Back to Journals » OncoTargets and Therapy » Volume 10
Expression and significance of B7-H3 and Tie-2 in the tumor vasculature of clear cell renal carcinoma
Authors Zhang X, Ji J, Zhang G, Fang C, Jiang F, Ma S, Hou J
Received 22 July 2017
Accepted for publication 6 October 2017
Published 13 November 2017 Volume 2017:10 Pages 5417—5424
DOI https://doi.org/10.2147/OTT.S147041
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr XuYu Yang
Xianyun Zhang,1,2 Jindong Ji,1 Guangbo Zhang,3 Chuntao Fang,4 Fujin Jiang,2 Song Ma,2 Jianquan Hou1
1Department of Urology, First Affiliated Hospital of Soochow University, Suzhou, 2Department of Urology, The Affiliated Huai’an Hospital of Xuzhou Medical University and The Second People’s Hospital of Huai’an, Huai’an, 3Clinical Immunology Laboratory, First Affiliated Hospital of Soochow University, Suzhou, 4Department of Gynaecology, The Affiliated Huai’an Hospital of Xuzhou Medical University and The Second People’s Hospital of Huai’an, Huai’an, Jiangsu, China
Abstract: Tumor angiogenesis is required for tumor growth and metastasis, and the Ang/Tie-2 axis plays a pivotal role in angiogenesis. B7-H3, a new member of the B7 family of costimulatory molecules, has a critical function in the T-cell-mediated antitumor immune response, and abnormal tumor B7-H3 expression is frequently associated with a poor prognosis. However, the relationship between B7-H3 and angiogenesis in clear cell renal carcinoma (ccRCC) remains unclear. In this study, we used immunohistochemical methods to detect tumor vascular expression of B7-H3 and Tie-2 in tissue microarrays of 82 ccRCC patient samples. According to the results, B7-H3 is highly expressed in the tumor vascular endothelium of ccRCC and is associated with the ccRCC grade and tumor-node-metastasis (TNM) stage. Although vascular Tie-2 expression was also correlated with T stage and lymph node metastasis, it was not related to ccRCC grade or distant metastasis. The microvessel density (MVD) labeled by CD34 was correlated with tumor grade and TNM stage. Expression of B7-H3 and Tie-2 was positively correlated, and the levels were positively associated with the MVD. Additionally, immunofluorescence staining revealed coexpression of B7-H3 and Tie-2 in the vascular endothelia of ccRCC. Collectively, our findings suggest that expression of B7-H3 and Tie-2 in ccRCC tumor vasculature is closely related to the progression and prognosis of the disease. Furthermore, B7-H3 possibly promotes ccRCC angiogenesis through the Tie-2 pathway. Thus, B7-H3 might serve as an effective endothelial marker for ccRCC prognosis and become a promising target for ccRCC anti-angiogenic-targeted therapy.
Keywords: B7-H3, Tie-2, microvessel density, clear cell renal carcinoma, prognosis, angiogenesis
Introduction
Renal cell carcinoma (RCC) is a malignant tumor originating from renal tubular epithelial cells. RCC accounts for 2%–3% of all malignant tumors; its incidence rate is the third highest among urologic tumors1 and continues to rise in most countries.2 The most common histological type, clear cell renal carcinoma (ccRCC) represents 75%–85% of all RCCs.3 Although early stage renal cancer can be treated effectively with surgery, metastatic renal carcinoma (mRCC) has a poor prognosis and is insensitive to radiotherapy or chemotherapy. Moreover, RCC, especially ccRCC, is characteristically hypervascular, and thus, anti-angiogenic-targeted therapy is implemented in the treatment of mRCC. However, as the current anti-angiogenic therapy targeting vascular endothelial growth factor (VEGF) is not completely satisfactory due to its inherent defects, identification of new targets has become a popular research focus.
