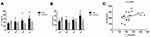Back to Journals » Journal of Inflammation Research » Volume 14
Expression and Correlation of IgG4 and IL-21 in Collagen-Induced Arthritis Rats
Authors Long ZY, Zhou YF, Yuan H , Peng YM, Wu SX, Peng F
Received 25 April 2021
Accepted for publication 21 September 2021
Published 1 October 2021 Volume 2021:14 Pages 5051—5058
DOI https://doi.org/10.2147/JIR.S317420
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Monika Sharma
Zhen-Yi Long,1,* Yi-Feng Zhou,2,* Hao Yuan,1 Ya-Meng Peng,1 Si-Xian Wu,1 Fang Peng1
1Department of Clinical Laboratory, Hunan Provincial People’s Hospital, The First Affiliated Hospital of Hunan Normal University, Changsha, 410005, People’s Republic of China; 2Operating Room, Hunan Provincial People’s Hospital, The First Affiliated Hospital of Hunan Normal University, Changsha, 410005, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hao Yuan
Department of Clinical Laboratory, Hunan Provincial People’s Hospital, The First Affiliated Hospital of Hunan Normal University, Changsha, 410005, People’s Republic of China
Tel +86-13677366519
Fax +86-731-82278012
Email [email protected]
Purpose: We explored the expression levels of IgG4 and interleukin (IL)-21 in the serum and ankle joints of collagen-induced arthritis (CIA) rats at different disease stages.
Materials and Methods: Wistar rats were randomly divided into normal and model groups, and the latter group was administered bovine type II collagen to induce arthritis. Enzyme-linked immunosorbent assay was performed at 21, 28, 35, and 42 days to detect IgG4 and IL-21 in the serum, followed by histological and immunohistochemical analyses of IgG4 and IL-21r in the ankle joint of rats.
Results: The contents of IgG4 and IL-21 in the serum of the CIA model group were positively correlated and increased with disease progression. The expression of IgG4 and IL-21 receptors in the ankle joint of the CIA model group was significantly higher than that in the control group. These proteins were closely related to the pathological score. The serum IL-21 level in the model group was closely related to the level of IL-21 receptor in the ankle joint.
Conclusion: IL-21 may promote the occurrence and development of rheumatoid arthritis by combining with IL-21r to regulate the content of IgG4.
Keywords: rheumatoid arthritis, immunoglobulin, interleukin, interleukin receptor
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease primarily manifesting as multiple chronic arthritis and has a worldwide incidence of approximately 0.5–1%.1 Approximately 50% of patients report the involvement of extra-articular organs, such as the skin, eyes, lungs, and kidneys; hence, the disease is a major cause of disability and loss of function.2–4 RA is a chronic immune-mediated disease in which multiple immune cell types and signaling network malfunction to elicit a maladaptive tissue repair process.5 The fundamental hallmark of RA is the production of autoantibodies such as rheumatoid factor and antibodies that recognize post-translationally modified proteins, including anti-citrullinated protein antibodies and anti-carbamylated protein antibodies.6 Interleukin (IL)-21, a member of the IL-2 family, is involved in biological activities in cancer and autoimmunity by binding to its receptor, IL-21r.7 IL-21 stimulates RA-FLS proliferation and promotes the production of tumor necrosis factor-α and IL-6 and blockade of IL-21/IL-21R pathway with IL-21r.8 IL-21 was previously confirmed to be upregulated in not only the serum but also the synovial fluid of patients with RA.7 Moreover, studies on the role of IL-21 in RA pathogenesis have reported that this protein can promote osteogenesis in patients, leading to bone destruction.9 However, another study showed that IL-21r is expressed in RA synovial fibroblasts and synovial macrophages. IL-21 was not expressed in the RA and osteoarthritis synovium, as determined by in situ hybridization with IL-21-specific probes.10 Therefore, we detected the expression of IL-21r-positive cells in the joints. These findings suggest that targeting IL-21 and IL-21r can alleviate the progression of bone damage in patients with RA. IgG can be divided into four subtypes, which can be classified into IgG1–4. IL-21 may promote the secretion of IgG4 by plasma cells, it activates the complement system by binding to IgG4-type immune complexes, mediates tissue damage, and promotes disease progression.11 At present, Rispens et al12 hypothesized that two different specific IgG4 molecules can form a bispecific antibody through “half molecule” Fc-Fc exchange; this bispecific antibody may induce an inflammatory response. However, this hypothesis has not been confirmed; we explored the role of IgG4 and IL-21 in disease progression by observing the distribution of IgG4 and IL-21r in the joints of collagen-induced arthritis (CIA) rats at various stages.
