Back to Journals » Cancer Management and Research » Volume 11
Expression of CD133 and CD24 and their different phenotypes in urinary bladder carcinoma
Authors Farid RM , Sammour SA, Shehab ElDin ZA , Salman MI , Omran TI
Received 15 December 2018
Accepted for publication 23 April 2019
Published 23 May 2019 Volume 2019:11 Pages 4677—4690
DOI https://doi.org/10.2147/CMAR.S198348
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Rituraj Purohit
Rola M Farid,1 Sanaa Abd-Elmaged Sammour,1 Zeinab Abdelkader Shehab ElDin,1 Manal Ibrahim Salman,1 Tag Ibrahim Omran2
1Department of Pathology, Ain Shams University, Cairo, Egypt; 2Department of Pathology, Military Medical Academy, Cairo, Egypt
Introduction: Several lines of evidence suggest the contribution of cancer stem cells (CSCs) to the tumorigenicity of bladder cancer. Although CD133 and CD24 CSC biomarkers are associated with survival disadvantages in some cancers, the biological attributes of a specific tumor alters the expression of these markers and any associated phenotypic characteristics.
Aim: To analyze CD133 and CD24 expression and their different phenotypes in urinary bladder carcinoma.
Material and methods: Expression of CD133 and CD24 and their divergent phenotypes were analyzed in patients with urinary bladder carcinoma (n=60) and correlated with clinicopathological parameters.
Results: CD133+, and CD24+, tumor cells were more frequent in high grade, less differentiated carcinomas (18/22, and 15/17, p=0.022 and 0.01, respectively), muscle invasive tumors (20/22, p=0.017 and 17/17, p=0.001, respectively), and tumors with advanced stage (p=0.001 and 0.007, respectively). The expression of CD24 slightly correlated with lymphovascular invasion (p=0.04), whereas CD133 was associated with distant metastasis. The CD133+, CD24+, phenotype exhibited more aggressive tumorigenic behavior than other phenotypes.
Conclusion: CD133+, and CD24+, cells correlated with determinants of aggressive behavior and may be involved in tumor progression and distant metastasis. The CD133+, subpopulation is likely to have a more potent tumorigenic capacity. Although divergent, the strong correlation between the two populations may support phenotypic plasticity among them. Compared to the CD133+, CD24−, and CD133−, CD24+, phenotypes, the CD133+, CD24+, phenotype is the most aggressive. These putative biomarkers can potentially aid in the selection of high-risk patients for more aggressive targeted therapy.
Keywords: CD133, CD24, bladder carcinoma, phenotypes
Introduction
Adult stem cells are rare, highly specialized subsets of cells. They possess the functional and molecular ability of embryonic stem cells and induce pluripotent stem cells to self-renew, differentiate, and regenerate adult tissues.1 Some tumors contain cells that have maintained the self-renewal capacity of non-malignant adult stem cells but have bypassed their growth regulatory pathways.1 These stem cell-like tumor cells are also known as cancer stem cells (CSCs) or tumor initiating cells.2 The ability of these rare undifferentiated tumorigenic cells to self-renew and differentiate into heterogeneous lineages of neoplastic cells is the explicit biological criterion responsible for tumor initiation, maintenance, dissemination, and initiation of relapse.3
The heterogeneous molecular pathogenesis of bladder cancer1 and the frequent recurrence of superficial urothelial carcinoma (UC)2 suggest the contribution of CSCs to their tumorgenicity.4 The escape of CSCs, which retain full capacity to restore the tumor cell bulk, from antitumor treatments may account for the contemporary failure of therapies in this type of cancer.4 Ultimately, understanding the biological behavior of CSCs is crucial for the development of promising CSC‐targeted therapies. In addition, analyzing CSCs within a residual disease is a valuable determinant for therapy efficacy rather than the gross measurement of tumor regression.5
Surface markers are currently used to isolate and sort CSCs. Nevertheless, the lack of well-defined surface markers for distinct tumors hinders progress.3 The CSC marker CD133 was the first to discriminate the stem cell phenotype in solid tumors. Remarkably, the current debate in the CSC field regarding its role has not prohibited its conventional recognition as a marker of stemness in a diverse set of malignancies.6 Transplantation of CD133-expressing CSCs into immunodeficient mice generated a histologically analogous tumor mass with self-renewal potential. The biological function of CD133 has been linked to the fate determination of stem cells and, moreover, their ability to survive viability challenges.6
Formerly recognized as a cell marker for hematopoietic cell lineages (specifically pre-B lymphocytes prior to their maturation to plasma cells),7 CD24 is currently documented as a CSC biomarker8 in various cancers.7–9 Aggressive course and ominous outcome are linked to the CD24+ subset in cancer cell nests.10
CSCs differentiate aberrantly, thus generating heterogeneous growth of tumor bulk and essentially constructing a tumor microenvironment fundamental for their self-maintenance.11 Experimental evidence indicates that CSCs are not exactly identical, as various subpopulations reside within a cancer mass and possess a divergent capacity for tumorigenesis.12 Consecutively, even among histologically identical tumors, the biological characteristics of a tumor will influence the existence and phenotype of CSCs.3
The acquisition of stem-like cell capabilities is “context-dependent”.13 For example, although earlier studies in breast cancer have shown that CD24 overexpression was associated with node spread and advanced pathologic stages, stemness capabilities were found to be associated with CD24 downregulation combined with CD44 expression (ie, CD44+ CD24−).13 Similarly, in a PC1-Expl-1 cell line, Ortiz-Montero et al found that the CD44+ CD24−, and CD44+ CD24+ phenotypes have “dramatically” different tumorigenic capacities.13 They concluded that it seems imperative to associate the presence or absence of CD24 expression with other events responsible for tumor initiation and/or progression.13 In keeping with the same concept, another study found that, in gall bladder carcinoma, a CD44+ CD133+ population possesses CSC-like features and may be “true” CSCs.14,15 Different populations of CSCs in a tumor give it a specific oncogenic phenotype signature, which has a particular stemness capacity; this, in turn, is translated into divergent tumorigenic potential. Meanwhile, both CD24 and CD133 are customarily accepted as markers of stemness in different tumors.6
Hence, the aim of this study was to examine the combined expression of CD133 and CD24 in the same tumors and to analyze, for the first time, the phenotypes of these two specific CSC markers for histopathological stratification of urinary bladder carcinoma by immunohistochemistry.