Angiogenesis is required for tumor growth and metastasis, processes that are regulated by a variety of vascular factors. In addition to VEGF receptors, newly identified tyrosine kinase receptor Tie-2 binds ligands that are members of the angiopoietin family. Studies have shown that Tie-2 acts independently of VEGF in the regulation of tumor microvascularization, and inhibition of the Tie-2 pathway alone is reported to inhibit tumor growth.4 As an angiogenin receptor, Tie-2 is mainly expressed by endothelial cells; some macrophages and monocytes also express Tie-2, namely Tie-2-expressing monocytes (TEMs). TEMs purified from human tumor specimens promote tumor angiogenesis in transplanted tumors, whereas a lack of TEMs reduces angiogenesis and delays tumor growth.5
B7-H3 is a new member of the B7 family of costimulatory molecules that was first cloned from a human dendritic cell cDNA library by Chapoval et al.6 B7-H3 has the dual function of stimulating and inhibiting T-cell activation, with an important function in the T-cell-mediated antitumor immune response. B7-H3 is overexpressed in a variety of human malignancies and is closely correlated to tumor progression and prognosis.7–12 Currently, the nonimmune roles of B7-H3 are attracting increasing attention. Seaman et al13 showed that B7-H3 is specifically expressed at high levels in the vascular endothelium of various malignant tumor tissues, whereas no expression was found in the normal human vascular endothelium, suggesting that B7-H3 is critical for tumor angiogenesis. In addition, Zang et al14 reported that 93 of 103 cases of ovarian tumor samples expressed B7-H3 molecules and that the tumor vascular endothelial cells of 44% of patients expressed B7-H3, whereby patients with high B7-H3 expression had high recurrence rates and short survival times. Recent studies have also shown that B7-H3 is highly expressed in the blood vessels of ccRCC tissues and is associated with prognosis.15,16 Nonetheless, the relationship between B7-H3 and RCC angiogenesis as well as the underlying mechanism remains unclear. In this study, vascular B7-H3 and Tie-2 expression in ccRCC was evaluated by immunohistochemistry and immunofluorescence. We found vascular B7-H3 and Tie-2 expression to be correlated with the clinicopathological features of ccRCC and the microvessel density (MVD). B7-H3 and Tie-2 were found to be coexpressed in ccRCC blood vessels, indicating that these factors are both involved in the ccRCC angiogenic process. Our study provides a theoretical basis for the use of B7-H3 and Tie-2 as predictive factors for ccRCC prognosis and as targets in anti-angiogenic therapy.
Materials and methods
Sample collection
We collected paraffin-embedded samples of cancer tissues surgically removed from 82 patients with ccRCC. The patients were admitted to and underwent surgery at First Affiliated Hospital of Soochow University from June 2012 to August 2013. The patients were not treated with radiotherapy or chemotherapy prior to the operation. In addition, 20 adjacent normal renal tissues were collected as controls. Tumor grading was performed according to the latest grading standard of the World Health Organization (WHO) and the International Society of Urological Pathology (ISUP) (the four-tiered WHO/ISUP grading system).17 Tumor staging was based on the seventh edition of the American Cancer Joint Committee (AJCC) 2010 Tumor-Node-Metastasis (TNM) staging system. The study protocol was approved by the Academic and Ethics Committee of our hospital, and all patients signed written informed consent forms.
Tissue microarray construction
Tissues embedded in paraffin were sectioned and stained with hematoxylin and eosin (HE), followed by diagnosis by two pathologists. A site with typical pathology was identified in the corresponding paraffin block and marked. The acceptor paraffin block was perforated with a tissue arrayer (Beecher Instruments Inc, Sun Prairie, WI, USA), and a paraffin-embedded tissue core with a diameter of 1.5 mm was taken from the sampling site of the donor paraffin block and implanted into the corresponding hole in the acceptor paraffin block. The tissue array paraffin block was generated according to a predesigned array diagram. A conventional method was used to cut the array into 4-μm-thick continuous sections, which were attached to anti-dewaxing slides and stored at room temperature.
