Back to Journals » International Journal of General Medicine » Volume 13
Expression and Clinical Prognostic Value of Platelet NLRP3 in Acute Coronary Syndrome
Authors Peng H, Wu H, Zhang G, Zhang W , Guo Y, Chang L, Chen S, Xue R, Zhang S
Received 4 August 2020
Accepted for publication 9 September 2020
Published 9 October 2020 Volume 2020:13 Pages 791—802
DOI https://doi.org/10.2147/IJGM.S275481
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Huitong Peng,1 Hongyi Wu,2 Ge Zhang,1 Wei Zhang,1 Yifan Guo,2 Lin Chang,1 She Chen,1 Ruyi Xue,3 Si Zhang1
1Department of Biochemistry and Molecular Biology, NHC Key Laboratory of Glycoconjugates Research, School of Basic Medical Sciences, Fudan University, Shanghai, People’s Republic of China; 2Department of Cardiology, Zhongshan Hospital, Fudan University, Shanghai, People’s Republic of China; 3Department of Gastroenterology and Hepatology, Zhongshan Hospital, Fudan University, Shanghai, People’s Republic of China
Correspondence: Ruyi Xue
Department of Gastroenterology and Hepatology, Zhongshan Hospital, Fudan University, No. 160, Feng Lin Road, Xuhui, Shanghai, People’s Republic of China
Tel +86-21-54237624
Email [email protected]
Si Zhang
Department of Biochemistry and Molecular Biology, School of Basic Medical Sciences, Fudan University, No. 130, Dong’an Road, Xuhui, Shanghai, People’s Republic of China
Tel +86 138 1709 2601
Email [email protected]
Purpose: Little is known about the relationship between the level of platelet NOD-like receptor protein 3 (NLRP3) and the severity of acute coronary syndrome (ACS) or the prognostic value of platelet NLRP3 for percutaneous coronary intervention (PCI).
Methods: Platelets collected from 25 healthy subjects, 23 patients with stable angina pectoris (SAP), and 72 patients with ACS were analyzed by Western blotting and real-time fluorescence quantitative PCR (qPCR). A total of 152 patients with ACS who had undergone PCI were included in this study to evaluate the prognostic value of platelet NLRP3.
Results: The levels of platelet NLRP3 in both the healthy and SAP groups were clearly lower than in the ACS group (P< 0.001). According to the Pearson correlation analysis, the expression of platelet NLRP3 was closely related to the mean platelet volume (MPV), left ventricular ejection fraction (LVEF), the Gensini score, and the Global Registry of Acute Coronary Events (GRACE) score (all P< 0.001). Multivariate logistic regression analysis identified NLRP3 as an independent risk factor for adverse cardiovascular events (ACEs) after PCI (P=0.004). The proportion of patients with high NLPR3 expression (the NLRP3-high group) remaining free of adverse events for 3 years was remarkably lower than that in patients with low NLPR3 expression (the NLRP3-low group; P=0.024). The NLRP3-high group had a significantly higher proportion of patients with interleukin-1β–expressing (20.4%± 6.1%) platelets than the NLRP3-low group (10.7%± 3.5%, P< 0.001). Moreover, the NLRP3-high group exhibited higher platelet activity, as indicated by increased PAC-1 binding and CD62P expression, compared with the NLRP3-low group (P< 0.001).
Conclusion: These results indicated that platelet NLRP3 was a novel potential prognostic factor for patients with ACS that underwent PCI.
Keywords: platelet NLRP3, acute coronary syndromes, percutaneous coronary intervention, inflammation, prognosis
Introduction
Defined as the acute ischemic syndrome of the heart resulting from the rupture of thrombosis or atherosclerotic plaque in the coronary arteries,1 acute coronary syndrome (ACS) is one of the most serious and potentially fatal diseases. Inflammation contributes to building plaques, regards as determinants of acute coronary.
NOD-like receptor protein 3 (NLRP3) inflammasome, an inflammasome with the clear structure and function, consists of NLRP3, pro-caspase-1, and apoptosis-associated speck-like protein with a CARD (ASC).2 NLRP3 is closely related to the occurrence and development of cardiovascular disease, in which inflammatory bodies are activated by cholesterol crystals in a dose-dependent manner in the early stages of atherosclerosis.3 Mice lacking the activation domains, including ASC and caspase-1, showed reduced inflammation, infarct size, and myocardial fibrosis after ischemic injury.4
Wang et al5 discovered that patients with ACS and acute myocardial infarction (AMI) had increased expression of NLRP3 and downstream cytokines in monocytes. After activation by external stimuli, NLRP3 forms a complex with proteins, including ASC, BTK, and caspase-1, and regulates the production of pro-inflammatory factors, such as active interleukin (IL)-1β, thereby controlling platelet activation, aggregation, and thrombosis during ACS.6 Besides their presence in monocytes, NLRP3 expression has been recently found in platelets, however, the expression and clinical prognostic value of platelet NLRP3 in ACS remains unclear.