Materials and methods
Patients and tumor samples
A total of 60 cases were retrieved from the pathology department of Ain Shams Specialized Hospital in 2012 and 2013, according to the availability of slides and blocks with adequate tumor tissue. Data relating to tumor size and clinical parameters, including patient age and gender, were extracted from pathology reports.
Hematoxylin and eosin staining method and diagnostic criteria
Sections of 4 µm in thickness were sliced from formalin-fixed, paraffin-embedded blocks and stained with hematoxylin and eosin. The slides were examined to determine histological tumor type, grade, stage, and evidence of schistosomiasis infection within the tumor or adjacent nontumor tissue. To avoid data fragmentation, cases were segregated as UCs and non-UCs. Pathological grade was defined according to the latest World Health Organization (WHO) criteria, 2016.16 Tumor stage was determined according to the American Joint Committee on Cancer (AJCC) TNM nodal staging system17 depending on the degree of infiltration of the bladder wall layer and extension into the perivesical fat and the adjacent organs. Accordingly, pTa tumors are defined as tumors that do not invade the lamina propria, pT1 tumors grow under the basement membrane into the lamina propria, pT2 tumors invade the muscularis propria, pT3 tumors invade perivesical tissue, and pT4 tumors show direct invasion into the prostatic stroma, seminal vesicles, uterus, vagina, pelvic wall or abdominal wall.
The WHO 2016 system categorizes non-muscle invasive UCs (ie, pTa and pT1) as either low grade or high grade. If different grades are detected in the same lesion, the tumor is graded according to the highest grade noted. Non-invasive papillary low grade UC is characterized by orderly arranged papillae with mild cytological atypia and rare mitosis. In contrast, non-invasive papillary high grade UCs are characterized by a disorderly appearance due to both cytological atypia and architectural disorganization. However, all muscle invasive UCs (pT2-4) are classified as high grade tumors. Non-UC was categorized according to the grade of differentiation into low grade (well/moderately differentiated) and high grade (poorly differentiated).
Immunohistochemical staining
Primary antibodies targeting CD133 (rabbit, polyclonal, diluted 1/100, CP 348, A, B, C BioCare Medical) and CD24 (mouse, monoclonal, diluted 1/100, SN3b, BioCare Medical) were used.
Briefly, sections were deparaffinized, dehydrated, and immersed in methanol. Slides were microwaved for 10 minutes in citrate buffer (pH=6) for antigen retrieval and incubated with primary antibodies overnight at room temperature. Bound antibody was detected by avidin-biotin using diaminobenzidine as the chromogen, followed by nuclear counterstaining with hematoxylin. In each run, positive and negative controls were used throughout. Normal kidney tissues and breast carcinoma tissues were used as the appropriate positive controls for CD133 and CD24 antibodies, respectively.
Evaluation of CD133 and CD24
Analysis was independently performed using computerized Image Analysing Software (Special SIS starter, version 3.2, Olympus Corporation, Tokyo, Japan) connected to an Olympus microscope (model BX51) by three pathologists blinded to the patients’ clinical and pathological information.
Cells showing a membranous and/or cytoplasmic staining pattern were considered positive. The percentage of positively stained cells, with unequivocal strong to moderate staining intensities, was determined in ten medium-power fields. Tumor cells were evaluated in the most prominent staining areas. Since calculation of the cut-off values by receiver operating characteristic analysis is less relevant,18,19 the median percentage of positively stained tumor cells for each marker was chosen as the cut-off value to categorize cases into low and high expression. The median cut-off percentages of positive CD133 and CD24 expression were 50% and 10%, respectively.
Statistical analysis
Continuous variables were expressed as the mean and SD (± SD). Categorical variables were expressed as frequencies and percentages and compared using the chi-squared test or Fisher’s exact test. Spearman’s correlation was used as appropriate. A P-value (level of significance) >0.05 was designated non-significant and <0.05 was designated significant. Vassar Stats and IBM SPSS statistics (version 21.0, IBM Corporation, Armonk, NY, USA) were used for data analysis.
Compliance with ethical standards
This research complied with all the relevant national regulations and institutional policies. It is in accordance with the tenets of the Helsinki Declaration. The study was approved by the Research Ethics Committee, Faculty of Medicine, Ain Shams University (FMASU 870/2010). Patient data confidentiality and anonymity were maintained. This is a retrospective study that used paraffin blocks retrieved from the archives of the Department of Pathology and followed strict regulations that guarantee the patients’ rights and welfare. Accordingly, this research carries no risk of harm to patients and will not affect their rights or welfare. Hence, the Institutional Review Board waived patient consent.