Immunohistochemical staining
The tissue paraffin blocks were continuously sectioned into a 4-μm thickness, dewaxed in xylene, and hydrated in an ethanol gradient. Endogenous peroxidase activity was blocked by incubation in 3% hydrogen peroxide for 20 minutes, and antigen retrieval was conducted by heating the tissue sections at 100°C for 30 minutes in EDTA solution. The sections were incubated in 5% bovine serum albumin (BSA) at room temperature for 30 minutes. Primary antibodies (goat anti-human B7-H3 polyclonal antibody [working concentration 1:400; R&D Systems, Inc., Minneapolis, MN, USA], goat anti-human Tie-2 polyclonal antibody [working concentration 1:100; R&D Systems, Inc.], and sheep anti-human CD34 polyclonal antibody [working concentration 1:100; R&D Systems, Inc.]) were added dropwise, followed by overnight incubation at 4°C. According to the manufacturer’s manual, the secondary antibody was also added dropwise, and the samples were incubated for 30 minutes at room temperature. After adding the streptavidin biotin complex reagent dropwise, the samples were allowed to stand at room temperature for 30 minutes. 3,3′-Diaminobenzidine tetrahydrochloride solution was applied, and the samples were stained with hematoxylin, dehydrated, and clarified. Coverslips were added, and the sections were viewed under a microscope. Known ccRCC tissue sections were used as positive controls; phosphate-buffered saline (PBS) was used as a negative control instead of the primary antibody.
Evaluation of immunohistochemical staining
Two senior pathologists reviewed the sections, and the results were determined in a double-blind manner. Under an optical microscope, blood vessels with positive expression were identified based on brownish to brown staining of the vascular endothelium. Compared to the CD34-labeled vascular endothelium, distribution of B7-H3 and Tie-2 expression in the vascular endothelium of the tumor was categorized as follows: 0, absent; 1, focal expression; 2, moderate expression; and 3, diffuse expression.15 Staining intensity was scored as follows: 0, no staining; 1, weak staining; 2, moderate staining; and 3, strong staining. These two scores were then multiplied to yield the final score. For statistical analysis, sections with a final score of ≤3 were defined as the low-expression group, whereas sections with a final score of >3 were included in the high-expression group.
MVD measurement
In the RCC tissues, isolated brown-yellow vascular endothelial cells or cell clusters were considered to represent a single microvessel. CD34-positive microvessels were observed under low magnification (100×). Regions with the highest microvascular distribution were selected, and the numbers of blood vessels staining brown were counted under high magnification (200×). The mean MVD value from three fields of high magnification (200×) is reported in the results.
Immunofluorescence double labeling and laser confocal scanning microscopy
Samples embedded in paraffin were sectioned and subjected to xylene deparaffinization, gradient ethanol hydration, antigen retrieval, blocking in 5% BSA, and incubation at room temperature (similar to the process described above for immunohistochemistry). After washing with PBS, B7-H3/Tie-2 and B7-H3/CD34 primary antibodies were added for overnight incubation at 4°C. Secondary antibodies labeled with two different fluorescent probes were diluted with PBS, and 100 μL of the corresponding fluorescent secondary antibody was added dropwise to each sample. Alexa Fluor 594 AffiniPure Rabbit Anti-Goat IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA), fluorescein (FITC) AffiniPure Rabbit Anti-Sheep IgG (Jackson ImmunoResearch Laboratories, Inc.), and goat anti-mouse CFL-488 (Santa Cruz Biotechnology Inc., Dallas, TX, USA) were used, and the samples were incubated in the dark at 37°C for 30 minutes. Next, each slide was incubated with 50 μL of 4′,6-diamidino-2-phenylindole added dropwise in the dark for 10 minutes. After anti-fluorescence quenching, coverslips were added, and the samples were observed and scanned in two channels using a laser confocal microscope (Olympus FV1200; Olympus Corporation, Tokyo, Japan). FVl0-ASWl.4 viewer software was used for z stack superimposition and processing.
Statistical analysis
SPSS 21.0 software (IBM Corporation, Armonk, NY, USA) was used for statistical analysis. The relationship between the expression of B7-H3 and Tie-2 and clinicopathological parameters was assessed using the χ2 test or Fisher’s exact test. A correlation test was performed using Spearman’s correlation analysis. MVD data are expressed as the mean ± SD. Comparisons of means between the groups were achieved using Student’s t-test. P<0.05 was considered a significant difference.