Patients with ACS participating in the PLATelet inhibition and patient Outcomes trial were at a high risk of residual disease, with 10% of patients experiencing an ischemic endpoint, even after receiving powerful dual antiplatelet therapy.7 Interestingly, the Canakinumab Anti-inflammatory Thrombosis Outcomes Study trial suggested that the IL-1β monoclonal antibody canakinumab contributed to a 15% reduction in the risk of cardiovascular events in patients with myocardial infarction and C-reaction protein concentration >2 mg/dL based on standard drug therapy.8 This is the first direct demonstration that anti-inflammatory treatment can reduce cardiovascular events, indicating that inflammation and thrombosis are inseparable in ACS.
Percutaneous coronary intervention (PCI) is an effective treatment for some patients with ACS, although there are still issues surrounding prognosis. There are many factors related to the prognosis after PCI, and the indices studied remain inadequate in terms of sensitivity and specificity. Therefore, there is an urgent need to find ideal indicators to evaluate the prognosis of PCI in patients with ACS. Since platelets are involved in the pathological process of inflammation and thrombosis in ACS, the biomarkers in platelets may be important predictors of the prognosis of ACS.
Platelets can express and release inflammatory factors and chemokines after activation,9 and inflammatory cells are recruited by platelets-secreted chemokines to vascular lesions,10 promoting vascular endothelial dysfunction and inducing atherosclerosis. We speculated that platelet NLRP3 may participate in the platelet hyperactivity in ACS and affect the prognosis of patients with ACS. Based on this hypothesis, for the first time, we observed the expression of platelet NLRP3 in patients with ACS. Furthermore, we performed retrospective analysis to examine the relationship between platelet NLRP3 expression and the clinical endpoints in patients with ACS.
Materials and Methods
Participants and Design
In this study, two different patient cohorts were included: one cohort comprised healthy people and patients with coronary heart disease, and the other comprised patients with ACS who underwent PCI. Study subjects were patients hospitalized in Zhongshan Hospital of Fudan University between January 2016 and July 2017. This study was approved by the appropriate Ethics Committee and strictly adhered to the Declaration of Helsinki, and written informed consent was obtained from each patient after a detailed presentation regarding this study.
The first cohort enrolled 25 healthy subjects; 23 patients with stable angina pectoris (SAP); and 72 patients with ACS, comprising 23 patients with unstable angina pectoris (UAP), 26 patients ST-segment elevation myocardial infarction (STEMI), and 23 patients with non-STEMI (NSTEMI). The second cohort comprised 152 patients with ACS who successfully underwent PCI. The inclusion and exclusion criteria defined for both cohorts are shown in Figure S1.11–13
Data Collection and Evaluation
The demographic and clinical characteristics of patients included age (years), body mass index (BMI), sex, smoking status, hypertension, diabetes, blood lipids, low- and high-density lipoprotein cholesterol (LDL-C and HDL-C, respectively), medication taken during this study (administration of β receptor blocker, ACE1, ARB, PPIs, H2RA, statins, and nitrates), platelet parameters, and left ventricular ejection fraction (LVEF).
The blood samples were collected from all patients at the time of enrollment. The venous blood from patients with SAP and ACS was drawn immediately upon hospital admission before medication and angiography. Blood samples were drawn from healthy subjects in the control group on the day of physical examination. For patients in the PCI group, venous blood was drawn immediately upon hospital admission before revascularization (ie, thrombolysis or PCI).
We evaluated the 3-year incidence of cardiovascular events (ACEs) (eg, stroke, AMI, cardiovascular event, and unplanned coronary revascularization)14 in 152 patients who underwent PCI.