Results
Clinicopathological characteristics
The patients’ mean age (± SD) was 57 (±7.43) years with a male to female ratio of 4:1.
Histopathological examination revealed 50 UC and ten non-UC carcinoma (squamous cell carcinoma (n=8) and adenocarcinoma (n=2)) cases. Thirty-seven percent of the tumors were low grade, and 63% were high grade. Twenty-seven percent of the tumors were non-muscle invasive, and 73% were muscle invasive tumors. Seventeen percent of the tumors were associated with schistosomiasis. Lymphovascular invasion (LVI) and perineural invasion were observed in 10% and 7% of the patients, respectively. Nodal spread and distant metastasis occurred in 12% and 8% of the patients, respectively. The patients’ clinicopathological characteristics are shown in Table 1.
 | Table 1 Clinicopathological characteristics of the studied population (n=60) |
Immunohistochemical expression and cellular distribution of CD133 and CD24
CD133 was expressed in carcinoma cells in 22 (36.67%) out of 60 cases. CD133 protein expression was categorized as low (<50%) or high (≥50%) in 12 and ten cases, respectively.
CD24 was expressed by carcinoma cells in 17 cases (28.3%), and the staining pattern was categorized as low (<10%) or high (≥10%) in seven and ten cases, respectively.
Association of CD133 and CD24 expression with clinicopathological parameters of bladder cancer
Statistical analysis revealed a nonsignificant correlation between all clinicopathological parameters and the diverse patterns of expression (high vs low) of CD133 and CD24. Accordingly, the presence or absence of CD133+ and CD24+ cells was correlated with the studied clinicopathological parameters.
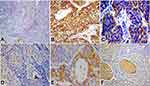
In urothelial and non-urothelial bladder cancer, the presence of CD133+ and CD24+ tumor cells was more frequent in high grade, less differentiated carcinomas (18/22, and 15/17, respectively) than in low grade, well to moderately differentiated carcinomas (p=0.022 and 0.01, respectively) (Table 2, Figure 1). The expression of CD133 and CD24 was frequent in all tumors invading the muscle (20/22, 90.9% p=0.017 and 17/17, 100%, p=0.001, respectively) (Table 2, Figure 2). Similarly, in UC, the prevalence of CD133 and CD24 tumor cells increased in non-papillary high grade US (n=16/18, %, and 13/13, 100%, respectively) compared to papillary low grade UC (p=0.016 and 0.002, respectively) (Figures 1 and 2). Advanced stage was significantly associated with CD133+ and CD24+ tumor cells (p=0.001 and 0.007, respectively) (Table 2). Although neither CD133 nor CD24 expression affected lymph node spread, only CD24 expression was weakly correlated with LVI (p=0.04) (Table 2, Figure 3). On the other hand, only CD133 expression was associated with distant metastasis. Five (8.3%) patients developed distant metastasis at different time intervals after complete resection, and this was strongly associated with CD133 expression in the primary tumor (n=5/5, 100%, p=0.004) (Table 2). The patient’s age, gender, histological tumor type, associated bilharzia infestation, and perineural invasion did not influence the presence of either CD133+ or CD24+ tumor stem cells (Table 2).
 | Table 2 Correlation of CD133 and CD24 expression with clinicopathological parameters |
Correlation between CD133 and CD24 expression
The pattern of CD133 expression showed a significant positive correlation with the pattern of CD24 expression (r=0.4337, p=0.00054, 95% CI, 0.202–0.619). In cases with coexpression of CD133 and CD24 (n=10), the CD133+ high/CD24+ high and CD133+ low/CD24+ low patterns were expressed in 80% (n=8/10) and 20% (n=2/10) of the cases, whereas CD133+ high/CD24+ low and CD133+ low/CD24+ high patterns were not detected. The CD133−/CD24− pattern was detected in 51.6% (n=31/60) of cases (Table 3).
 | Table 3 Correlation between CD133 and CD24 expression |
Association between CD133/CD24 phenotypes and other clinical characteristics
To investigate the relationship between CD133/CD24 expression and clinicopathological parameters, tumors were categorized according to their CD133 and CD24 status. This classification yielded four different phenotypic groups: CD133+/CD24+ (10/60, 16.6%), CD133+/CD24− (12/60, 20%), CD133−/CD24+ (7/60, 11.6%), and CD133−/CD24− (31/60, 51.6%) (Table 4). Statistical analysis identified the following factors that were associated with CD133/CD24 status: histological grade, LVI, lymph node spread, muscle invasion, distant metastasis, and tumor stage in all bladder carcinomas and pattern of growth and grade in UCs. All carcinomas with the CD133+/CD24+ phenotype were high grade (n=10/10, 100%, p=0.006), including high grade non-papillary UC (n=9/9, 100%, p=0.01). Moreover, in these tumors, the incidence of the CD133+/CD24− phenotype was greater than that of the CD133−/CD24+ phenotype. The CD133+/CD24+ phenotype, compared with the other phenotypes, exhibited increased LVI (n=4/6, 66.6%, p=0.0004) and lymph node spread (n=4/7, 57%, p=0.01). In addition, CD133−/CD24+ was associated with LVI in 33.3% (n=2/6) of cases, whereas CD133+/CD24− cases were never associated with LVI. The CD133+/CD24+ phenotype and CD133+/CD24− phenotype exhibited similar increases in muscle invasion (n=10/44, 22.7%, p=0.005) and distant metastasis (n=2/5, 40%, p=0.03). The CD133+/CD24− phenotype, compared with either the CD133+/CD24+ or CD133−/CD24+ phenotypes, was frequently observed in tumors with advanced tumor stages, ie, stage III and IV, p<0.0001.