In this study, a model of RA was generated using Wistar rats. By assessing the expression levels of IgG4 and IL-21 in the serum and IgG4 and IL-21r level in the ankle joint, we investigated the pathomechanism of RA and diagnostic value of these indicators of RA, providing a new insight into the pathogenesis and treatment of RA.
Materials and Methods
Reagents
Bovine type II collagen (Sigma, St. Louis, MO, USA) and Freund’s incomplete adjuvant (Chondrex, Redmond, WA, USA) were applied to build the rat model. A rabbit anti-IgG4 polyclonal antibody from MXB Biotechnologies (Fuzhou, China, MAB-0651) was used, rabbit anti-IL-21r polyclonal antibody was obtained from Abcam (Cambridge, UK, ab5980), the mouse IgG4 enzyme-linked immunosorbent assay (ELISA) kit was purchased from Jianglai Biotechnology (Jianglaibio, China, JL15562), and a mouse IL-21 ELISA kit (Jianglaibio, China, JL20239) were used.
Animals
Male Wistar rats (Tianqin Biotechnology Co., Changsha, China) were used. All rats were housed in a specific pathogen-free environment under controlled conditions (temperature, 20–26°C; humidity, 35–75%; light/dark cycle, 12 h/12 h) with ad libitum access to chlorinated water and irradiated food. Before the study, rats were allowed a 7-day acclimatization period. The health of the rats was monitored daily.
Ethics Approval
The study was approved by the Ethics Committee of the Hunan Provincial People’s Hospital (approval number 2019S46). The studies were conducted in compliance with institutional review board regulations.
Induction of CIA
Fifty rats were randomly divided into control and model groups. CIA was induced as described previously.3 In the CIA group, 30 Wistar rats (4–5-week-old) were immunized with 200 µL bovine type II collagen in 0.05 M acetic acid and emulsified with an equal volume of Freund’s incomplete adjuvant and administered intradermally at the base of the tail (day 0). A booster injection of 100 μL collagen-Freund’s incomplete adjuvant suspension was administered in the same manner on day 8. In the control group, 20 Wistar rats were given normal saline during the same period. The rats were anesthetized by intraperitoneal injection of 0.3 mL/100 g chloral hydrate after skin disinfection before the operation. Arthritic swelling was evaluated visually on a scale of 0–4 for each limb (0, no change; 1, limited swelling and erythema of the toe joints; 2, swelling and erythema of the toe joints and digital joints; 3, swelling and erythema of the foot below the ankle joints; 4, severe swelling and erythema of the whole foot including ankle joints); therefore, the maximum score per mouse is 16.4 If the AI score was 4 or greater within 21 days after initial immunization, the rats were considered as successfully modeled. In general, the swelling score was evaluated in a blinded manner.
ELISA
Two group rats were killed by cervical dislocation on days 21 (n = 10), 28 (n = 10), 35 (n = 10), and 42 (n = 10) after the first injection. Serum from the rats were obtained and stored at −40°C until use. Serum IL-21 and IgG4 levels were determined using ELISA kits in accordance with the manufacturer’s protocol, and absorbance was measured at 450 nm using a microplate reader.
