals » Cancer Management and Research » Volume 11
Expression Of Cyclin D1 Protein Isoforms And Its Prognostic Significance In Cervical Cancer
Authors Gu J, Zhang X, Yang Z, Wang N
Received 21 July 2019
Accepted for publication 19 September 2019
Published 24 October 2019 Volume 2019:11 Pages 9073—9083
DOI https://doi.org/10.2147/CMAR.S224026
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Jiahui Gu,1 Xinyu Zhang,2 Zhuo Yang,1 Ning Wang1
1Department of Obstetrics and Gynecology, Shengjing Hospital of China Medical University, Shenyang, Liaoning Province 110001, People’s Republic of China; 2Department of Obstetrics and Gynecology, Daqing People’s Hospital, Daqing, Heilongjiang Province 163711, People’s Republic of China
Correspondence: Ning Wang
Department of Obstetrics and Gynecology, Shengjing Hospital Affiliated to China Medical University, Sanhao Street No. 36, Heping District, Shenyang, Liaoning, People’s Republic of China
Email [email protected]
Introduction: Cyclin D1 had been associated with different clinical and pathological stages of cervical cancer; however, few studies had focused on its correlation with cervical cancer prognosis. Therefore, this study aimed to assess the expression of cyclin D1a and D1b in normal tissue, cervical cancer and cervical intraepithelial neoplasia and their effect on prognosis.
Methods: Expression of cyclin D1a and D1b was detected by immunohistochemical staining in 78 cases of primary cervical cancer, 40 cases of cervical intraepithelial neoplasia, and 40 cases of normal cervical tissue.
Results: No significant difference was observed in the expression of cyclin D1a between normal and cervical cancer tissues (P = 0.201); however, its expression was significantly higher in cervical cancer than in cervical intraepithelial neoplasia tissues (P = 0.000). Expression of cyclin D1b was higher in normal tissues than in cervical cancer tissues (P = 0.000). No significant difference was observed in the expression of cyclin D1a in cervical cancer tissues with respect to age, pathological type, clinical-stage, depth of tumor invasion, or presence of lymph node metastases (P = 0.111,0.119,0.539,0.084,0.539). COX survival analysis showed that lymph node metastasis might be an independent factor affecting postoperative recurrence (hazard risk [HR] = 0.240; 95% confidence interval [CI] = 0.968–30.156; P = 0.034).
Discussion: Cyclin D1a expression was associated with tumor tissue size and degree of differentiation. The expression of cyclin D1b in cervical cancer was associated with the presence of lymph node metastases. Cyclin D1a and D1b expression in cervical cancer tissue was significantly correlated. Cox survival analysis showed that the presence of lymph node metastases might serve as an independent factor affecting postoperative recurrence. The expression of cyclin D1a and D1b was not associated with cervical cancer prognosis.
Conclusion: Assessment of cyclin D1a and D1b expression in cervical cancer and cervical intraepithelial neoplasia revealed that cyclin D1 could not be used as a reference to assess cervical cancer patient prognosis.