Detection of Platelet NLRP3 Expression
Platelet-rich plasma was prepared by centrifuging whole blood samples collected from healthy subjects and patients at 300 × g for 20 min. Then, the platelet-rich plasma was centrifuged at 740 × g for 10 min to remove the supernatant and resuspended in Tyrode’s buffer (135 mM NaCl, 12 mM NaHCO3, 2.9 mM KCl, 0.3 Na2HPO4, 1 mM MgCl2, 5 mM D-glucose, 10 mM HEPES, and 1.5% BSA, pH 7.4, 37ºC) to obtain a platelet suspension. The concentration was adjusted to 1×105 cells/μL. Isolated platelets were immediately analyzed by flow cytometry or stored at –80°C for further analysis by qPCR and Western blotting.
The manufacturer’s protocol (cat. no. 15596–026, Invitrogen) was used to extract the platelet RNA. The total RNA was assessed for purity using an ultraviolet spectrophotometer (Eppendorf, Germany), and then stored at −80ºC in a refrigerator until use.
In accordance with the PCR amplification kit (cat. no. 206143, Qiagen), primer sequences were matched and amplified using a real-time fluorescence qPCR instrument (Eppendorf, Germany).
Primers were designed and synthesized by a bioengineering company. The following primer sequences were used: NLRP3 upstream and downstream primers: 5ʹ-AACGACCCCTTCATTGAC-3ʹ and 5ʹ-GAGGAAGAGGAGGAAAAGGACA-3ʹ; internal reference GAPDH upstream and downstream primers: 5ʹ-GAAGGTGAGGTCGGAGTC-3ʹ and 5ʹ-GGAGATGGTGATGGGATT −3ʹ.
The PCR conditions were as follows: 95°C, 95°C, 58°C, and 72°C for 5 min, 20 s, 30s, and 30 s, respectively. Each cycle was repeated 40 times. An analysis was performed for each sample in three parallel duplicate wells. NLRP3 expression in platelets was calculated using the 2−ΔΔCt method.15
Western Blotting
Platelet samples were lysed in ice-cold radioimmunoprecipitation assay buffer and vortexed occasionally for 30 min to ensure complete lysis before centrifugation (2.1×104 × g, 10 min) to remove insoluble debris. Each sample was analyzed by SDS-PAGE and the gel was transferred to a polyvinylidene difluoride membrane, which was incubated with primary antibodies (cat. no. IMG-6668A, 1:1000, NLRP3, from Novus Biologicals or cat. no. 5174S, 1:1000, GAPDH, from Cell Signaling Technology) in 3% BSA in TBS-T at 4°C for overnight. Anti-rabbit HRP (cat. no. 7074S, 1:5000, from Cell Signaling Technology) was used as a secondary antibody. An ECL Plus kit was used to develop the blot, and bands were quantified by pixel intensity using ImageJ software.
Flow Cytometry
Platelets were analyzed using a FACSAria. Approximately 300 μL of platelets was fixed with 4% paraformaldehyde for 20 min, permeabilized with 0.1% Triton for 10 min, and incubated with APC-tagged anti-CD41 (cat. no. MA1-19779, 1:100, Thermo), PE-tagged CD62p antibody (cat. no. 12–0626-82, 1:200, Thermo), FITC-tagged PAC-1 antibody (cat. no. MA5-28564, 1:200, Thermo), or FITC-tagged IL-1β antibody (cat. no. 340515, 1:100, BD) in darkness for 30 min, and then added to PBS (10 mM) for flow cytometric analysis. The activation of platelets can be shown by the percentage of CD62p- or PAC-1 positive platelets. The specific binding of APC-tagged anti-CD41 and characteristic side and forward scattering were used to distinguish platelets. FlowJo version V10 (TreeStar) was used to perform the data analysis.
Statistical Analysis
SPSS software (SPSS Inc., Chicago, IL, USA) was used for statistical analyses. Data normality was tested using the one-sample Kolmogorov–Smirnov test. The mean ± standard deviation (SD) was used to express normally distributed continuous variables, whereas the median (interquartile range) was used to express abnormally distributed variables. The number (percentage) for frequencies was adopted to express the results. Normally distributed continuous variables were compared using Student’s t-test, whereas abnormally distributed variables were compared using the Mann–Whitney U-test. The Pearson chi-square test was conducted to compare differences in intergroup categorical data. The association between platelet NLRP3 and clinical parameters was investigated using Pearson’s and Spearman’s rank correlation coefficients (normally distributed and non-normal data, respectively). Receiver operating characteristic (ROC) curves were used to determine the most meaningful cutoff point. Multivariate logistic regression analysis was performed to identify the independent predictors of ACEs after PCI. The Kaplan-Meier test was conducted to perform survival analysis, and the Log rank test was performed to assess whether the differences in curves were statistically significant. Each test was two-sided and P-values of <0.05 were considered to indicate statistical significance.