Results
Vascular B7-H3 expression and its relationship with clinicopathological features of ccRCC
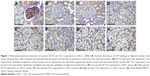
B7-H3 was found to be expressed in the renal tubular epithelia of normal renal tissues but was not expressed in normal glomeruli or renal cortical vessels. In contrast, B7-H3 was overexpressed in ccRCC tissues, especially in tumor vascular endothelial tissues (Figure 1). The expression rate of B7-H3 was 97.56% in the blood vessels of ccRCC, of which 26.83% showed focal expression, 25.61% moderate expression, and 45.12% diffuse expression. The B7-H3 low-expression group accounted for 32.93% of the samples, with the high-expression group comprising 67.07%. Vascular B7-H3 expression was positively correlated with ccRCC histological grade, TNM stage, and lymph node metastasis (P<0.05) but not with gender, age, or tumor size, suggesting an association of B7-H3 overexpression with the degree of malignancy and progression of ccRCC and thus indicating a poor prognosis (Table 1).
  | Table 1 Vascular B7-H3 and Tie-2 expressions in ccRCC and correlations with clinicopathological parameters |
Vascular Tie-2 expression and its relationship with the clinicopathological features of ccRCC
Regarding Tie-2, we observed expression in renal tubular tissue and the glomerular vascular endothelium of normal renal tissues; in ccRCC tissues, it was mainly expressed in the tumor vascular endothelia (Figure 1). Overall, 58.54% of the ccRCC blood vessels exhibited high Tie-2 expression, with 41.46% having low expression. Vascular Tie-2 expression was not related to gender, age, tumor size, or ccRCC grade but was positively correlated with tumor stage and lymph node metastasis (P<0.05). These findings suggest that Tie-2 might predict tumor invasion and metastasis but that it is not related to tumorigenesis or the degree of malignancy (Table 1).
Correlation between vascular B7-H3 and Tie-2 expression in ccRCC
Among the 82 ccRCC cases examined, 38 (46.34%) were positive for vascular expression of both B7-H3 and Tie-2 expression; 17 cases (20.73%) were negative for both. Therefore, vascular B7-H3 and Tie-2 expression were positively correlated (r=0.306, P=0.005). As vascular expression of B7-H3 in ccRCC was positively correlated with that of Tie-2, these factors might have synergistic roles in tumor angiogenesis and B7-H3 might regulate angiogenesis through the Tie-2 pathway (Table 2).
  | Table 2 Correlation between B7-H3 and Tie-2 expression in ccRCC |
Correlations of MVD with the clinicopathological features of ccRCC
The MVD labeled by CD34 did not correlate with gender, age, tumor size, or ccRCC grade but were positively correlated with tumor stage, lymph node metastasis, and distant metastasis (P<0.05). These findings suggest that MVD might predict tumor invasion and metastasis in ccRCC (Table 3).
  | Table 3 MVD in ccRCC and correlations with clinicopathological parameters |
Correlations of B7-H3 and Tie-2 with MVD
MVD was significantly greater for the B7-H3 high-expression group compared to the low-expression group (P=0.009) and also for the Tie-2 high-expression group than the low-expression group (P=0.002) (Table 4). These results suggest that B7-H3 and Tie-2 are both involved in ccRCC tumor angiogenesis.
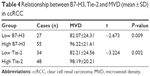  | Table 4 Relationship between B7-H3, Tie-2 and MVD (mean ± SD) in ccRCC |
Immunofluorescence detection of B7-H3 and Tie-2 expression in the vascular endothelium of ccRCC
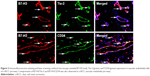
Positive fluorescent signals of immunofluorescence double labeling were observed in green and red channels by laser scanning confocal microscopy, and the overlap of the two fluorescent signals was yellow. B7-H3 and Tie-2 were both found to be expressed in ccRCC vascular endothelial tissues. Coexpression of the two proteins was also detected in these tissues (Figure 2).
