Back to Journals » Eye and Brain » Volume 12
Exploring The Current Management Idiopathic Intracranial Hypertension, And Understanding The Role Of Dural Venous Sinus Stenting
Authors Gurney SP, Ramalingam S , Thomas A, Sinclair AJ , Mollan SP
Received 31 July 2019
Accepted for publication 5 November 2019
Published 14 January 2020 Volume 2020:12 Pages 1—13
DOI https://doi.org/10.2147/EB.S193027
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Margaret Wong-Riley
Sam P Gurney, 1 Sateesh Ramalingam, 2 Alan Thomas, 2 Alex J Sinclair, 3–5 Susan P Mollan 1
1Birmingham Neuro-Ophthalmology, Queen Elizabeth Hospital, University Hospitals Birmingham, Birmingham B15 2WB, UK; 2Neuroradiology Department, Queen Elizabeth Hospital, University Hospitals Birmingham, Birmingham B15 2WB, UK; 3Metabolic Neurology, Institute of Metabolism and Systems Research, College of Medical and Dental Sciences, University of Birmingham, Birmingham B15 2TT, UK; 4Centre for Endocrinology, Diabetes and Metabolism, Birmingham Health Partners, Birmingham B15 2TH, UK; 5Department of Neurology, University Hospitals Birmingham NHS Foundation Trust, Queen Elizabeth Hospital, Birmingham B15 2WB, UK
Correspondence: Susan P Mollan
Birmingham Neuro-Ophthalmology, Queen Elizabeth Hospital, University Hospitals Birmingham, Birmingham B15 2WB, UK
Tel +44 1213716912 Email [email protected]
Abstract: Idiopathic Intracranial Hypertension (IIH) is a debilitating disorder characterised by raised intracranial pressure (ICP), papilloedema with the potential risk of permanent visual loss, and headaches that are profoundly disabling and reduce the quality of life. The first consensus guidelines have been published on investigation and management of adult IIH and one key area of uncertainty is the utility of dural venous sinus stenting for the management of headache and visual loss. There are an increasing number of series published and to help understand the successes and complications. During a patient physician priority setting, the understanding of the best type of intervention to treat IIH was assigned to the top 10 of most desired research questions for the disease. Ultimately randomised clinical trials (RCTs) in neurovascular stenting for IIH would be instructive, as the literature to date may suffer from publication bias. Due to the increasing incidence of IIH, there is no better time to systematically investigate interventions that may reverse the disease process and achieve remission. In this review we discuss the pathophysiology of IIH in relation to venous sinus stenosis, the role of venous sinus stenting with a review of the relevant literature, the advantages and disadvantages of stenting compared with other surgical interventions, and the future of stenting in the treatment of IIH.
Keywords: pseudotumor cerebri, raised intracranial pressure, venous sinus stenosis, neurovascular stenting, headache, papilloedema
Introduction
Idiopathic intracranial hypertension (IIH) is a disorder of unknown aetiology, primarily affecting women of childbearing age with raised body mass index (BMI), characterised by signs and symptoms of raised intracranial pressure (ICP).1 IIH is a diagnosis of exclusion, typically made using the modified Dandy criteria,2 requiring the presence of raised ICP (>25 cm H2O on lumbar puncture, performed in the lateral decubitus position), with papilloedema typically present, and normal cerebrospinal fluid (CSF) profile and neuroimaging. In a retrospective case review, the incidence of IIH was reported as 1.56 per 100,000 in the general population, increasing to 11.90 per 100,000 in obese young women.3 A recent paper highlighted the growing epidemic of IIH, most notably in areas of social deprivation (thereby mirroring trends in obesity); over their 14-year study period, incidence in the general UK population increased by greater than 100% to 4.7 per 100,000.4 With more and more patients presenting, there is an unmet management need in IIH, namely safe and effective therapies.