Results
NLRP3 Expression in the Platelets of Patients with ACS
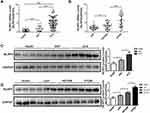
Overall, 25 healthy subjects, 23 patients with SAP, and 72 patients with ACS (23 patients with UAP, 23 patients with STEMI, and 26 patients with NSTEMI) were included in the analysis of platelet NLRP3 expression. The baseline characteristics of the patients are listed in Table 1. Compared with the healthy and SAP groups, the ACS group demonstrated a significantly higher expression level of platelet NLRP3, as detected by qPCR (both P<0.001, Figure 1A), and the STEMI group exhibited the highest expression of NLRP3 among all patients with ACS (Figure 1B). The results were confirmed by Western blotting, which indicated similar differences in NLRP3 expression in different groups (Figure 1C and D).
 |
Table 1 Baseline Characteristics and the Level of Platelet Parameters in the Study Population |
Clinical Significance of Platelet NLRP3 in Patients with ACS
In total, 152 patients who successfully underwent PCI were examined (average 66.32±4.58 years of age, 57.24% males) to analyze the potential clinical significance of platelet NLRP3 in patients with ACS.
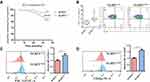
The focus was on the relationship between platelet parameters and NLRP3 expression level (detected by qPCR, which is a more sensitive and reproducible technique than Western blotting). Mean platelet volume (MPV), an indicator of platelet activity, was positively associated with platelet NLRP3 expression (r=0.599, P<0.001) (Figure 2A), whereas platelet count (PLT), platelet volume distribution width (PDW), or platelet crit (PCT) showed no association with platelet NLRP3 expression (P>0.05) (Figure 2B-D). In other clinical parameters, the expression of NLRP3 in patients with ACS was negatively correlated with LVEF (r=−0.288, P<0.001) (Figure 2E), and was not related to HDL-C concentration, LDL-C concentration, or WBC count (P>0.05) (Figure 2F-H).
The expression of platelet NLRP3 gradually increased with the severity of the Gensini score, reflecting the severity of coronary lesions and the positive correlation between the expression of platelet NLRP3 and the Gensini score (r=0.353, P<0.001) (Figure S2A and S2C). Similar results were observed in the Global Acute Coronary Event (GRACE) score when obtained based on a variety of indicators at the time of admission (r=0.561, P<0.001) (Figure S2B and S2D).
ROC Curves for NLRP3
The expression of NLRP3 (2−ΔΔCt) was used as the detection variable to define ACEs within 36 months as the endpoint, set state variable value as 1, and establish an ROC curve (Figure 3A), which suggested that the best cutoff value was 3.8850, with a sensitivity and specificity of 75.4% and 70.5%, respectively.
The patients were divided into groups, with low (n=81) and high expression (n=71) based on platelet NLRP3 expression (Figure 3B), and the clinical parameters of the two groups were compared. Compared with the group with low NLRP3 expression, the group with high NLRP3 expression showed significantly higher STEMI ratio, MPV, hs-CRP, and IL-1β, and significantly lower LVEF (Table 2).
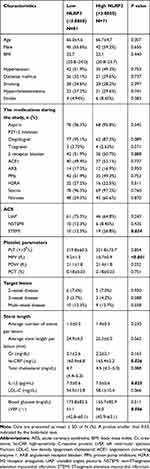 |
Table 2 Baseline Demographic and Clinical Characteristics of Low and High NLRP3 Groups |
Prognostic Value of Platelet NLRP3 in Patients with ACS
Multivariate analyses were performed to further investigate whether platelet NLRP3 expression was a relatively independent prognostic factor for endpoint events within 3-year after PCI. Patient age (odds ratio [OR] =1.056, 95% confidence interval [CI]: 1.003–1.113, P=0.039), hypertension (OR=1.737, 95% CI: 1.046–2.883, P=0.033), and platelet NLRP3 (OR=1.084, 95% CI: 1.035–1.136, P=0.001), which showed a significant prognostic value in univariate analyses, were included in the multivariate analyses. The results indicated that platelet NLRP3 expression (OR=1.071, 95% CI: 1.022–1.123, P=0.004) can be considered as a relatively independent factor in prediction of the prognosis of patients that underwent PCI (Table 3).