Discussion
Angiogenesis, the process by which new blood vessels are formed, is an important hallmark of tumor progression,18 and a sufficient vascular response is key for the development and continuous growth of solid tumors. Therefore, combining anti-angiogenic treatments with other treatment methods to prevent the progression of malignant tumors is receiving increasing attention.
Vascular growth factors and receptor signaling are fundamental for tumor-mediated angiogenesis. The second most important endothelium-specific growth factor receptor system after the VEGF/VEGFR axis, the Ang/Tie-2 axis is a dual system that allows healthy blood vessels to remain in a resting state but still respond to angiogenic stimulation.19 TEMs comprise a myeloid-derived cell subset characterized by the expression of angiopoietin receptor Tie-2. In mouse tumor models, TEMs are responsible for almost all angiogenic activities of myeloid-derived cells.20 In a breast cancer mouse model, Tie-2 gene knockout in TEMs inhibits tumor angiogenesis, indicating that the Ang/Tie-2 axis regulates the angiogenic activity of these cells.21 Our preliminary study also showed that in renal cancer, a significant increase in the frequency of TEMs among infiltrating mononuclear cells occurs with tumor progression and angiogenesis, which was positively correlated with MVD.22 The results of the present study demonstrated that Tie-2 is highly expressed in the vascular endothelium of ccRCC and that its expression is related to tumor staging and lymph node metastasis. These results indicate that Tie-2 is involved in ccRCC angiogenesis and is associated with invasion and metastasis in ccRCC patients.
B7-H3 is an immunomodulatory protein belonging to the B7 family of T-cell co-stimulatory factors.23 Although early studies reported that B7-H3 can act as a co-stimulatory factor for CD4+ and CD8+ T-cells and can promote T-cell proliferation and cytokine production in vitro, most authors have reported that B7-H3 can inhibit T-cell activation and proliferation.24,25 Indeed, the role of B7-H3 in tumors remains controversial. In patients with pancreatic cancer, B7-H3 overexpression is associated with a better prognosis compared to low B7-H3 expression.26 Conversely, abnormally high B7-H3 expression is found in most tumors, such as prostate cancer, liver cancer, and non-small cell lung cancer and is associated with metastasis and a poor prognosis.8,9,11 In this study, we evaluated B7-H3 expression in ccRCC and normal paracancerous tissues, and the results showed that B7-H3 is mainly expressed in the vascular endothelial tissue of ccRCC. Moreover, tumor vascular B7-H3 expression was correlated with ccRCC pathological grade, staging, and lymph node metastasis. Thus, tumor vascular B7-H3 expression may be a useful indicator for the diagnosis and prognosis of renal cancer, consistent with previous studies.15,16 As an endothelial marker, Tie-2 is expressed in both normal and cancer tissues. Based on our results, Tie-2 expression is associated with tumor staging and lymph node metastasis but not with tumor grade. Although not expressed in normal tissues, up to 97.56% of tumor tissues express B7-H3, and its expression is correlated with tumor grade, stage and lymph node metastasis. Overall, B7-H3 is superior to Tie-2 as an effective molecular marker for estimating the degree of malignancy and predicting invasion and metastasis capacities.