Histological and Immunohistochemical Analyses
Tissue from the left ankle joints on days 21, 28, 35, and 42 were decalcified in bone decalcifier, embedded in paraffin, and cut into 4-mm-thick sections. Paraffin-embedded sections were stained with hematoxylin-eosin (H&E) for histological analysis. Immunohistochemical analysis was performed for IL-21r and IgG4 in the joints in accordance with the manufacturer’s instructions. Paraffin-embedded sections were dewaxed and repaired using tissue antigen by routine methods and incubated for 10 min with 3% H2O2. Each section was incubated with primary rat anti-IL-21r and anti-IgG4 antibodies for 1 h at room temperature. After incubation with poly-horseradish peroxidase anti-rabbit IgG for 15 min at room temperature, 50 µL of a pre-made working solution of chromogenic agent diaminobenzidine was added at room temperature for 3–5 min. The sections were dehydrated after staining with hematoxylin for 1 min. The sample was placed in xylene for 2 min and then sealed with neutral gum was sealed for observation under the microscope. The histopathological scores of each group were independently measured twice.13 (a) Synovial cell proliferation score: (0): less than 3 layers of synovium, (1): 3–4 layers, (2): 5–6 layers, (3): more than 6 (b) layers. Inflammation score: (0): no lymphocyte infiltration; (1): lymphocyte aggregation; (2): diffuse distribution forming lymphatic follicles; (3): formation of lymphatic follicular-associated hair centers. (c) Angiogenesis score: (0): no neovascularization, (1): mild neovascularization, (2): moderate, (3): severe. The total score of each rat was calculated. Two experienced pathologists selected three visual fields in the cell-intensive area of each specimen and counted the number of positive IgG4 and IL-21r inflammatory cells under a high-power visual field to calculate the average value.8
Statistical Analysis
Data were presented as the mean ± standard error of the mean. The two groups were compared using Mann–Whitney U-test for two independent samples. Correlations were determined using Spearman correlation analysis with SPSS version 18.0 software (SPSS, Inc., Chicago, IL, USA). The Kolmogorov–Smirnov test in SPSS was used to estimate the normality of the data. P-values less than 0.05 were considered to indicate significant results.
Results
General Characteristics of Experimental Rats
Twenty of the 30 rats were successfully induced with CIA with a success rate of 66.7%. According to the analysis of the pre-experiment, the sample size was determined to be 5 per group (see Appendices 1–4). The model group was lethargic and irritable compared with the control group. Redness, swelling, and heat were observed in some joints on day 8 after initial immunization in the CIA group. Redness and swelling at each foot joint increased over time. However, the joints of the control group did not change significantly over time (Figure 1). In this experiment, the CIA model was established successfully for further exploration of the disease. However, because the disease model was induced under certain experimental conditions, the rats would not show fluctuation and recurrence of the disease or extraarticular manifestations such as rheumatoid nodules and vasculitis in patients with RA. These are limitations of our model.
Serum IL-21 and IgG4 Levels of Experimental Rats
Serum IgG4 and IL-21 levels were lower in CIA rats than in control rats at 21 days, and the median value of two indices in the model group increased over time (Figure 2). IL-21 and IgG4 levels were positively correlated in the CIA group.
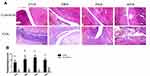
Histological Analysis of the Ankle Joint
H&E staining was performed for the ankle joints of rats in each group (Figure 3). The synovial layer was thin, and the articular cartilage layer was thick in the negative control group. No structural damage, inflammatory cell infiltration, and angiogenesis were apparent, and no obvious changes were observed at 21, 28, 35, and 42 days. In the model group, synovial hyperemia edema elevated to more than 10 layers at 21 days. Between 28 and 35 days, the synovial membrane was thicker than before, extending into the articular cavity in a villous shape. The surface of the articular cartilage displayed obvious damage, the transparent cartilage surface was severely damaged, and local fibrosis was observed. At 42 days, significant necrosis and exfoliation of articular cartilage was detected, and increased cells were found in the synovial membrane. These results are consistent with those of a previous study showing that fibrous hyperplasia and neovascularization occurred in the synovial layer and that the articular cavity was narrower than before.14
IL-21r and IgG4 Expression Levels in Ankle Joints
Immunohistochemical staining was performed for the ankle joints of rats in all groups (Figure 4). IgG4 and IL-21r were not significantly upregulated in the synovial membrane, cartilage, and bone in the negative control group. IgG4 and IL-21r were upregulated in the CIA model group. IgG4 and IL-21r inflammatory cells in the model group showed a positive relationship. IgG4 was primarily expressed in the cytoplasm15 and IL-21r is expressed in the cell membrane. A significant correlation was obtained between IgG4 and IL-21r inflammatory cells in the model group. In the model group, serum IL-21 levels were positively correlated with the arthritis index, pathological score, and IL-21r expression in the ankle joint (r = 0.447, 0.461, 0.451, P < 0.05), whereas serum IgG4 levels were not significantly correlated with the arthritis index, pathological score, and IgG4 expression in the ankle joint. IL-21r expression in the ankle joint of CIA rats was positively correlated with the arthritis index and pathological score (r = 0.548, 0.540, P < 0.05), and IgG4 expression in the ankle was positively correlated with the pathological score (r = 0.549, P < 0.05).