 |
Table 3 Univariate and Multivariate Cox Regression Analysis in ACS Patient with PCI |
The Kaplan-Meier curves of groups with low and high platelet NLRP3 expression were plotted, and Log rank tests were performed. The results indicated that patients with high expression of platelet NLRP3 had a significantly poorer prognosis after PCI than those with low expression of platelet NLRP3 (P=0.024), as evidenced by the significantly lower proportion of patients remaining free of postoperative events (Figure 4A). Patients in the NLRP3-low group had one all-cause mortality, two AMI, and two stroke outcomes, whereas patients in the NLRP3-high group had three all-cause mortality, two AMI, five stroke, and three re-PCI/CABG outcomes. Patients in the NLRP3-high group (20.4%±6.1%) showed a significantly higher proportion of IL-1β–expressing platelets (P<0.001) than those in the NLRP3-low group (10.7±3.5%) (Figure 4B). Moreover, the NLRP3-high group exhibited higher platelet activity, as indicated by integrin αIIbβ3 activation (PAC-1 binding, Figure 4C) and P-selectin exposure (CD62P expression, Figure 4D), compared with the NLRP3-low group.
Discussion
In our study, high expression of NLRP3 in platelets was found in patients with ACS. Moreover, correlation analysis found that platelet NLRP3 levels were correlated with the MPV, LVEF, Gensini score, and GRACE score. Multivariate logistic regression analysis showed that platelet NLRP3 was an independent risk factor for ACEs after PCI in patients with ACS. The Kaplan-Meier Log rank tests indicated that patients with high expression of platelet NLRP3 had a significantly poorer prognosis after PCI than those with low expression of platelet NLRP3.
Several studies have indicated a close relationship between the NLRP3 inflammasome and the occurrence and development of cardiovascular diseases.3,16 Inhibition of NLRP3 inflammatory bodies in a rat ischemia-reperfusion model reduced the area of myocardial infarction and the level of cardiac troponin I in serum.17 In ACS, hypoxia and ischemia can increase reactive oxygen species (ROS) production, potassium efflux, and other stressful processes, thereby forming NLRP3 inflammatory bodies and accelerating atherosclerosis. Liu et al18 detected upregulated expression of NLRP3 and an increase in caspase-1 activity and the production of IL-1β and IL-18 in the ischemic heart of a mouse ischemia-reperfusion model, indicating that the inflammatory response mediated by NLRP3 inflammatory bodies was of great importance in the development of ACS.
The IL-1 receptor antagonist (IL-1ra) is the endogenous counter-regulator of IL-1. Recently it has been suggested IL-1 blockade could be beneficial in acute coronary syndrome.19 Over the last three decades, the precise role of AMI-related inflammation response, such as IL-1β production, has been an important topic of pre-clinical animal studies, and clinical research in ACS therapy.20,21 In the MRC-ILA Heart Study, 182 NSTEMI patients were assigned to anakinra, a recombinant IL-1 receptor antagonist, and the results showed that IL-1 blockade could be sufficient to blunt the acute inflammatory response in ACS.22 Other pre-clinical studies of endogenous or exogenous regulation of IL-1β in ACS models also show the beneficial effects,8,20,23,24 therefore, IL-1β antagonism could be a promising therapeutic strategy for ACS.
Besides their presence in monocytes, NLRP3 expression has been recently found in platelets.25,26 During the activation and thrombosis of platelets, the expression of platelet NLRP3 is upregulated and the secretion of platelet inflammatory factors, such as IL-1β, increases.25 Despite the absence of changes in the important platelet surface receptors such as αIIbβ3 integrin, GPIba and GPVI in NLRP3−/- mice, NLRP3-/- mice have a prolonged bleeding time and arterial thrombosis, accompanied by reduced levels of c-Src, Syk, and PLCγ2 phosphorylation. Previous studies have found that engagement of G protein coupled receptors by thrombin can induce the ROS production in platelets, thereby activating platelet NLRP3, leading to the assembly of NLRP3 inflammatory bodies and subsequent caspase-1 activation. Activated caspase-1 transformed immature pre-IL-1β into mature IL-1β, which leads to the phosphorylation of c-Src and Syk and promotes platelet aggregation, spread, and clot shrinkage.27,28 In our study, the expression of platelet NLRP3 in patients with ACS was significantly higher than that in healthy individuals. In addition, the STEMI group exhibited the highest NLRP3 expression, followed by the NSTEMI and UAP groups. These evidences, combined with previous evidence that NLRP3 is involved in platelet function, indicates an important role of platelet NLRP3 in the development of ACS.