 | Table 4 Association between CD133/CD24 phenotypes and other clinical characteristics |
Discussion
Early research has indicated that CSCs are a rare population.20 It is currently unknown whether all cancers contain subpopulations of CSCs.20 Herein, CD133 and CD24 were identified in 44% and 28.3% of the tumor tissues tested, respectively. Accumulating evidence has confirmed that a stem cell hierarchy is not applicable to all tumors. Tumor type and cell of origin together with the oncogenic events driving “stemness” may indeed determine the absolute frequency of CSCs.21 CSCs, as hypothesized, originate from normal stem cells/progenitor cells,22 whereas oncogenic mutations ensure their uncontrolled proliferation.23 These oncogenic mutations either prevent normal stem cells from entering the postmitotic differentiated state or counteract antigrowth-constraining signals,22,24 thereby creating a pool of self‐renewing cells with a further accumulation of mutations.4
Although CD133− CSCs retained stem-like features in primary glioblastomas in nude mice,25 only the CD133+ subpopulation fulfilled the criteria consistent with CSCs in the J82 human bladder cancer cell line.26 In the present study, CD133+ cells were frequently associated with high grade/less differentiated tumors, including non-papillary high grade UC. The number of CD133+ cells was increased in all tumors that invaded the muscle and persisted with advancement of tumor stage. Furthermore, CD133+ cells resided in the primary tumor of all patients who developed distant metastasis. In urinary bladder carcinoma, tumor staging and grading are the paramount prognostic indicators signifying a high risk of local invasion and distant metastasis,27 specifically in tumors that have invaded the muscle layer.10 Our results suggest a potent tumorigenic potential of the CD133+ subset and are consistent with the aggressive in vitro proliferation capacity of these cells.26 Another study that found a nonsignificant correlation between CD133 and clinicopathological parameters is seemingly conflicting,19 but the diverse study methodology may conceptualize the contradictions. In a tissue microarray, the sampled small cores may not represent the whole tumor, particularly with the complexity of clonal evolution driving the “stemness” of malignancy.3,21
CD24 is another putative marker to isolate CSCs from solid tumors.8 Similar to CD133+ CSCs, CD24+ CSCs were frequent in high grade muscle invasive carcinomas with advanced stage. A meta-analysis of the studies on the association between CD24 expression and prognostic factors in diverse carcinomas published in 1990–2009 determined that CD24 is a significant marker of malignancy and poor prognosis in numerous cancers.7 Specifically, CD24 may endorse cancer development and progression in ovary, breast, and urinary bladder. Overall, CD24 significantly correlated with lymph node metastasis (OR=2.41; CI, 1.013–5.720; P=0.047), advanced clinical stages (OR=1.59; 95% CI, 1.244–2.032; P<0.001), and shortened overall survival (HR=2.13; 95% CI, 1.656–2.730; P<0.001). In UC, CD24 expression was highly correlated with advanced clinical stages (OR=2.22; 95% CI, 1.442–3.418; P<0.001) and LVI (OR=2.78; 95% CI, 1.522–5.068; P=0.001).7
Overall, CD133 and CD24 were expressed in tumor cells of bladder carcinoma and correlated with determinants of tumor behavior. The preferential expression of these two putative CSC markers in certain tumors is consistent with Bomken’s suggestion.3 The biological characteristics of histologically identical tumors are the driving force influencing the phenotype of CSCs and, furthermore, the absence or presence of these CSCs within a specific cancer.3
Herein, immunohistochemical evaluation demonstrated that CD133+ and CD24+ cells, although expressed in biologically identical tumors, are a heterogeneous population. Previous studies have revealed that distinct populations of CSCs, with miscellaneous genetic anomalies and tumorigenic potentials, reside within the same tumor and display different, sometimes non-overlapping, sets of markers.4,25,28–31 The heterogeneity between CD133+ and CD24+ cells is intriguing, especially given that CD133 is significantly correlated with CD24 and the CD133+ CD24− phenotype (20%) is more frequent than the CD133+ CD24+ phenotype (16.6%). These findings provide conjectures for inter-conversion between CD133 and CD24 CSCs in urinary bladder carcinoma and support the existence of phenotypic plasticity in tumor cell populations, as proposed by Gupta et al.32 Moreover, upregulation of the CD24+ subpopulation, similar to the populations expressing the pluripotent stem cell markers Oct-4 and Sox-2,26 is likely linked to the CD133+ subset of cells. The recently anticipated model validating plasticity between stem cells and their committed progeny may provide us with empirical support.32 Such plasticity creates a dynamic equilibrium that may be shifted in one direction or another to generate unstable, epigenetic, or intrinsic genetic differences between tumorigenic and non‐tumorigenic cells within the same tumor.4,33,34
Although the search for CSCs can pull up different markers,20 an optimal combination of markers has not yet been established to ascertain CSCs in relation to invasion, metastasis, and therapeutic resistance in a certain tumor.8 The genetic heterogeneity and different mutational patterns of bladder carcinoma are likely adjoined by the heterogeneous complexity of CSCs, which bolsters the prognostic significance of different CSC phenotypes.24 While injection of a few breast CSCs with the ESA+/CD44+/CD24low-neg phenotype into NOD/SCID mice reinitiated the original tumor, 10,000 cells with alternate phenotypes failed.24,34 Several phenotypes have been proposed in other cancers (eg, CD44+/CD24low-neg phenotype in breast cancer) but not in urinary bladder carcinoma. For the first time, the present study addressed the clinical characteristics of different CD133/CD24 phenotypes. In summary, our comparative analysis may provide initial insights into the potential impact of each marker.