Keywords: cyclin D1 protein isoforms, cervical cancer, expression, prognosis
Introduction
Cervical cancer is one of the most severe threats to the health of women. Its incidence ranks second among malignant tumors in women.1,2 Accumulating evidence showed that human papillomavirus (oncogenic types) plays a crucial role in the induction and development of human cervical cancer.3–5 As reported, some patients are not infected with human papillomavirus, suggesting that other factors promote the malignant progression of cervical cancer.6,7 The exact pathogenesis of cervical cancer has not yet been fully elucidated, and it is currently considered to be the result of a combination of factors, involving the regulation of many genes.8,9
With developments in cancer research, the relationship between disorders in cell-cycle regulation and malignant tumors is becoming increasingly understood. It is believed that abnormal cell-cycle regulation is an essential mechanism for tumor development and progression. Some have even suggested that “cancer is a disease of the cell cycle”.10 Cell-cycle progression is a process tightly regulated by cyclins, cyclin-dependent kinases, and cyclin-dependent kinase inhibitors at two key restriction points, G1/S and G2/M.11,12
Cancer development and progression are stepwise processes involving oncogene activation, tumor suppressor gene inactivation, and imbalance of regulatory mechanisms of the immune system, the combination of which causes cell variability and leads to cancerous transformation of tissue.13,14 Studies have shown that uncontrolled cell proliferation is closely associated with cell-cycle dysregulation. Cyclins are relevant positive regulators of the cell cycle, and cyclin D1, essential member of this family, is a regulator of cyclin-dependent kinases.15 Its primary function is promoting cell proliferation, but it also acts independently of cyclin-dependent kinase activity that promotes gene transcription.16 Mutation, amplification, or overexpression of the cyclin D1 gene changes the cell-cycle progression. This has been observed in various cancers, such as breast, lung, bladder, cervical, and parathyroid cancer and lymphoma.17,18
Many studies have focused on the expression of cyclin D1 and its splice variants cyclin D1a and cyclin D1b, which arise from a single-nucleotide polymorphism in the CCND1 gene in cervical cancer.16,19 While cyclin D1 has been associated with different clinical and pathological stages of cervical cancer, and few studies have focused on its correlation with cervical cancer prognosis. Currently, the accepted view is that cyclin D1 and its isoforms play an essential role in the development and progression of cervical cancer. During the normal cell cycle, cyclin D1 forms a complex with cyclin-dependent kinase 4, which promotes the phosphorylation of the tumor suppressor retinoblastoma protein and thereby relieves G1 arrest.20 The transcription factor EZF is then initiated to promote DNA synthesis, allowing the completion of cell division by moving from the G1 phase to the S phase. Cyclin D1 can thus be seen as shortening the G1 phase of the cell cycle.21 When control of the cyclin D1 protein is abnormal and multiple cancer-related genes result in its increased expression, the time cells spend in the G1 phase of the cell cycle is significantly decreased, causing the cells to enter the S phase early, in turn resulting in uncontrolled cell proliferation and transformation, leading to carcinogenesis.22 Currently, cyclin D1 is recognized as a proto-oncogene, and its overexpression can alter progression through the cell cycle, leading to uncontrolled cell proliferation and malignancy. The pathogenesis of the development and progression of cervical cancer is still not clearly understood. Therefore, early detection of cervical lesions, early diagnosis, and early treatment plays a vital role in ensuring a good prognosis. This warrants thorough research on specific cancer markers for cervical lesions, as well as malignant uterine tumors. We wondered whether abnormal cyclin D1 expression can be used as an indicator of cancer diagnosis and studied the role of cyclin D1 in cell-cycle regulation in order to find drugs for the treatment of cancer.
In this study, immunohistochemistry was used to measure the expression of the cyclin D1 isoforms cyclin D1a and cyclin D1b in normal tissue, cervical intraepithelial neoplasia tissue, and cervical cancer tissue (stages Ia–IIb). Moreover, a five-year cumulative survival rate was obtained by follow-up to study the effect of cyclin D1 isoform expression on cervical cancer prognosis.
Methods
Tissue Samples
Archived paraffin blocks from 78 cases of primary cervical cancer, 40 cases of cervical intraepithelial neoplasia, and 40 cases of normal cervical tissue, confirmed by surgery or pathology, were selected from the Shengjing Hospital of China Medical University, Department of Obstetrics and Gynecology, between September 2005 and June 2010. All pathology specimens selected were confirmed by the Department of Pathology of the hospital. The study was approved by the Responsible Committee on Human Experimentation of Shengjing Hospital of China Medical University. Written informed consent was obtained from each participant before data collection.
The inclusion criteria for primary cervical cancer were: 1) undergoing radical surgery for cervical cancer (stages Ia–IIb); 2) some cases with high-risk postoperative factors (intravascular tumor thrombus, infiltration > 1/2–2/3, > 5 cm, positive margin, positive lymph nodes, periuterine positive); and 3) no adjuvant chemotherapy after radiotherapy. Clinical data of patients were complete.