The major risk factor for IIH is obesity,5 with truncal fat mass being associated with lumbar puncture opening pressure.6 Hence, management strategies focus on disease modification through weight loss, although this by lifestyle alone is difficult to achieve. The underlying aetiology is not fully understood1,5 but neuroendocrine metabolic pathways are being studied,7 and recently a unique signature of androgen excess in IIH has been implicated and provides evidence that androgens can modulate CSF secretion via the choroid plexus.8 Intriguingly, women with IIH appear to have a 2-fold increase in the cardiovascular disease risk, over and above age, gender and BMI matched controls, providing further evidence that this may well be a neurometabolic disease.9
The clinical presentation varies but symptoms classically include headache; however, our understanding of the traditional phenotype of raised ICP headache has progressed and often those with IIH have headaches that fulfil the criteria for migraine.10,11 Other common symptoms include pulse synchronous tinnitus, transient visual obscurations, double vision (from unilateral or bilateral 6th nerve palsy), reduction in visual acuity or visual field, and neck pain1,12 Previously termed as benign intracranial hypertension, IIH was renamed to reflect the fact that the condition may be associated with severe morbidity, most commonly permanent visual loss, affecting up to 24% of patients in early studies13 and a reduced quality of life which is linked to headache morbidity.14
Following the success of collaborative patient-clinician research15 and the clinical uncertainty highlighted in managing IIH,16 the patient group founded a James Lind Alliance Priority Setting Partnership for adult IIH.17 Understanding of the best type of intervention to treat IIH was placed within the top 10 priorities for research17 highlighting the interest in modern intervention such as dural venous sinus stenting for IIH.
Medical Management Of IIH
Consensus guidance published in 2018 stated uncertainties in all aspects of IIH management.16 Indeed, a Cochrane review of interventions for IIH18 judged the literature in medical management of IIH with acetazolamide, the preferred drug, to be of low quality, despite two randomised controlled trials (RCT).19,20 However, it has been observed that management with weight loss was effective in the treatment of IIH. For example, a prospective study21 has demonstrated that a very low-calorie diet resulting in significant weight loss (15.3±7.0% of body weight) significantly lowered ICP and led to a significant improvement in papilloedema, vision and headache outcomes. There is an ongoing multicentre, randomised controlled trial22 designed to assess if weight loss through bariatric surgery is a more effective sustainable treatment for IIH than lifestyle modification through a community weight management program.
Medical management to reduce ICP is typically with acetazolamide, a carbonic anhydrase inhibitor, which is thought to act by reducing CSF secretion at the choroid plexus. Two RCTs have been carried out to study the effect of acetazolamide versus placebo (with both groups receiving weight-reduction diet and lifestyle modification) for the treatment of IIH. Ball et al19 showed a small benefit for the use of acetazolamide at 12 months, with a reduction in headache, vision loss, transient visual obscurations, and improvement in contrast sensitivity. They did not find evidence of reduction in papilloedema, headache severity, improvement in visual acuity, or visual field parameters. Wall et al20 showed (in the treatment group at 6 months) an improvement in visual field, papilloedema, quality of life, weight loss, and CSF opening pressure. The authors of a Cochrane review based on these two RCTs were however unable to recommend or reject acetazolamide for the treatment of IIH.18 One study suggested non-inferiority of topiramate, an anti-epileptic and migraine prophylaxis, versus acetazolamide.23 The proposed advantage of this drug is its combined effects of carbonic anhydrase inhibition, migraine prophylaxis, and appetite suppression.