The presence of CD133+ and/or CD24+ cells in the nests of biologically violent tumors theoretically rationalizes the presence of the CD133+/CD24+ phenotype in aggressive tumorigenicity. In all high grade, less differentiated tumors, including non-papillary high grade UC, the incidence of the CD133+/CD24− phenotype was higher than that of the CD133−/CD24+ phenotype, although the CD133+/CD24+ CSC phenotype was the most frequent. This finding, in turn, may suggest a stronger deleterious tumorigenic potential for CD133+ than CD24+ subpopulations distressing tumor growth. The aggressive growth of tumors after subcutaneous transplantation of CD133+ subsets of J82 cancer cells into nude mice is in accordance with our work.23 Comparatively, the CD133+/CD24− phenotype, in contrast with CD133+/CD24+ and CD133−/CD24+ phenotypes, was frequently associated with advanced tumor stages. This finding ultimately supports our prior hypothesis associating the CD133+ subset with more aggressive behavior. It is hypothesized that CD133 has an inherent effect on the attachment of stem cells to their niche35,36 and an ability to fine-tune tumor biology by controlling diverse cellular processes, eg, glucose and transferrin uptake, autophagy, membrane-membrane interaction, and matrix metalloproteinase functions.24 Accordingly, several CD133+ solid tumors in other systems have poor clinical outcomes and shorter overall survival.6
CD24 is a ligand of an adhesion receptor present on activated endothelial cells and platelets.10 Accordingly, CD24+ tumor cells theoretically can form tumor thrombi and advance toward metastasis.10 Herein, CD24 did not correlate with lymph node metastasis and showed a borderline correlation with LVI. Studies that exist lack a rationale for why there is a nonsignificant correlation between CD24 and either LVI or lymph node metastasis.10 However, following a brief review of the existing evidence, the intriguing occurrence of LVI and lymph node spread in tumors exhibiting the CD133+/CD24+ phenotype supports the notion of behavioral intentions between the CD133+ and CD24+ subsets. First, in human brain cancer, CD133+ CSCs reside within a perivascular niche, diligently interact with endothelial cells,33 and secrete very high levels of VEGF.37 Neutralizing VEGF with specific antibodies37 or inhibiting its signaling36 reduced the abundance of CD133+ CSCs, inhibited its in vivo self-renewal capacity, and substantively ameliorated tumor xenograft growth.36
By binding to endothelial cells, CD24 may predominantly affect cell–cell interactions and adhesions,38 thus promoting metastatic seeding, whereas CD133 may be more involved in cell survival.39 Additionally, the significant correlation between CD133 and CD24 cell nests may support the possible engagement of CD133+ subsets in the intracellular signaling of the CD24+ population in relation to other stemness gene pathways through which CD133 expression controls tumorigenesis, eg, the Akt/PKB and Bcl-2 pathways, the Akt and MAPK pathways, Ras and its downstream effectors, OCT4, and/or SOX2.39,40 Finally, while the CD133−/CD24+ phenotype, although less frequent than the CD133+/CD24+ phenotype, was associated with LVI, the CD133+/CD24− phenotype was not associated with LVI. This observation may strengthen the causal inference describing CD24 as a “lynchpin” of metastatic progression and colonization rather than just a mediator of endothelial cell adhesion.41
The similar association of the CD133+/CD24+ and CD133+/CD24− phenotypes with distant metastasis may further confer the metastatic potential of CD133+ to their progenies. Several in vitro and in vivo studies have shown that CD133+ CSCs acquire their metastatic potential through NF-kappa B and matrix metalloproteinase secretion.39,42–44 Additionally, CD133+/CD44+ cancer cells have been characterized in several highly metastatic tumors, and CD133+/CXCR4+ cancer cells undergo epithelial-mesenchymal transitions in vitro and in vivo with high metastatic capacities.39,45 Additionally, the analogous association between the CD133+ subset with distant metastasis and the CD24+ subset with lymph node metastasis supports the biological diversity between lymphatic spread and hematogenous dissemination.10
Indeed, the CD133+/CD24+ and CD133+/CD24− phenotypes, but not the CD133−/CD24+ phenotype, were almost equally associated with muscle invasion and distant metastasis. In breast cancer cell lines, during tumor initiation, CD44+/CD24+ cells are suggested to engender CD44+/CD24− cells, and CD24+ cells originate from CD44+ cells. The genetic similarity between CD24+ and CD44+ cells provides supporting evidence for this progression.8 Based on the same concept, it is possible that in human urinary bladder cancer, CD133+/CD24+ cells differentiated to CD133+/CD24− cells during the tumor invasion-metastasis process. This observation may provide additional support to the “plasticity” between CD133+ and CD24+ CSCs and, moreover, enforce the influential regulation of CD133+ CSCs, even if the underlying mechanism is still ambiguous. The distinction of the molecular signaling between CD133+ subsets and the CD24+ and/or CD24− subpopulation may have important relevance to pinpoint the biological functions and effectors of CD133, thereby stirring the continuing debate over CD133 as a stem cell biomarker. It is possible that factors that are upregulated in the CD133+ subset support the genomic DNA molecular scaffold in stem cells through activation of the DNA repair complex,27 and are thus linked to radioresistance due to amplified DNA repair following irradiation.26