Discussion
The first rat model of CIA was established using Wistar, Sprague-Dawley, and Wistar-Lewis rats in 1977 by Trentham.16 As the pathophysiological and immunological characteristics of CIA model rats are similar to those of RA in humans, these animals are considered as the best animal model for studying RA.17 In this study, Wistar rats were used to establish an animal model of CIA, reflecting the disease characteristics in humans.
RA is common and often causes serious damage such as systemic symptoms of synovial joint inflammation.18 A study reported that the risk of death in RA patients is 1.5–1.6-fold that of the general population,19 particularly for patients with advanced RA, severe disease, or on long-term bed rest. Currently, RA pathogenesis is not completely understood; however, it is generally considered that the deciding factor for resolving initial injury is the obliteration of autoimmune tolerance.20,21 However, subsequent studies reported that IL-6-mediated IgG4 upregulation is partially corroborated by IL-21.22 Furthermore, Carbone et al23 can selectively reduce IL-21 and IgG4 levels in the absence of IL-6 and affect RA progression. In comparison, IL-21 may induce IgG4 production in B cells more strongly than IL-4. In this study, IgG4 and IL-21 expression was assessed in the serum samples of rats at four time points, and this result is consistent with previous experiments. Herein, the serum contents between the normal and model groups did not markedly differ during the 21 days of the disease (Figure 2). However, this finding contrasts those of previous studies,9,24 likely because of differences among individual rats and the combination of two factors in inflammatory tissue in the early stage of the disease.25 Comparison of the levels of IgG4 and IL-21 at days 21 and 42 of disease progression revealed upregulation of both proteins. Serum IgG4 levels are associated with IL-21 in rats, and the levels of IL-21 and IgG4 in the model group were significantly higher than those in the control group in day 42 in the CIA group (z = −2.402; −1.984, P < 0.05). Serum IL-21 levels were positively correlated with the arthritis index. Thus, changes in IgG4 and IL-21 may be associated with disease progression.
H&E staining of the ankle joint revealed no obvious synovial thickening, inflammatory cell infiltration, or neovascularization in the ankle joint in the control group. Synovial inflammation occurred at the ankle joint of rats in the CIA group (Figure 3). Upon disease progression, articular cartilage injury, local fibrosis, and neovascularization gradually occurred.2 The articular cartilage displayed necrotic shedding, consistent with disease progression in patients with RA, indicating that the model was successfully established and is valuable for basic studies of RA. It was reported that IL-21 regulates the innate and adaptive immune responses via heterodimers of IL-21r and the common cytokine receptor γc1.7 Upon binding, the IL-21-IL-21r complex activates Janus kinase 1 and 3, which in turn phosphorylates STAT1 and STAT3. Previous studies suggested that IL-21r knockout mice do not produce auto-specific immunoglobulin and cannot be induced with collagen to develop arthritis.26 Thus, we detected the expression of IL-21r-positive cells in the joints. In this study. IL-21r-positive cells were present at various stages of the disease in CIA rats, showing higher expression levels than in the negative control group (Figure 4A), suggesting that IL-21r is involved in pathological hyperplasia in the disease synovium. The serum IL-21 level in the model group was closely related to the level of IL-21 receptor in the ankle joint. This expression of IL-21r in tissue indicates that binding of IL-21 to its receptor is prerequisite for RA progression. Targeting IL-21 and IL-21r may mitigate the progression in patients with RA. These results are consistent with those of previous studies. Immunohistochemical staining revealed that IgG4-positive cells were present at various stages of the disease in CIA rats, particularly in the synovial membrane and bone, showing higher expression levels than the negative control group. Among these, infiltration of IgG4-positive cells in the bone was observed in the CIA group at 42 days, suggesting that IgG4 may be involved in bone destruction in RA (Figure 4B). IL-21r expression in the ankle joint of CIA rats was positively correlated with IgG4 expression, the arthritis index, and pathological score (r = 0.633, 0.548, 0.540, P < 0.05), and IgG4 expression in the ankle was positively correlated with the pathological score (r = 0.549, P < 0.05). Thus, changes in IgG4 and IL-21r may be associated with disease pathogenesis and progression and the disease may be treated by modulating IL-21, IL-21r, and IgG4 levels.