Angiogenesis plays a crucial role in promoting cell infiltration and distant metastasis.27 MVD is considered a useful indicator that reflects the degree of tumor angiogenesis and possibly tumor invasion and metastasis capacities. High MVD was also reported to predict worse prognosis in various types of cancer.28–30 In the present study, the staining of endothelial cells for CD34 was used to evaluate the MVD in ccRCC tissues. Our results showed that MVD correlated with tumor grade and TNM stage in ccRCC, suggesting that MVD is a reliable indicator of tumor angiogenesis and a useful prognostic predictor for ccRCC, consistent with a previous study.28 This study also found B7-H3 and Tie-2 expression in ccRCC to be positively correlated with CD34-labeled MVD, suggesting that B7-H3 and Tie-2 are both involved in ccRCC angiogenesis. Immunofluorescence staining and confocal scanning revealed co-localization of B7-H3 and Tie-2 in tumor vascular endothelial cells, indicating that both the factors have key roles in tumor angiogenesis. Previous studies have shown that TEMs in mouse tumors can induce IL-10 production via ANGPT2 and inhibit T-cell proliferation and regulatory T-cell (Treg) growth, thereby exhibiting the various properties of M2 macrophages.31 In addition, M2 macrophages promote tumor progression through angiogenesis and inhibition of antitumor immunity,32 and B7-H3 signaling significantly increases IL-10 secretion and matrix metalloproteinase expression, promoting the transformation of macrophages from the M1 to the M2 phenotype.33 In hepatocellular carcinoma, B7-H3 can act as a chemokine to prompt macrophages to migrate from the peripheral blood to the tumor tissue and induce M1 to M2 cell phenotype transformation, resulting in tumor formation and development. Additionally, the B7-H3/STAT3 pathway may be involved in macrophage M2 transformation and negative regulation of T-lymphocyte-mediated immune responses.34 Therefore, both B7-H3 and Tie-2 induce angiogenesis by causing the M1 to M2 transformation. Recently, Zawada et al35 reported that CD14+ monocytes participate in tumor angiogenesis by upregulating Tie-2 and endothelial adhesion gene expression. Our previous study showed that B7-H3 has an important function in CD14+ monocyte-mediated renal cell tumor angiogenesis.36 Thus, we hypothesized that B7-H3 and Tie-2 have synergistic effects in ccRCC angiogenesis and that B7-H3 mainly regulates tumor angiogenesis by promoting the M1 to M2 transformation in macrophages and the TEM-mediated pathway. However, elucidation of the specific mechanisms requires further investigation.
mRCC has a poor prognosis and is resistant to chemotherapy; in some cases, the disease is insensitive to interferon and interleukin-2-based immune therapy. Although a certain treatment effect has recently been achieved via anti-angiogenic therapy targeting the VEGF and mTOR signaling pathways, targeting VEGF still has many limitations: only a temporary clinical response can be obtained, with the tumor eventually developing resistance to and circumventing angiogenic inhibition.37 Due to the inherent weakness of VEGF therapy, the Ang/Tie-2 pathway has become a new target. For example, the new Tie-2 inhibitor BAY-826 improves treatment responses in tumors rich in blood vessels, such as gliomas, and in animal models.38 Furthermore, AMG-386, a recombinant antibody consisting of immunoglobulins linked to Tie-2, blocks interaction between Ang-2 and Tie-2; this antibody has been shown to have an antitumor effect in mRCC, and multiple clinical trials using AMG-386 combined with anti-VEGF therapy are ongoing.3 Our results showed that B7-H3 and Tie-2 both have critical functions in ccRCC tumor angiogenesis. B7-H3 is specifically expressed in tumor vascular endothelial tissues but not in normal tissue vessels, indicating that it may be a highly selective target for anti-angiogenic-targeted therapy. Such targeted therapies should have limited effects outside of the tumor, avoiding impacts on normal vascular tissues. Because of its dual roles in immune regulation and anti-angiogenesis, targeting B7-H3 may be an effective treatment for renal cancer. However, the mechanisms underlying renal cancer are very complex, and the use of a single targeting drug is insufficient to completely control tumor progression, whereas multitarget combination blockade can improve efficacy and reduce drug resistance as well as side effects. As B7-H3 and Tie-2 have synergistic effects on ccRCC angiogenesis, targeting both the B7-H3 and Tie-2 pathways may be highly effective in efforts to inhibit tumor angiogenesis.
In summary, this study for the first time reveals the role of B7-H3 in ccRCC angiogenesis and its correlation with the angiogenic factor Tie-2 and MVD, and the findings suggest that B7-H3 may promote tumor angiogenesis through the Tie-2 pathway. B7-H3 might serve as an effective endothelial marker for the prognosis of ccRCC and become a promising target for ccRCC anti-angiogenic combination therapy. However, this study has limitations. Many factors affect angiogenesis, and we did not investigate other vascular factors or conduct in vitro and in vivo experiments to further verify the results, which will be the focus and direction of our future study.