“Cancer stem cells are hard to identify because a practical operating definition has never been achieved”, says Scott Kern.20 Studies analyzing surface markers to isolate CSCs, such as CD133 and CD24, are contradictory even among the same type of carcinomas.7 We believe that the diversity in evaluating the immunoexpression of these surface markers created these controversies. Although scientists disagree over how a small and distinct population of cells can enhance tumors, they tend to agree that the assays to discern such cells “can be lousy”.20 Various cut-off levels of the same marker have been proposed among multiple studies. Moreover, some studies have used the cut-off to define expression positivity, while other studies used the same cut-off value to segregate cases into low vs high expression. Moreover, in some reports, the immunoreactivity was described as the percentage of tumor cells with positive expression, while other studies described only the staining intensity. Even research that evaluated the percentage of area stained at each intensity level sub-classified the study cohorts differently. The isolation of CSCs is still an enduring ambiguity necessitating a valid method for evaluating their immunoexpression. These variations yielded distinct results vis-à-vis the relevance of a single marker to clinical disease in the respective malignancies. Standardization of the analytic methodology for various surface markers will undoubtedly enhance the prospective characterization and isolation of CSCs in human malignancies.
The Cancer Genome Atlas (TCGA) consortium and individual research groups have recently proposed several genome-based molecular classifications of UC (ie, University of North Carolina, MD Anderson Cancer Center, TCGA, Lund University, Baylor College Of Medicine, the CIT Classification, and others).46 However, these classifications still have several limitations that prevent their current integration into clinical practice.46,47 Sequentially, detection of CSCs in a tumor is complex because CSCs seem to have extreme heterogeneity with a variable range of phenotypes in each cancer type. Identification of the main niches and pure CSC subsets is still ambiguous.48,49 CSC research and molecular classification of a tumor, on their own, herald promise to improve clinical management. Therefore, both are current areas of active investigation. Parallel advances in both fields will definitely improve patient management in multiple aspects, eg, the selection and combinations of tailored therapies. Knowledge incorporating the CSC hypothesis with molecular classifications may translate to better patient outcomes and survival. However, so far, data are still missing, and many questions remain unanswered.
In conclusion, CD133+ and CD24+ cells reside more frequently in high grade, less differentiated bladder carcinomas and are correlated with determinants of aggressive behavior. Although the CD133+ and CD24+ populations were phenotypically divergent, their expression showed a strong correlation. The results support phenotypic plasticity between CD133+ and CD24+ cells; however, the CD133+ subpopulation is likely to have more aggressive tumorigenic potential. The CD133+/CD24+ phenotype exhibited more aggressive tumorigenicity with more advanced tumor stage and distant metastasis. LVI and lymph node metastasis are almost associated with tumors co-expressing the CD133+/CD24+ phenotype. Although CD24 is a “lynchpin” of metastatic progression, CD133+ cells may be involved in CD24+ cellular interactions during LVI. During the tumor invasion-metastasis cascade, it seems that CD133+/CD24+ cells differentiated to the CD133+CD24− phenotype, further supporting the phenotypic plasticity phenomenon. The distinction of the molecular signaling between CD133+ subsets and CD24+ and/or CD24− subpopulations may have important biological relevance to overcome therapeutic resistance and tumor recurrence. The CD133 and CD24 molecules, as markers of a putative CSC population, need to be exploited in the clinical setting on a larger scale with further molecular and functional studies to identify high-risk patients for more aggressive therapy, and to ultimately provide therapeutic targets against refractory bladder cancer.
Highlights
- The rare population of CD133+ CD24+ CSCs are common in biologically aggressive, high grade, less differentiated bladder carcinomas.
- Although phenotypically divergent, the expression levels of CD133+ and CD24+ are strongly correlated, but tumorigenic potential is likely more aggressive in CD133+ tumors.
- CD133+ CD24+ tumors have the most aggressive phenotype associated with advanced stage and distant metastasis.
- Although CD24 is a “lynchpin” of metastatic progression, CD133+ cells may aid CD24-mediated cellular interactions during LVI.