Platelets are of vital importance for the formation of atherosclerosis, and may promote intracoronary thrombosis if large and super-active.29 An elevated MPV has been demonstrated widely as a prognostic factor for death, myocardial infarction, and composite endpoint events.30 Therefore, the measurement of MPV at the time of admission is valuable for predicting the degree of arterial openness and the possibility of no-reflow in patients with coronary artery disease (CAD) after PCI.31,32 Patients with AMI have greater platelet density and higher MPV values than healthy individuals.33 With more particles and mitochondria per unit volume, large MPV platelets have a greater ability to produce thromboxane A2, and secrete more transforming growth factor-β, and more are expressed per unit serosal area of glycoprotein IIb/IIIa.34 At present, no reports have been published on the expression of platelet NLRP3 in patients with ACS and its relationship with the clinical characteristics and prognosis. In our study, the analysis of the platelet parameters in patients with ACS revealed a positive correlation between NLRP3 expression and MPV (r=0.599, P<0.001), which was consistent with the involvement of platelet NLRP3 in platelet activation,25 suggesting that the upregulation of NLRP3 may promote platelet activation in ACS.
Patients with ACS may have a poor cardiac function, which manifests as a decreased ejection fraction. The inhibition of NLRP3 inflammatory bodies/caspase-1/IL-1β can ameliorate cardiac remodeling and attenuate left ventricular systolic dysfunction in mice.35 Owing to its important role in the measurement of cardiac function, LVEF is an important factor that can be used to predict the prognosis of myocardial infarction.36 Long-term follow-up showed that LVEF can predict all-cause mortality when echocardiographic measurements were performed for patients after admission as early as possible.37 We found that platelet NLRP3 expression levels were negatively correlated with LVEF (r=−0.288, P<0.001), suggesting that platelet NLRP3 expression may be associated with the degree of impaired heart function in patients with ACS.
In this study, we analyzed the relationship between the expression of platelet NLRP3 and Gensini or GRACE risk scores in patients with ACS that underwent PCI for the first time. The Gensini score is a classic criterion used to assess coronary arteries,38 whereas the GRACE risk score is used to evaluate the severity of CAD. We observed that platelet NLRP3 expression was positively correlated with the Gensini score (r=0.353, P<0.001) and GRACE score (r=0.561, P<0.001), indicating a close relationship between NLRP3 expression and the severity of CAD.
In the analyses of risk factors for ACEs after PCI in patients with ACS, the Multivariate logistic regression analysis identified platelet NLRP3 as an independent risk factor (P=0.004). The incidence of ACEs was increased (P=0.024) in the platelet NLRP3-high group after 3 years of follow-up, suggesting that high NLRP3 expression in platelets was closely related to poor prognosis. The expression of PAC-1 and CD62p was higher in the platelet NLRP3-high group, which indicated that high NLRP3 expression in platelets may be associated with the platelet hyperactivity.
Up to August 30, 2020, coronavirus disease 2019 (COVID-19) has been confirmed in 24854 140 people worldwide, carrying a mortality of approximately 0.15%.39 COVID-19 has been linked to a number of critical cardiovascular complications, including ACS, and even individuals without a history of cardiovascular disease are at risk of cardiovascular complications.40 COVID-19-stimulated innate immune response can triggers activation of the NLRP3 inflammasome.41 The relationship between COVID-19 and prognosis of ACS patients is still largely unknown. It is worth exploring to find out the connection between COVID-19 associated ACS and platelet NLRP3.
This study, for the first time, detected the expression of platelet NLRP3 in patients with ACS, and explored the relationship between platelet NLRP3 expression levels and the clinicopathological characteristics/prognosis of ACS, to provide a novel potential prognostic biomarker and therapeutic target in ACS.
Limitations
Our study had several potential limitations. First, the sample size of the second cohort was small and subgroup analyses were not carried out. Second, circulating levels of IL-1β at different timeframes from ACS were not monitored, which may further explain the role of IL-1β in ACS. Finally, the patients included in the current analysis were whole spectrum of ACS and the prognostic role of platelet NLRP3 in patients with NSTEMI and UAP should be verified in future studies.
Acknowledgments
This study was financially supported by grants from the National Science Foundation of China (grant nos. 81770137, 81572308, 81871934, 81573423, 81772615, 81970298, and 81672334).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Yang N, Liu J, Liu J, et al. Performance on management strategies with Class I Recommendation and a level of evidence among hospitalized patients with non-ST-segment elevation acute coronary syndrome in China: findings from the improving care for Cardiovascular Disease in China-Acute Coronary Syndrome (CCC-ACS) project. Am Heart J. 2019;212:80–90.