Acknowledgment
This work was supported by grants from the National Natural Science Foundation of China (81272839; 81472401).
Disclosure
The authors report no conflicts of interest in this work.
References
Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67(5):913–924. | ||
Znaor A, Lortet-Tieulent J, Laversanne M, Jemal A, Bray F. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol. 2015;67(3):519–530. | ||
Penticuff JC, Kyprianou N. Therapeutic challenges in renal cell carcinoma. Am J Clin Exp Urol. 2015;3(2):77–90. | ||
Siemeister G, Schirner M, Weindel K, et al. Two independent mechanisms essential for tumor angiogenesis: inhibition of human melanoma xenograft growth by interfering with either the vascular endothelial growth factor receptor pathway or the Tie-2 pathway. Cancer Res. 1999;59(13):3185–3191. | ||
De Palma M, Naldini L. Angiopoietin-2 TIEs up macrophages in tumor angiogenesis. Clin Cancer Res. 2011;17(16):5226–5232. | ||
Chapoval AI, Ni J, Lau JS, et al. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2(3):269–274. | ||
Chen LJ, Chen J, Xu B, et al. B7-H3 expression associates with tumor invasion and patient’s poor survival in human esophageal cancer. Am J Transl Res. 2015;7(12):2646–2660. | ||
Jin Y, Zhang P, Li J, et al. B7-H3 in combination with regulatory T cell is associated with tumor progression in primary human non-small cell lung cancer. Int J Clin Exp Pathol. 2015;8(11):13987–13995. | ||
Kang FB, Wang L, Jia HC, et al. B7-H3 promotes aggression and invasion of hepatocellular carcinoma by targeting epithelial-to-mesenchymal transition via JAK2/STAT3/Slug signaling pathway. Cancer Cell Int. 2015;15:45. | ||
Huang C, Zhou L, Chang X, Pang X, Zhang H, Zhang S. B7-H3, B7-H4, Foxp3 and IL-2 expression in cervical cancer: associations with patient outcome and clinical significance. Oncol Rep. 2016;35(4):2183–2190. | ||
Benzon B, Zhao SG, Haffner MC, et al. Correlation of B7-H3 with androgen receptor, immune pathways and poor outcome in prostate cancer: an expression-based analysis. Prostate Cancer Prostatic Dis. 2017;20(1):28–35. | ||
Loos M, Hedderich DM, Ottenhausen M, et al. Expression of the costimulatory molecule B7-H3 is associated with prolonged survival in human pancreatic cancer. BMC Cancer. 2009;9:463. | ||
Seaman S, Stevens J, Yang MY, Logsdon D, Graff-Cherry C, St Croix B. Genes that distinguish physiological and pathological angiogenesis. Cancer cell. 2007;11(6):539–554. | ||
Zang X, Sullivan PS, Soslow RA, et al. Tumor associated endothelial expression of B7-H3 predicts survival in ovarian carcinomas. Mod Pathol. 2010;23(8):1104–1112. | ||
Crispen PL, Sheinin Y, Roth TJ, et al. Tumor cell and tumor vasculature expression of B7-H3 predict survival in clear cell renal cell carcinoma. Clin Cancer Res. 2008;14(16):5150–5157. | ||
Qin X, Zhang H, Ye D, Dai B, Zhu Y, Shi G. B7-H3 is a new cancer-specific endothelial marker in clear cell renal cell carcinoma. Onco Targets Ther. 2013;6:1667–1673. | ||
Delahunt B, Cheville JC, Martignoni G, et al. The International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. Am J Surg Pathol. 2013;37(10):1490–1504. | ||
Carla C, Daris F, Cecilia B, Francesca B, Francesca C, Paolo F. Angiogenesis in head and neck cancer: a review of the literature. J Oncol. 2012;2012:358472. | ||
Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. | ||