Data availability
Dataset reference: Farid, Rola M; Sammour, Sanaa; Shehab El-Din, Zeinab; Salman, Manal; Omran, Tag (2018), “Evaluation of CD133 and CD24 cancer stem cells and their different phenotypes in bladder carcinoma.”, Mendeley Data, v1http://dx.doi.org/10.17632/xj82cmj29g.1DOI
Ethics statement
The study was approved by the Research Ethics Committee, Faculty of Medicine, Ain Shams University (FMASU 870/2010), and patient consent was waived because there is no risk of harm to patients, and this study will not affect their rights and welfare.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Part of this work has been presented at XXXII International Academy of Pathology Congress, 14–18 October 2018, Amman, Jordan.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kashyap V, Rezende NC, Scotland KB, et al. Regulation of stem cell pluripotency and differentiation involves a mutual regulatory circuit of the NANOG, OCT4, and SOX2 pluripotency transcription factors with polycomb repressive complexes and stem cell microRNAs. Stem Cells Dev. 2009;18(7):1093–1108. doi:10.1089/scd.2009.0113
2. Marcinkiewicz K, Scotland KB, Boorjian SA, et al. The androgen receptor and stem cell pathways in prostate and bladder cancers (review). Int J Oncol. 2012;40(1):5–12. doi:10.3892/ijo.2011.1212
3. Bomken S, Fiser K, Heidenreich O, Vormoor J. Understanding the cancer stem cell. Rev Br J Cancer. 2010;103(4):439–445. doi:10.1038/sj.bjc.6605821
4. Bentivegna A, Conconi D, Panzeri E, et al. Biological heterogeneity of putative bladder cancer stem-like cell populations from human bladder transitional cell carcinoma samples. Cancer Sci. 2010;101(2):416–424. doi:10.1111/j.1349-7006.2009.01414.x
5. Blagosklonny MV, Target for cancer therapy: proliferating cells or stem cells. Leukemia. 2006;20(3):385–391. doi:10.1038/sj.leu.2404075
6. Glumac PM, LeBeau AM. The role of CD133 in cancer: a concise review. Clin Transl Med. 2018;7(1):18. doi:10.1186/s40169-018-0198-1
7. Lee JH, Kim SH, Lee ES, Kim YS. CD24 overexpression in cancer development and progression: a meta-analysis. Oncol Rep. 2009;22(5):1149–1156.
8. Jaggupilli A, Elkord E. Significance of CD44 and CD24 as cancer stem cell markers: an enduring ambiguity. Clin Dev Immunol. 2012;2012:708036. doi:10.1155/2012/708036
9. Choi D, Lee HW, Hur KY, et al. Cancer stem cell markers CD133 and CD24 correlate with invasiveness and differentiation in colorectal adenocarcinoma. World J Gastroenterol. 2009;15(18):2258–2264.
10. Choi YL, Lee SH, Kwon GY, et al. Overexpression of CD24: association with invasiveness in urothelial carcinoma of the bladder. Arch Pathol Lab Med. 2007;131(2):275–281. doi:10.1043/1543-2165(2007)131[275:OOCAWI]2.0.CO;2
11. Meacham CE, Morrison SJ. Tumor heterogeneity and cancer cell plasticity. Nature. 2013;501(7467):328–337. doi:10.1038/nature12624
12. Liu HG, Zhang XH. How to estimate cancer stem cell frequency correctly. Asian Pac J Cancer Prev. 2009;10(4):711–714.
13. Ortiz-Montero P, Liu-Bordes WY, Londoño-Vallejo A, Vernot JP. CD24 expression and stem-associated features define tumor cell heterogeneity and tumorigenic capacities in a model of carcinogenesis. Cancer Manag Res. 2018;10:5767–5784. doi:10.2147/CMAR.S176654
14. Shi C, Tian R, Wang M, et al. CD44+ CD133+ population exhibits cancer stem cell-like characteristics in human gallbladder carcinoma. Cancer Biol Ther. 2010;10(11):1182–1190.
15. He Y, Xue C, Yu Y, et al. CD44 is overexpressed and correlated with tumor progression in gallbladder cancer. Cancer Manag Res. 2018;10:3857–3865. doi:10.2147/CMAR.S175681
16. Humphrey PA, Moch H, Ulbright TM, Reuter VE. The 2016 WHO classification of tumors of the urinary system and male genital organs- part b.Prostate and bladder tumors. Eur Urol. 2016;70(1):106–119. doi:10.1016/j.eururo.2016.02.028
17. Amin MB, Edge SB, Greene FL, et al. AJCC Cancer Staging Manual.
18. Saigusa S, Tanaka K, Toiyama Y, et al. Correlation of CD133, OCT4, and SOX2 in rectal cancer and their association with distant recurrence after chemoradiotherapy. Ann Surg Oncol. 2009;16(12):3488–3498. doi:10.1245/s10434-009-0617-z
19. Edaghat S, Gheytanchi E, Asgari M, Roudi R, Keymoosi H, Madjd Z. Expression of cancer stem cell markers OCT4 and CD133 in transitional cell carcinomas. Appl Immunohistochem Mol Morphol. 2017;25(3):196–202. doi:10.1097/PAI.0000000000000291
20. Bake M. Cancer stem cells, becoming common. Nat Rep Stem Cells. 2008. doi:10.1038/stemcells.2008.153
21. Heuser M, Sly LM, Argiropolous B, et al. Modeling the functional heterogeneity of leukaemia stem cells: role of STAT5 in leukaemia stem cell self-renewal. Blood. 2009;114(19):3983–3993. doi:10.1182/blood-2009-04-215525
22. Ayob AZ, Ramasamy TS. Cancer stem cells as key drivers of tumor progression. Rev J Biomed Sci. 2018;25(1):20. doi:10.1186/s12929-018-0426-4
23. Sell S. On the stem cell origin of cancer. Am J Pathol. 2010;176(6):2584–2594. doi:10.2353/ajpath.2010.091064
24. Baccelli I, Trumpp A. The evolving concept of cancer and metastasis stem cells. Rev J Cell Biol. 2012;198(3):281–293. doi:10.1083/jcb.201202014
25. Beier D, Hau P, Proescholdt M, et al. CD133(+) and CD133(−) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007;67(9):4010–4015. doi:10.1158/0008-5472.CAN-06-4180
26. Huang P, Watanabe M, Kaku H, et al. Cancer stem cell-like characteristics of a CD133(+) subpopulation in the J82 human bladder cancer cell line. Mol Clin Oncol. 2013;1(1):180–184. doi:10.3892/mco.2012.29
27. Herr HW. Tumor progression and survival of patients with high grade, noninvasive papillary (TaG3) bladder tumors: 15-year outcome. J Urol. 2000;163(1):60–62. doi:10.1016/S0022-5347(05)67972-4
28. Chan KS, Espinosa I, Chao M, et al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor‐initiating cells. PNAS. 2009;106(33):14016–14021. doi:10.1073/pnas.0906549106
29. Piccirillo SGM, Combi R, Cajola L, et al. Distinct pools of cancer stem‐like cells coexist within human glioblastomas and display different tumorigenicity and indipendent genomic evolution. Oncogene. 2009;28(15):1807–1811. doi:10.1038/onc.2009.27
30. Botchkina IL, Rowehl RA, Rivadeneira DE, et al. Phenotypic subpopulations of metastatic colon cancer stem cells: genomic analysis. Cancer Genomics Proteomics. 2009;6(1):19–29.