A recent in vivo study investigated the commonly cited medications for IIH at both clinical (single equivalent human dose) and high (daily equivalent human dose) namely, subcutaneous acetazolamide 1 g and 4 g; Topiramate 50 mg and 200 mg; and oral preparations of Topiramate 200 mg and Acetazolamide 4 g. At clinical and high doses, subcutaneous administration of topiramate significantly lowered ICP over 2 hrs to 68.6 ± 2.0% of baseline which was a 32% reduction (p < 0.001) and by 79.2 ± 7.5% of baseline, which was a 21% reduction (p < 0.05), respectively, compared to the control. Of note, there was no significant reduction in ICP noted with equivalent to human doses of acetazolamide, amiloride, furosemide, or octreotide. Oral administration of topiramate lowered ICP, and over the first two hours, topiramate significantly reduced ICP compared to baseline (22% reduction, p < 0.05). Compared to placebo, acetazolamide did not significantly lower ICP over the same study period.24 Human physiological studies would be further informative in assessing ICP levels in response to the common medications used. On the horizon may be more medical options, including 11β-Hydroxysteroid Dehydrogenase Type 1 inhibitors.25
Surgical Management For IIH
In less than 10% of IIH4 present with rapidly progressive loss of visual function (termed fulminant IIH) and in whom an acute reduction in ICP is required to preserve vision, surgical interventions are necessary.16,26 Such options include CSF diversion with the commonest surgeries being a ventriculoperitoneal shunt or lumboperitoneal shunt, or optic nerve sheath fenestration (ONSF).
The disadvantages of any intervention are that they can be invasive, carry inherent risks to the patient and may not relieve headache symptoms. Shunt revision surgeries are very common, with a third of patients requiring multiple revision surgeries.27 Ventriculo-peritoneal shunts are preferred16,26 due to the reported lower revision rates compared to lumbo-peritoneal shunts (1.8 versus 4.3 revisions per patient, respectively).28 It is important to counsel patients that ventriculo-peritoneal shunt insertion leads to a temporary driving restriction in some countries such as the UK.16
ONSF surgery is another option, particularly for asymmetric papilloedema or when visual symptoms predominate. It carries the risk of severe complications such as loss of vision, as well as diplopia and pupillary dysfunction.29
Dural Venous Sinus Stenting
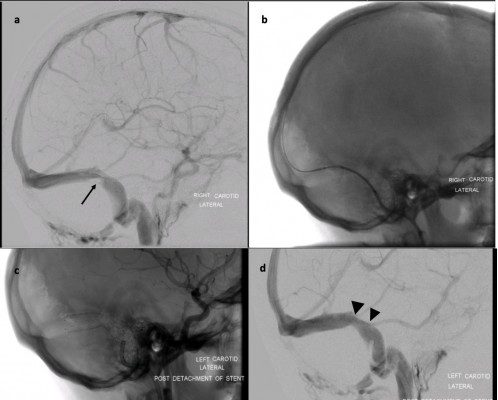
Venous sinus stenting was first described by Higgins et al30 in 2002, with the technique of inserting a catheter into the internal jugular vein to direct a self-expanding stent over a guidewire across a venous sinus stenosis. In this seminal case, the dural venous pressure gradient reduced from 18 mmHg pre-procedure to 3 mmHg post-procedure, with the patient reporting an improvement in their headache. Since then it has been added to the surgical armamentarium, in some centres, for IIH. Several studies (Table 1) have demonstrated seemingly good efficacy and favourable safety profile.30–49 In this paper, we discuss the pathophysiology of IIH in relation to venous sinus stenosis, the rationale and role of venous sinus stenting with a review of the relevant literature, the advantages and disadvantages of stenting compared with other surgical interventions, and the future of stenting in the treatment of IIH.