Venneri MA, De Palma M, Ponzoni M, et al. Identification of proangiogenic TIE2-expressing monocytes (TEMs) in human peripheral blood and cancer. Blood. 2007;109(12):5276–5285. | ||
Mazzieri R, Pucci F, Moi D, et al. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell. 2011;19(4):512–526. | ||
Ji J, Zhang G, Sun B, et al. The frequency of tumor-infiltrating Tie-2-expressing monocytes in renal cell carcinoma: its relationship to angiogenesis and progression. Urology. 2013;82(4):974.e9–974.e13. | ||
Ceeraz S, Nowak EC, Noelle RJ. B7 family checkpoint regulators in immune regulation and disease. Trends Immunol. 2013;34(11):556–563. | ||
Prasad DV, Nguyen T, Li Z, et al. Murine B7-H3 is a negative regulator of T cells. J Immunol. 2004;173(4):2500–2506. | ||
Fukushima A, Sumi T, Fukuda K, et al. B7-H3 regulates the development of experimental allergic conjunctivitis in mice. Immunol Lett. 2007;113(1):52–57. | ||
Loos M, Hedderich DM, Ottenhausen M, et al. Expression of the costimulatory molecule B7-H3 is associated with prolonged survival in human pancreatic cancer. BMC Cancer. 2009;9:463. | ||
Xue J, Wu XL, Huang XT, et al. Correlation of RUNX3 expression with microvessel density in colorectal adenocarcinoma tissues and clinical significance. Asian Pac J Trop Med. 2017;10(1):98–101. | ||
Nativ O, Sabo E, Reiss A, Wald M, Madjar S, Moskovitz B. Clinical significance of tumor angiogenesis in patients with localized renal cell carcinoma. Urology. 1998;51(5):693–696. | ||
Canoglu A, Gogus C, Beduk Y, Orhan D, Tulunay O, Baltaci S. Microvessel density as a prognostic marker in bladder carcinoma: correlation with tumor grade, stage and prognosis. Int Urol Nephrol. 2004;36(3):401–405. | ||
Pereira T, Dodal S, Tamgadge A, Bhalerao S, Tamgadge S. Quantitative evaluation of microvessel density using CD34 in clinical variants of ameloblastoma: an immunohistochemical study. J Oral Maxillofac Pathol. 2016;20(1):51–58. | ||
Coffelt SB, Chen YY, Muthana M, et al. Angiopoietin 2 stimulates TIE2-expressing monocytes to suppress T cell activation and to promote regulatory T cell expansion. J Immunol. 2011;186(7):4183–4190. | ||
Coffelt SB, Hughes R, Lewis CE. Tumor-associated macrophages: effectors of angiogenesis and tumor progression. Biochim Biophys Acta. 2009;1796(1):11–18. | ||
Sun J, Mao Y, Zhang YQ, et al. Clinical significance of the induction of macrophage differentiation by the costimulatory molecule B7-H3 in human non-small cell lung cancer. Oncol Lett. 2013;6(5):1253–1260. | ||
Kang FB, Wang L, Li D, Zhang YG, Sun DX. Hepatocellular carcinomas promote tumor-associated macrophage M2-polarization via increased B7-H3 expression. Oncol Rep. 2015;33(1):274–282. | ||
Zawada AM, Rogacev KS, Rotter B, et al. SuperSAGE evidence for CD14++CD16+ monocytes as a third monocyte subset. Blood. 2011;118(12):e50–e61. | ||
Li M, Zhang G, Zhang X, et al. Overexpression of B7-H3 in CD14+ monocytes is associated with renal cell carcinoma progression. Med Oncol. 2014;31(12):349. | ||
Huang H, Lai JY, Do J, et al. Specifically targeting angiopoietin-2 inhibits angiogenesis, Tie2-expressing monocyte infiltration, and tumor growth. Clin Cancer Res. 2011;17(5):1001–1011. | ||
Schneider H, Szabo E, Machado RA, et al. Novel TIE-2 inhibitor BAY-826 displays in vivo efficacy in experimental syngeneic murine glioma models. J Neurochem. 2017;140(1):170–182. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.