There were some limitations to this study. Fluctuations and recurrence were not observed in the rats, and no extraarticular manifestations such as rheumatoid nodules and vasculitis were detected in this model, as the disease model was induced under specific experimental conditions. Rats had certain individual differences and the microscopic examination results were subjective. These factors likely influenced the experimental results. The expression levels of IgG4, IL-21, and IL-21r in the serum and ankle joints should be validated in further studies.
Conclusion
We assessed the expression levels of IgG4, IL-21, and IL-21r in the serum and ankle joints of CIA rats at various disease stages. The results show that with disease progression, the serum levels of IgG4 and IL-21 increased gradually, and the expression levels of IL-21r and IgG4 in the tissues were significantly higher than those in the control group. The serum IL-21 level in the model group was closely related to the level of IL-21 receptor in the ankle joint. These results suggest that IL-21 may promote RA occurrence and development by combining with IL-21r to regulate IgG4 levels. Our results provide insight into potential therapeutic targets for RA and RA pathogenesis.
Abbreviations
IgG4, Immunoglobulin 4; IL-21, Interleukin-21; CIA, collagen-induced arthritis; ELISA, Enzyme-linked immunosorbent assay; RA, rheumatoid arthritis; IL-21r, Interleukin-21 receptor; FLS, fibroblast like synoviocytes; TNF-α, tumor necrosis factor-α; BIIC, Bovine type II collagen; IFA, Freund’s incomplete adjuvant; AI, arthritis index; H&E, hematoxylin and eosin; STAT, signal transducer and activator of transcription.
Acknowledgments
This work was supported by Scientific Research Fund of Hunan Provincial Education Department (No.18A035), 2020 Science and Technology Project of Changsha Science and Technology Bureau (No. kq2004120), and Fund Project of Hunan Provincial Department of Science and Technology (No.2019JJ80009).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All authors report no potential conflicts of interest with regard to this work.
References
1. Wang X, Yan X, Wang F, et al. Role of methotrexate chronotherapy in collagen-induced rheumatoid arthritis in rats. Z Rheumatol. 2016;77(3):249–255. doi:10.1007/s00393-016-0236-6
2. Catrina AI, Ytterberg AJ, Reynisdottir G, et al. Lungs, joints and immunity against citrullinated proteins in rheumatoid arthritis. Nat Rev Rheumatol. 2014;10(11):645–653. doi:10.1038/nrrheum.2014.115
3. Cheng BCY, Yu H, Guo H, et al. A herbal formula comprising rosae multiflorae fructus and lonicerae japonicae flos, attenuates collagen-induced arthritis and inhibits tlr4 signalling in rats. Sci Rep. 2016;6(1):20042. doi:10.1038/srep20042
4. Ahmad S, Alam K, Hossain MM, et al. Anti-arthritogenic and cardioprotective action of hesperidin and daidzein in collagen-induced rheumatoid arthritis. Mol Cell Biochem. 2016;423(1–2):115–127. doi:10.1007/s11010-016-2830-y
5. Weyand CM, Goronzy JJ. The immunology of rheumatoid arthritis. Nat Immunol. 2021;22(1):10–18. doi:10.1038/s41590-020-00816-x
6. Buch MH, Eyre S, McGonagle D. Persistent inflammatory and non-inflammatory mechanisms in refractory rheumatoid arthritis. Nat Rev Rheumatol. 2021;17(1):17–33. doi:10.1038/s41584-020-00541-7
7. Yuan M, Wang T. Advances of the interleukin-21 signaling pathway in immunity and angiogenesis. Biomedl Rep. 2016;5:3–6. doi:10.3892/br.2016.665
8. Xing R, Yang L, Jin Y, et al. Interleukin‐21 induces proliferation and proinflammatory cytokine profile of fibroblast‐like synoviocytes of patients with rheumatoid arthritis. Scand J Immunol. 2016;83(1):64–71. doi:10.1111/sji.12396
9. Kwok SK, Cho ML, Park MK, et al. Interleukin-21 promotes osteoclastogenesis in humans with rheumatoid arthritis and in mice with collagen-induced arthritis. Arthritis Rheum. 2014;64(3):740–751. doi:10.1002/art.33390