Of the 78 cervical cancer tissue specimens selected, 31 were cases of squamous cell carcinoma, 28 were cases of adenocarcinoma, and 19 were cases of adenosquamous carcinoma. The pathological stages included 35 cases with high differentiation, 23 cases with moderate differentiation, and 20 cases with poor differentiation. There were 29 cases with lymph node metastases.
Immunohistochemistry
The thickness of the paraffin sections was 5 µm, and subsequent streptavidin-peroxidase (S-P) staining was carried out per instructions provided with the S-P kit to ensure accurate and standardized operation. The main steps were as follows: paraffin sections were dewaxed in water and restored by rinsing for 8 mins. A 3% hydrogen peroxide solution was added to the sections, and the slides were incubated for 10 mins at room temperature. The slides were washed with phosphate-buffered saline three times for 5 mins each. Rabbit anti-human cyclin D1a monoclonal primary antibody (sc-717, Santa Cruz Biotech, Inc., Santa Cruz, CA) at a dilution of 1:100 was added, and the slides were incubated overnight at 4°C. Sections were removed the following morning, placed at room temperature, and washed with phosphate-buffered saline three times for 5 mins. The secondary antibody was added, and diaminobenzidine color development, hematoxylin counterstaining, alcohol gradient dehydration, xylene clearing, and neutral gum sealing were performed. The method for cyclin D1b detection was the same as above, using the rabbit anti-human cyclin D1b monoclonal primary antibody (ImB, Shanghai Immune Biotech, Ltd., Shanghai, China).
Evaluation Of Staining Results
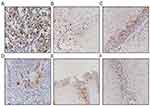
The surrounding interstitial cells and adjacent normal epithelia present in the sections served as internal controls. Scoring of the Cyclin D1 reactivity was performed by two of the authors (Gu and Zhang) on a multiheaded microscope using the Allred method.23 With this method, the intensity of the immunohistochemical reaction was recorded as 0, negative (no staining of any nuclei even at high magnification); 1, weak (only visible at high magnification); 2, moderate (readily visible at low magnification); or 3, strong (strikingly positive even at low power magnification). The proportion of tumor nuclei showing positive staining was also recorded as either: 0, no staining; 1, <1%; 2, 1–10%; 3, 11–33%; 4, 34–66%; 5, 67–100% nuclei staining. The proportion and intensity scores were subsequently added to obtain a total score, which ranged from 0 to 8. Tumors were categorized into four groups: negative/weak (total scores 0–2), moderate (total scores 3–5) and strong (total scores 6–8) expression as previously described.23 Only nuclear staining was considered specific. Positive immunohistochemical staining of cyclin D1a and cyclin D1b was located in the nucleus, and cells with yellow-brown granules were considered positive (Figure 1).
Statistical Analysis
The results of immunohistochemical staining were evaluated, and the chi-squared test was used to compare the positive expression rates between tissues. The Spearman method was used for correlation analysis. Cervical cancer prognosis was analyzed using Cox survival analysis. The Kaplan–Meier nonparametric method was used for survival curve construction, and the log-rank test was used for comparison of survival rates. Differences with p-values of <0.05 were considered statistically significant. SPSS software (version 19.0) was used for data analysis.
Results
Expression Of Cyclin D1a And D1b
The expression rate of cyclin D1a in normal tissue was not significantly different from that in cervical cancer cases (p = 0.201), but its expression in cervical intraepithelial neoplasia tissue was significantly lower than that in cervical cancer (p = 0.000). The expression rate of cyclin D1b in cervical cancer tissue was significantly lower than in normal tissue (p = 0.000) (Table 1).
 |
Table 1 Expression Of Cyclin D1a And D1b |
Relationship Between Cyclin D1 Expression In Cervical Cancer And Clinical Characteristics
The expression of cyclin D1a in cervical cancer tissues did not differ significantly with respect to age (p = 0.111), pathological type (p = 0.119), clinical-stage (p = 0.539), depth of tumor invasion (p = 0.084), or presence of lymph node metastasis (p = 0.539). However, it was associated with tumor tissue size and degree of differentiation: the larger the tumor tissue, the higher the positive expression rate (p < 0.05), and the expression was higher in well-differentiated tissues than in moderately differentiated tissues (p < 0.05).