 |
Table 1 Summary Of Key Studies |
Pathophysiology Relating To Venous Sinus Stenosis
The precise pathogenesis of IIH is still not fully understood, but clinical signs and symptoms point to raised ICP in the absence of hydrocephalus or ventriculomegaly. Therefore, the primary mechanisms are proposed to be CSF hypersecretion, CSF outflow obstruction and, more recently, venous sinus hypertension. Other contributing factors are thought to include cerebral oedema, obesity, hormonal factors, as well as important secondary causes (for example, corticosteroids, certain antibiotics, and vitamin A) and associated disorders (such as anaemia, obstructive sleep apnoea, and renal failure).5,16
Dural venous sinus occlusion, most commonly occurring in the superior sagittal sinus (SSS) or transverse sinus (TS) secondary to thrombosis or a compressive lesion, is known to cause raised ICP and clinical features synonymous with IIH. However, partial occlusion or stenosis with resultant venous sinus hypertension is now increasingly recognised in patients with IIH. It has been hypothesised for some time that patients with IIH have anatomically different dural venous sinuses.50 A link between venous sinus hypertension and IIH was demonstrated by King et al51 in 1995 when manometry was performed on nine patients with IIH. Raised pressure in the SSS and proximal TS was found in all of them, with a mean pressure gradient of 13.3 mmHg. The reported incidence of venous sinus stenosis in patients with IIH ranges from 30% to 93%38 compared with 6.8% among the general population.52 One imaging study50 noted bilateral venous sinus stenosis in 93% of IIH patients, compared with 7% of controls. As such, our understanding about the importance of dural venous haemodynamics in the pathophysiology of IIH has greatly increased in recent years; indeed, venous sinus hypertension has been hypothesised by some to be the cause of IIH.13 However, there is still debate as to whether dural venous sinus stenosis is the cause or consequence of refractory IIH.
CSF absorption occurs primarily via the arachnoid villi of the SSS and is determined by the following equation: CSF absorption = (PCSF–PSSS)/RO, where PCSF is the CSF pressure, PSSS is the SSS pressure, and RO is the resistance across the arachnoid villi.53 This implies that CSF absorption (and thus ICP) are governed by the venous pressure in the SSS.31 To facilitate CSF drainage via the arachnoid granulation, CSF pressure must be higher than venous sinus pressure. The walls of the venous sinuses must therefore be rigid in order to maintain patency when surrounded by higher CSF pressure.
An alternative theory reverses this mechanistic relationship, suggesting that rather than being causative, venous sinus stenosis may occur in susceptible regions of the sinuses as a result of raised ICP. Evidence to support this includes studies showing reversal of venous sinus hypertension, stenosis, and trans-stenotic pressure gradient with reduction in ICP.54–56 Conflicting evidence of persisting venous sinus stenosis despite normalisation of ICP57 suggests more than one mechanism is at play.
To resolve these seemingly opposing mechanisms, it is now thought that two distinct types of venous sinus stenosis exist in association with IIH:
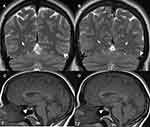
- The first, thought to occur in the majority of patients, is due to extrinsic compression as a consequence of raised ICP, with a long tapering stenosis and normal arachnoid granulations on neuro-imaging. This type of stenosis is reversible with normalisation of ICP (Figure 1). This has also been termed non-venogenic and is thought to be due to abnormal CSF absorption mechanisms.31
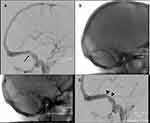
- The second, less common type, is due to intrinsic focal venous sinus stenosis (Figure 2), typically due to arachnoid granulation hypertrophy, fibrosis, or deposition. This “venogenic” type may be due to primary venous pathology (such as thrombosis or vasculitis) or anatomical variation.31 It is thought that this type may initially be pre-symptomatic, requiring a “second hit” such as weight gain or altered CSF dynamics to result in raised ICP. This is supported by evidence from a study which found bilateral venous sinus stenosis in a group of patients with no papilloedema or symptoms of raised ICP.58 This type of stenosis is unresponsive to changes in ICP.