10. Jüngel A, Distler J, Kurowska-Stolarska M, et al. Expression of interleukin-21 receptor, but not interleukin-21, in synovial fibroblasts and synovial macrophages of patients with rheumatoid arthritis. Arthritis Rheum. 2010;50(5):1468–1476. doi:10.1002/art.20218
11. van de Stadt LA, de Vrieze H, Derksen NI, et al. Antibodies to IgG4 hinge can be found in rheumatoid arthritis patients during all stages of disease and may exacerbate chronic antibody-mediated inflammation. Arthritis Rheumatol. 2014;66(5):1133–1140. doi:10.1002/art.38335
12. Rispens T, Ooievaar-de Heer P, Vermeulen E, et al. Human IgG4 binds to IgG4 and conformationally altered IgG1 via Fc-Fc interactions. J Immunol. 2009;182(7):4275–4281. doi:10.4049/jimmunol.0804338
13. Tsubaki T, Arita N, Kawakami T, et al. Characterization of histopathology and gene-expression profiles of synovitis in early rheumatoid arthritis using targeted biopsy specimens. Arthritis Res Ther. 2005;7(4):825–836. doi:10.1186/ar1751
14. Chang SK, Gu Z, Brenner MB. Fibroblast-like synoviocytes in inflammatory arthritis pathology: the emerging role of cadherin-11. Immunol Rev. 2010;233(1):256–266. doi:10.1111/j.0105-2896.2009.00854.x
15. Zhao PX, Adzavon YM, Ma JM, et al. IgG4 and IgE co-positive group found in idiopathic orbital inflammatory disease. Int J Ophthalmol. 2018;11(1):36–42. doi:10.18240/ijo.2018.01.07
16. Trentham DE, Townes AS, Kang AH. Autoimmunity to type II collagen an experimental model of arthritis. J Exp Med. 1977;146(3):857–868. doi:10.1084/jem.146.3.857
17. Chen X, Jiang X, Jusko WJ, et al. Minimal physiologically-based pharmacokinetic (mPBPK) model for a monoclonal antibody against interleukin-6 in mice with collagen-induced arthritis. J Pharmacokinet Pharmacodyn. 2016;43(3):291–304. doi:10.1007/s10928-016-9472-2
18. Kuo CF, Luo SF, See LC, et al. Rheumatoid arthritis prevalence, incidence, and mortality rates: a nationwide population study in Taiwan. Rheumatol Int. 2013;33(2):355–360. doi:10.1007/s00296-012-2411-7
19. Sokka T, Abelson B, Pincus T. Mortality in rheumatoid arthritis: 2008 update. Clin Exp Rheumatol. 2008;26:S35–61. doi:10.1002/art.24044
20. Kimata H, Fujimoto M, Ishioka C, et al. Histamine selectively enhances human immunoglobulin E (IgE) and IgG4 production induced by anti-CD58 monoclonal antibody. J Exp Med. 1996;184(2):357–364. doi:10.1084/jem.184.2.357
21. Takeuchi M, Sato Y, Ohno K, et al. T helper 2 and regulatory T-cell cytokine production by mast cells: a key factor in the pathogenesis of IgG4-related disease. Mod Pathol. 2014;27(8):1126–1136. doi:10.1038/modpathol.2013.236
22. Dienz O, Eaton SM, Bond JP, et al. The induction of antibody production by IL-6 is indirectly mediated by IL-21 produced by CD4+ T cells. J Exp Med. 2009;206(1):69–78. doi:10.1084/jem.20081571
23. Carbone G, Wilson A, Diehl SA, et al. Interleukin-6 receptor blockade selectively reduces IL-21 production by CD4 T cells and IgG4 autoantibodies in rheumatoid arthritis. Int J Biol Sci. 2013;9(3):279–288. doi:10.7150/ijbs.5996
24. Rasmussen TK, Andersen T, Hvid M, et al. Increased interleukin 21 (IL-21) and IL-23 are associated with increased disease activity and with radiographic status in patients with early rheumatoid arthritis. J Rheumatol. 2010;37:2014–2020. doi:10.3899/jrheum.100259
25. Weyand CM, Goronzy JJ. Immunometabolism in early and late stages of rheumatoid arthritis. Nat Rev Rheumatol. 2017;13(5):291–301. doi:10.1038/nrrheum.2017.49
26. Jang E, Cho SH, Park H, et al. A positive feedback loop of il-21 signaling provoked by homeostatic cd4+cd25- t cell expansion is essential for the development of arthritis in autoimmune k/bxn mice. J Immunol. 2009;182(8):4649–4656. doi:10.4049/jimmunol.0804350
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.