The expression of cyclin D1b in cervical cancer tissue was associated with the presence of lymph node metastases: the expression of cyclin D1b was significantly higher in tissues with lymph node metastasis (p < 0.05) (Tables 2 and 3). In the 78 cases of cervical cancer, the expression of cyclin D1a and D1b exhibited a significant negative correlation (p < 0.05) (Table 3).
 |
Table 2 Relationship Between Cyclin D1 Expression In Cervical Cancer And Clinical Characteristics |
 |
Table 3 Cyclin D1a Expression In Highly Differentiated And Moderately Differentiated Cervical Cancer |
Effect Of The Expression Of Different Cyclin D1 Isoforms On Cervical Cancer Prognosis
Multivariate Cox regression analysis of cervical cancer prognosis and Kaplan–Meier survival curve analysis showed that there was no significant difference in postoperative recurrence rate and overall survival between cervical cancer cases with positive and negative cyclin D1a expression (p = 0.517)(Figure 2) and cyclin D1b expression (p = 0.882)(Figure 4), and there was no statistically significance difference in the log-rank analysis about cyclin D1a expression (p = 0.889) (Figure 3) and cyclin D1b expression (p = 0.230) (Figure 5). However, the statistical analysis showed that the presence of lymph node metastasis might be an independent factor affecting postoperative recurrence (p = 0.034) (Table 4).
 |
Table 4 Multivariate Cox Regression Analysis Of Factors Affecting Postoperative Recurrence Rate In Patients With Cervical Cancer |
Finally, none of the assessed variables had a significant effect on the five-year overall cervical cancer survival rate (Table 5).
 |
Table 5 Multivariate Cox Regression Analysis Of Factors Affecting Postoperative Mortality Rate In Patients With Cervical Cancer |
Discussion
Cyclin D1 is a regulator of the cell cycle that promotes the transition from G1 to S phase by activating cyclin‐dependent kinase 4 or 6.24 Various studies have shown the role of cyclin D1 protein in neoplastic transformation and in the progression of variety of cancers.25,26 Cyclin D1 plays vital roles in cell biology, including cell proliferation and growth regulation, mitochondrial activity modulation, DNA repair, and cell migration control. In cervical squamous cell carcinoma, there is a discrepancy in the expression of cyclin D1, as a few authors have reported elevated levels,27 while others reported decreased levels.28 In the present study, it was clear that the different isoforms of cyclin D1 are expressed in normal, cervical intraepithelial neoplasia, and cervical cancer tissues. Although no significant difference was observed in the expression of cyclin D1a in cervical cancer tissue and normal tissue, it was significantly higher in cervical cancer than in cervical intraepithelial neoplasia tissue. However, the expression of cyclin D1b in cervical cancer tissue was significantly lower than in normal tissue. These findings indicate that different cyclin D1 isoforms may promote the development and progression of cervical cancer, which is consistent with our previous experimental results,16,24 in which cyclin D1 formed a complex with cyclin-dependent kinase 4 in the normal cell cycle and caused dysregulation of cell proliferation, transformation, and ultimately carcinogenesis through a series of interactions with various proteins.29,30
Based on the correlation between the expression of the two isoforms of cyclin D1 that we observed in cervical cancer tissue, we speculate that they may interact in cervical cancer. The total level of cyclin D1 protein may not be significantly elevated in patients with cervical cancer, but we propose that the effect of the decrease in cyclin D1b or the interaction between cyclin D1a and cyclin D1b in cervical tissue may indirectly promote the development of cervical cancer and further lead to the progression of tumors. Ramos-Garcia et al.31 reported the oncogenic activation of CCND1/cyclin D1 in oral squamous cell carcinoma, the potential diagnostic and prognostic value of cyclin D1, and the influence of CCND1/cyclin D1 on tumor size and clinical-stage. Our previous study showed that cyclin D1b has anti-tumor effects in cervical cancer, wherein it initiates cell-cycle arrest at the G0/G1 phase, induces apoptosis, and inhibits cell proliferation and colony formation.32 However, further experiments are required to assess whether the two isoforms interact at the molecular level and the mechanisms underlying their interaction. Thus, cyclin D1 seems to contribute in a contradictory way to the prognosis of different types of cancer. So why the difference? It may be related to the presence or absence of HPV infection. HPV is a DNA virus, which has a certain correlation with the occurrence of cervical cancer. In most cervical cancer cases, HPV may integrate with the host’s DNA, thus affecting the expression of cyclinD1, and thereby having different effects on the prognosis of cancer.