2. Bonaventura A, Vecchié A, Abbate A, Montecucco F. Neutrophil extracellular traps and cardiovascular diseases: an update. Cells. 2020;9(1):231. doi:10.3390/cells9010231
3. Duewell P, Kono H, Rayner KJ, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464(7293):1357–1361. doi:10.1038/nature08938
4. Kawaguchi M, Takahashi M, Hata T, et al. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation. 2011;123(6):594–604. doi:10.1161/CIRCULATIONAHA.110.982777
5. Wang L, Qu P, Zhao J, Chang Y. NLRP3 and downstream cytokine expression elevated in the monocytes of patients with coronary artery disease. Arch Med Sci. 2014;10(4):791–800. doi:10.5114/aoms.2014.44871
6. Deng M, Brickey WJ, Guo H, et al. Platelet activating factor as a novel danger signal for activation of NLRP3 inflammasome. J Immunol. 2018;200(1 Supplement):113–115.
7. Berger JS. Oral antiplatelet therapy for secondary prevention of acute coronary syndrome. Am J Cardiovasc Drugs. 2018;18(6):457–472. doi:10.1007/s40256-018-0291-2
8. Everett BM, Cornel JH, Lainscak M, et al. Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation. 2019;139(10):1289–1299. doi:10.1161/CIRCULATIONAHA.118.038010
9. Schrör K, Huber K. Platelets, inflammation and anti-inflammatory drugs in ACS and CAD. Thromb Haemost. 2015;114(3):446–448. doi:10.1160/TH15-08-0632
10. Heger LA, Hortmann M, Albrecht M, et al. Inflammation in acute coronary syndrome: expression of TLR2 mRNA is increased in platelets of patients with ACS. PLoS One. 2019;14(10):e0224181. doi:10.1371/journal.pone.0224181
11. Orrem HL, Nilsson PH, Pischke SE, et al. IL-6 Receptor inhibition by tocilizumab attenuated expression of C5a Receptor 1 and 2 in Non-ST-elevation myocardial infarction. Front Immunol. 2018;9:2035. doi:10.3389/fimmu.2018.02035
12. Wu H, Qian J, Sun A, Wang Q, Ge J. Association of CYP2C19 genotype with periprocedural myocardial infarction after uneventful stent implantation in Chinese patients receiving clopidogrel pretreatment. Circ J. 2012;76(12):2773–2778. doi:10.1253/circj.CJ-12-0635
13. Oz TK, Özbilgin N, Sungur A, et al. Prevalence of metabolic syndrome in young patients with ST-elevation myocardial infarction. Int J Cardiovasc Acad. 2018;4(3):53.
14. Lopes RD, de Barros ESPGM, Damiani LP, et al. Major adverse cardiovascular events after 12 months among patients with acute coronary syndrome receiving loading doses of atorvastatin prior to planned PCI. JAMA. 2020;323(8):787–789. doi:10.1001/jama.2020.0118
15. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi:10.1006/meth.2001.1262
16. Baldrighi M, Mallat Z, Li X. NLRP3 inflammasome pathways in atherosclerosis. Atherosclerosis. 2017;267:127–138. doi:10.1016/j.atherosclerosis.2017.10.027
17. Marchetti C, Chojnacki J, Toldo S, et al. A novel pharmacologic inhibitor of the NLRP3 inflammasome limits myocardial injury after ischemia-reperfusion in the mouse. J Cardiovasc Pharmacol. 2014;63(4):316–322. doi:10.1097/FJC.0000000000000053
18. Liu Y, Lian K, Zhang L, et al. TXNIP mediates NLRP3 inflammasome activation in cardiac microvascular endothelial cells as a novel mechanism in myocardial ischemia/reperfusion injury. Basic Res Cardiol. 2014;109(5):415. doi:10.1007/s00395-014-0415-z
19. Szekely Y, Arbel YJ. A review of Interleukin-1 in heart disease: where do we stand today? Cardiol Ther. 2018;7(1):25–44.