31. Günther HS, Schmidt NO, Phillips HS, et al. Glioblastoma‐derived stem cell‐enriched cultures form distinct subgroups according to molecular and phenotypic criteria. Oncogene. 2008;27(20):2897–2909. doi:10.1038/sj.onc.1210949
32. Gupta PB, Chaffer CL, Weinberg RA. Cancer stem cells: mirage or reality? Rev Nat Med. 2009;15(9):1010–1012. doi:10.1038/nm0909-1010
33. Shackleton M, Quintana E, Fearon ER, Morrison SJ. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell. 2009;138(5):822–829. doi:10.1016/j.cell.2009.07.042
34. Al‐Hajj M, Wicha MS, Benito‐Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100(7):3983–3988. doi:10.1073/pnas.0530291100
35. Wu Y, Wu P. CD133 as a marker for cancer stem cells: progresses and concerns. Stem Cells Dev. 2009;18(8):1127–1134. doi:10.1089/scd.2008.0338
36. Calabrese C, Poppleton H, Kocak M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11(1):69–82. doi:10.1016/j.ccr.2006.11.020
37. Bao S, Wu Q, Sathornsumetee S, et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66(16):7843–7848. doi:10.1158/0008-5472.CAN-06-1010
38. Friederichs J, Zeller Y, Hafezi-Moghadam A, Grone H-J, Ley K, Altevogt P. The CD24/P-selectin binding pathway initiates lung arrest of human A125 adenocarcinoma cells. Cancer Res. 2000;60(23):6714–6722.
39. Li Z. CD133: a stem cell biomarker and beyond. Exp Hematol Oncol. 2013;2(1):17. doi:10.1186/2162-3619-2-17
40. Ma S, Lee TK, Zheng BJ, Chan KW, Guan XY. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene. 2008;27(12):1749–1758. doi:10.1038/sj.onc.1210811
41. Overdevest JB, Thomas S, Kristiansen G, Hansel DE, Smith SC. Theodorescu D.CD24 offers a therapeutic target for control of bladder cancer metastasis based on a requirement for lung colonization. Cancer Res. 2011;71(11):3802–3811. doi:10.1158/0008-5472.CAN-11-0519
42. Kohga K, Tatsumi T, Takehara T, et al. Expression of CD133 confers malignant potential by regulating metalloproteinases in human hepatocellular carcinoma. J Hepatol. 2010;52(6):872–879. doi:10.1016/j.jhep.2009.12.030
43. Zhang M, Liu Y, Feng H, et al. CD133 affects the invasive ability of HCT116 cells by regulating TIMP-2. Am J Pathol. 2013;182(2):565–576. doi:10.1016/j.ajpath.2012.10.015
44. Long H, Xie R, Xiang T, et al. Autocrine CCL5 signaling promotes invasion and migration of CD133+ ovarian cancer stem-like cells via NF-kappaB-mediated MMP-9 upregulation. Stem Cells. 2012;30(10):2309–2319. doi:10.1002/stem.1194
45. Zhang SS, Han ZP, Jing YY, et al. CD133+CXCR4+ colon cancer cells exhibit metastatic potential and predict poor prognosis of patients. BMC Med. 2012;10:85. doi:10.1186/1741-7015-10-85
46. Lerner SP, McConkey DJ, Hoadley KA, et al. Bladder cancer molecular taxonomy: summary from a consensus meeting. Bladder Cancer. 2016;2(1):37–47. doi:10.3233/BLC-150037
47. McConkey DJ, Choi W. Molecular subtypes of bladder cancer. Curr Oncol Rep. 2018;20(10):77. doi:10.1007/s11912-018-0727-5
48. Polyak K. Heterogeneity in breast cancer. J Clin Invest. 2011;121(10):3786–3788. doi:10.1172/JCI60534
49. Prieto-Vila M, Takahashi RU, Usuba W, et al. Drug resistance driven by cancer stem cells and their niche (review). Int J Mol Sci. 2017;18(12) pii:E2574. doi:10.3390/ijms18122574
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.