No significant difference was observed in the expression of cyclin D1a in cervical cancer tissues with respect to age, pathological type, clinical-stage, depth of tumor invasion, and presence of lymph node metastases. However, it was associated with tumor tissue size and degree of differentiation. On the other hand, the expression of cyclin D1b in cervical cancer tissue was associated with the presence of lymph node metastasis. Although the results of this study did not show that the expression of cyclin D1 isoforms in tumor tissues was clearly correlated with cervical intraepithelial neoplasia or FIGO stage of cervical cancer, and no expected positive results were obtained, the results suggest that this may be because the sample size of each grade and stage was too small in the selected cases. If the sample size were increased to reduce bias, positive results might be obtained.
The postoperative survival rate of patients with cervical cancer may be affected by a variety of factors, and it is generally believed that tumor stage, degree of differentiation, and presence of lymph node metastases may be associated with disease prognosis, that is, the higher the tumor stage, the more poorly the tumor cells are differentiated, and the stronger the growth potential and the faster the division, the more likely lymph node metastases are present and the worse the prognosis. In the present study, 78 cases of cervical cancer were followed up for five years. The results showed that lymph node metastasis might be associated with the postoperative recurrence rate of cervical cancer. We could also observe the different trends of cyclin D1b isoform from the survivor curve, but no significant correlation between cyclin D1 isoform expression and survival rate was found. However, the subjects included only cases of stage II cervical cancer: the number of stages assessed was limited, and the sample size was small, thus introducing bias. Thus, it is not yet possible to determine whether there is a correlation between the expression of cyclin D1, especially cyclin D1b, and cervical cancer prognosis based on our findings, and a more significant number of prospective studies must be conducted to determine further whether cyclin D1 protein isoforms affect cervical cancer prognosis.
Contribution of cyclin D1 to cancer formation and cancer survival is not entirely known. In cancer tissues, overexpression of cyclin D1 is associated with both cancer genome instability and resistance to DNA-damaging cancer drugs. However, a new insight has been provided in recent years. Ramos-Garcia et al,31 reported the utilization of cyclin D1 as a therapeutic target and the combination of cyclin D1 inhibitors with cytotoxic agents. Jirawatnotai S et al,33 reported that cyclin D1 expression might contribute to DNA repair and chromosome instability, and these functions may facilitate cancer formation and drug resistance. We plan to pay particular attention to this aspect in future research.
Conclusion
In summary, we found that the expression of cyclin D1a is higher in normal tissues than in cervical intraepithelial neoplasia tissues, and its expression in cervical cancer tissues is related to tumor tissue size and degree of differentiation. On the other hand, the expression of cyclin D1b is higher in normal tissue than in cervical cancer tissue, and its expression in cervical cancer tissue is related to the presence of lymph node metastases. In addition, we revealed that the expression of cyclin D1a and cyclin D1b in cervical cancer tissue exhibits a significant correlation. Moreover, we found that the expression of cyclin D1a and cyclin D1b has no significant relationship with postoperative recurrence rate and overall survival rate; however, the presence of lymph node metastases may act as an independent factor affecting postoperative recurrence. The expression of the two proteins was not found to be associated with cervical cancer prognosis, indicating that cyclin D1 cannot be used as a reference for the assessment of cervical cancer patient prognosis, and further studies are required for confirmation.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (No. 81202047), the Program for Liaoning Excellent Talents in University (No. LJQ2013083), and the Natural Science Foundation of Liaoning Province (CN). (Grant 20170541003 for ZY).