20. Mahtta D, Sudhakar D, Koneru S, et al. Targeting inflammation after myocardial infarction. Curr Cardiol Rep. 2020;22(10):110. doi:10.1007/s11886-020-01358-2
21. Bullenkamp J, Mengoni V, Kaur S, et al. Interleukin-7 and interleukin-15 drive CD4+CD28null T lymphocyte expansion and function in patients with acute coronary syndrome. Cardiovasc Res. 2020. doi:10.1093/cvr/cvaa202
22. Morton AC, Rothman AM, Greenwood JP, et al. The effect of interleukin-1 receptor antagonist therapy on markers of inflammation in non-ST elevation acute coronary syndromes: the MRC-ILA Heart Study. Eur Heart J. 2015;36(6):377–384. doi:10.1093/eurheartj/ehu272
23. Mann DL. Innate immunity and the failing heart: the cytokine hypothesis revisited. Circ Res. 2015;116(7):1254–1268. doi:10.1161/CIRCRESAHA.116.302317
24. Abbate A, Van Tassell BW, Biondi-Zoccai G, et al. Effects of interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction [from the Virginia Commonwealth University-Anakinra Remodeling Trial (2) (VCU-ART2) pilot study]. Am J Cardiol. 2013;111(10):1394–1400. doi:10.1016/j.amjcard.2013.01.287
25. Murthy P, Durco F, Miller-Ocuin JL, et al. The NLRP3 inflammasome and bruton’s tyrosine kinase in platelets co-regulate platelet activation, aggregation, and in vitro thrombus formation. Biochem Biophys Res Commun. 2017;483(1):230–236. doi:10.1016/j.bbrc.2016.12.161
26. Paramel Varghese G, Folkersen L, Strawbridge RJ, et al. NLRP3 inflammasome expression and activation in human atherosclerosis. J Am Heart Assoc. 2016;5(5):e003031. doi:10.1161/JAHA.115.003031
27. Qiao J, Wu X, Luo Q, et al. NLRP3 regulates platelet integrin αIIbβ3 outside-in signaling, hemostasis and arterial thrombosis. Haematologica. 2018;103(9):1568–1576. doi:10.3324/haematol.2018.191700
28. Haneklaus M, O’Neill LA. NLRP3 at the interface of metabolism and inflammation. Immunol Rev. 2015;265(1):53–62. doi:10.1111/imr.12285
29. Ju HY, Kim JK, Hur SM, et al. Could mean platelet volume be a promising biomarker of progression of chronic kidney disease? Platelets. 2015;26(2):143–147. doi:10.3109/09537104.2014.890179
30. Koh YY, Kim HH, Choi DH, et al. Relation between the change in mean platelet volume and clopidogrel resistance in patients undergoing percutaneous coronary intervention. Curr Vasc Pharmacol. 2015;13(5):687–693. doi:10.2174/1570161112666141017121118
31. Colkesen Y, Coskun I, Muderrisoglu H. The effect of aspirin on mean platelet volume in patients with paroxysmal atrial fibrillation. Platelets. 2013;24(4):263–266. doi:10.3109/09537104.2012.682106
32. Haungsaithong R, Udommongkol C, Nidhinandana S, et al. The changes in mean platelet volume after using of antiplatelet drugs in acute ischemic stroke: a randomized controlled trial. J Med Assoc Thai. 2015;98(9):852–857.
33. Martin JF, Plumb J, Kilbey RS, Kishk YT. Changes in volume and density of platelets in myocardial infarction. Br Med J (Clin Res Ed). 1983;287(6390):456–459. doi:10.1136/bmj.287.6390.456
34. Murphy AJ, Tall AR. Disordered haematopoiesis and athero-thrombosis. Eur Heart J. 2016;37(14):1113–1121.
35. Carbone S, Mauro AG, Prestamburgo A, et al. An Orally Available NLRP3 inflammasome inhibitor prevents western diet-induced cardiac dysfunction in mice. J Cardiovasc Pharmacol. 2018;72(6):303–307. doi:10.1097/FJC.0000000000000628
36. Hamada S, Schroeder J, Hoffmann R, et al. Prediction of outcomes in patients with chronic ischemic cardiomyopathy by layer-specific strain echocardiography: a proof of concept. J Am Soc Echocardiography. 2016;29(5):412–420.
37. Zghal FM, Boudiche S, Haboubi S, et al. Diagnostic accuracy of strain imaging in predicting myocardial viability after an ST-elevation myocardial infarction. Medicine. 2020;99(19):e19528.
38. Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease. Rep Am Coll Cardiol Am Heart Assoc Task Force Clin PractGuidelines. 2017;70(2):252–289.
39. Coronavirus disease (COVID-19). Weekly epidemiological update. 2020. 8(31).
40. Zheng -Y-Y, Ma Y-T, Zhang J-Y, Xie XJNRC. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259–260.
41. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.