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Shen MR, Hsu YM, Hsu KF, et al. Insulin-like growth factor 1 is a potent stimulator of cervical cancer cell invasiveness and proliferation that is modulated by alphavbeta3 integrin signaling. Carcinogenesis. 2006;27(5):962–71. doi:10.1093/carcin/bgi336
2. Pan Y, Zhang Y, Chen L, et al. The critical role of Rab31 in cell proliferation and apoptosis in cancer progression. Mol Neurobiol. 2015.
3. Galloway TJ, Ridge JA. Management of squamous cancer metastatic to cervical nodes with an unknown primary site. J Clin Oncol. 2015;33:3328–3337. doi:10.1200/JCO.2015.61.0063
4. Biglia N, Bounous VE, Sgro LG, D’Alonzo M, Gallo M. Treatment of climacteric symptoms in survivors of gynaecological cancer. Maturitas. 2015;82:296–298. doi:10.1016/j.maturitas.2015.07.006
5. Yan J, Zhang Y, Ren C, et al. Involvement of nuclear protein C23 in activation of EGFR signaling in cervical cancer. Tumour Biol. 2015.
6. Nicolaidis S. Biomarkers of cervical cancer multiforme. Metabolism. 2015;64:S22–S27. doi:10.1016/j.metabol.2014.10.031
7. SH S, Liu D, Deng YT, et al. SIX1 coordinates with TGFβ signals to induce epithelial-mesenchymal transition in cervical cancer. Oncol Lett. 2016;12:1271–1278. doi:10.3892/ol.2016.4797
8. Zhang -Y-Y, Zhang F, Zhang Y-S, et al. Mechanism of juglone-induced cell cycle arrest and apoptosis in Ishikawa Human endometrial cancer cells. J Agric Food Chem. 2019;67(26):7378–7389. doi:10.1021/acs.jafc.9b02759
9. Wang J, Zhang YS, Thakur K, et al. Licochalcone A from licorice root, an inhibitor of human hepatoma cell growth via induction of cell apoptosis and cell cycle arrest. Food Chem Toxicol. 2018;120:407–417. doi:10.1016/j.fct.2018.07.044
10. Shuo Q, Diehl JA. Cyclin D1, cancer progression and opportunities in cancer treatment. J Mol Med (Berl). 2016;94(12):1313–1326. doi:10.1007/s00109-016-1475-3
11. Jia R, Suhui WU, Guo H, et al. Effects of MIF recombinant plasmid transfection on human cervical cancer cells SiHa and Its influence on CyclinD1 expression. Cancer Res Prev Treat. 2013;28(32):834–838.
12. Bali A, O’Brien PM, Edwards LS, Sutherland RL, Hacker NF, Henshall SM. Cyclin D1, p53, and p21Waf1/Cip1 expression is predictive of poor clinical outcome in serous epithelial ovarian cancer. Clin Cancer Res. 2004;10:5168–5177. doi:10.1158/1078-0432.CCR-03-0751
13. Casimiro MC, Di Sante G, Di Rocco A, et al. Cyclin D1 restrains oncogene-induced autophagy by regulating the AMPK-LKB1 signaling axis. Cancer Res. 2017;77(13):3391–3405. doi:10.1158/0008-5472.CAN-16-0425
14. Yi Y, Li H, Lv Q, et al. miR-202 inhibits the progression of human cervical cancer through inhibition of cyclin D1. Oncotarget. 2016;7(44):72067–72075. doi:10.18632/oncotarget.12499
15. Bates S, Parry D, Bonetta L, Vousden K, Dickson C, Peters G. Absence of cyclin D/cdk complexes in cells lacking functional retinoblastoma protein. Oncogene. 1994;9:1633–1640.
16. Wang N, Qian X, Wang S, et al. CCND1 rs9344 polymorphisms are associated with the genetic susceptibility to cervical cancer in Chinese population. Mol Carcinog. 2012;51:196–205. doi:10.1002/mc.20801
17. Sherr CJ, Roberts JM. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004;18:2699–2711. doi:10.1101/gad.1256504
18. Deshpande A, Sicinski P, Hinds PW. Cyclins and cdks in development and cancer: a perspective. Oncogene. 2005;24:2909–2915. doi:10.1038/sj.onc.1208618
19. Hosokawa Y, Arnold A. Mechanism of cyclin D1 (CCND1, PRAD1) overexpression in human cancer cells: analysis of allele-specific expression. Genes Chromosomes Cancer. 1998;22:66–71.
20. Chen H, Xu X, Wang G, et al. CDK4 protein is degraded by anaphase-promoting complex/cyclosome in mitosis and reaccumulates in early G1 phase to initiate a new cell cycle in HeLa cells. J Biol Chem. 2017;292(24):10131–10141. doi:10.1074/jbc.M116.773226
21. Y E S-W, M W W, Chen QR. Expression of USP2 and CyclinD1 in Gastric Carcinoma and Its Clinicopathologic Significance. China Cancer. 2014;20(35):830–838.
22. Howe D, Lynas C. The cyclin D1 alternative transcripts [a] and [b] are expressed in normal and malignant lymphocytes and their relative levels are influenced by the poly-morphism at codon 241. Haematologica. 2001;86:563–569.
23. Reis-Filho JS, Savage K, Lambros MB, et al. Cyclin D1 protein overexpression and CCND1 amplification in breast carcinomas: an immunohistochemical and chromogenic in situ hybridisation analysis. Mod Pathol. 2006;19:999–1009. doi:10.1038/modpathol.3800621
24. Hosooka T, Ogawa W. A novel role for the cell cycle regulator complex cyclin D1-CDK4 in gluconeogenesis. J Diabetes Investig. 2016;7(1):27–28. doi:10.1111/jdi.12369
25. Bonilla C, Lefèvre JH, Winney B, et al. Cyclin D1 rare variants in UK multiple adenoma and early-onset colorectal cancer patients. J Hum Genet. 2011;56:58–63. doi:10.1038/jhg.2010.144
26. Hayakawa Y, Hirata Y, Nakagawa H, et al. Apoptosis signal-regulating kinase 1 and cyclin D1 compose a positive feedback loop contributing to tumor growth in gastric cancer. Proc Natl Acad Sci USA. 2011;108:780–785. doi:10.1073/pnas.1011418108
27. Skomedal H, Kristensen GB, Lie AK, Holm R. Aberrant expression of the cell cycle associated proteins TP53, MDM2, p21, p27, cdk4, cyclin D1, RB, and EGFR in cervical carcinomas. Gynecol Oncol. 1999;73:223–228. doi:10.1006/gyno.1999.5346
28. Bae DS, Cho SB, Kim YJ, et al. Aberrant expression of cyclin D1 is associated with poor prognosis in early stage cervical cancer of the uterus. Gynecol Oncol. 2001;81:341–347. doi:10.1006/gyno.2001.6196
29. Massague J. G1 cell-cycle control and cancer. Nature. 2004;432:298–306. doi:10.1038/nature03094
30. Lange C, Huttner WB, Calegari F. Cdk4/cyclinD1 overexpression in neural stem cells shortens G1, delays neurogenesis, and promotes the generation and expansion of basal progenitors. Cell Stem Cell. 2009;5:320–331. doi:10.1016/j.stem.2009.05.026
31. Ramos-Garcia P, Gil-Montoya JA, Scully C, et al. An update on the implications of cyclin D1 in oral carcinogenesis. Oral Dis. 2017;23(7):897–912. doi:10.1111/odi.12620
32. Wang N, Wei H, Yin D, et al. Cyclin D1b overexpression inhibits cell proliferation and induces cell apoptosis in cervical cancer cells in vitro and in vivo. Int J Clin Exp Pathol. 2014;7(7):4016–4023.
33. Jirawatnotai S, Sittithumcharee G. Paradoxical roles of cyclin D1 in DNA stability. DNA Repair (Amst). 2016;42:56–62. doi:10.1016/j.dnarep.2016.04.011
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.