It could be argued that dural venous sinus stenting has no role in a patient with extrinsic venous sinus compression associated with IIH. However, as evidence exists to the contrary, a positive feedback loop, the so-called “collapsible model theory”. A mild increase in ICP causes a degree of venous sinus stenosis in a compressible region, resulting in impaired CSF outflow causing a further rise in ICP with more venous sinus compression, and a resultant increase in the trans-stenotic pressure gradient.59 This has been likened to a so-called Starling resistor whereby raised ICP restricts venous outflow, maintaining equilibrium between blood inflow and CSF outflow. Dural venous sinus stenting in these patients is theorised to increase vessel rigidity, reduce compression and collapse, and therefore interrupt this positive feedback loop. There is still debate as to what initiates this chain of events, but there is evidence of a positive correlation between BMI, mean intracranial venous pressure, and trans-stenotic pressure gradient in patients with IIH suggesting that weight-gain may be the inciting event.60
Role Of Neuro-Imaging In Venous Sinus Stenosis
The choice of neuro-imaging modality is important both in pre-procedure patient evaluation and post-procedure monitoring for the detection of re-stenosis. By using specific imaging protocols to grade venous sinus stenosis, one study reported a sensitivity and specificity of 93% for identifying IIH using MRV.50 It is recognised that the sensitivity of MRV is dependent on technical factors. In order to achieve an acceptable yield when investigating dural venous sinus pathology, neuroimaging should include the whole area from the SSS to the jugular bulb. There is ongoing research into the role of computer-assisted detection to create venous sinus “normograms” in control patients in order to better characterise the sinus changes in patients with suspected IIH.61 Other research suggests that time-of-flight MRV is a reliable representation of endoluminal dural venous sinus dimensions, whereas contrast-enhanced MRV tends to overestimate endoluminal sinus dimensions compared with intravascular ultrasound.62 Conventional cerebral arteriography is associated with small risk of stroke and has been shown to miss some venous sinus lesions in patients with IIH.63 Direct retrograde cerebral venography (DRCV) is more sensitive and has fewer risks, but alone can also miss lesions.51 A gold standard of neuro-imaging has therefore been proposed which combines both DRCV and dural venous manometry.31
When it comes to estimating venous sinus pressure gradients, non-invasive imaging is not thought to be suitable for ruling out clinically significant venous sinus stenosis; one study which aimed to correlate angiographic venous sinus measurements to physiological venous outflow obstruction determined by venous manometry, calculated a sensitivity for detecting a significant trans-stenotic pressure gradient with non-invasive magnetic resonance venography (MRV) or computerised tomography venography (CTV) to be 42%, with a negative predictive value of 22%.64 This compared unfavourably to invasive imaging modalities such as venous phase arteriography (sensitivity 81%, specificity 91%) and venography (sensitivity 92%, specificity 73%). Intravascular ultrasound provides 360° visualization with accurate measurements and information regarding the type of stenosis (intrinsic versus extrinsic).46
There is evidence that quantitative MRV may be a useful tool in post-stenting surveillance; using this imaging modality, venous outflow increases after venous sinus stenting and correlates with significantly improved sinus venous pressure.65 More recently, contrast-enhanced MRV has been shown to be a reliable first-line investigation for monitoring patients following venous sinus stenting, with one group reporting 100% sensitivity and 100% negative predictive value for detection of recurrent dural venous sinus stenosis.66 The obvious advantage of MRV is that it is non-invasive and avoids exposing patients to ionising radiation or contrast medium. Changes in optical coherence tomography imaging parameters have been shown to correlate with visual improvement, and therefore may be used as an objective, anatomical measure of outcomes in patients following venous sinus stenting.67
Establishing A Role Of Venous Sinus Stenting In IIH
One key question is where does venous stenting sits in the management algorithm for IIH, given that currently there is no class 1 evidence for its use in IIH: for example, should it be reserved for those requiring emergency treatment for fulminant disease? Or those refractory to medical management? As outlined above, it is now accepted that venous sinus stenosis and hypertension may play a role in the pathophysiology of IIH. Therefore, it can be argued that when a dural venous stenosis is identified in a patient and where emergency surgical treatment is required, the most appropriate intervention is one that acts directly on venous sinus haemodynamics, namely venous sinus stenting. They argue that other surgical options such as ONSF and CSF diversion procedures act to reduce CSF pressure; they do not act directly on venous sinus haemodynamics and therefore may not modulate the underlying cause.
Similarly, in deciding on the next course of action for an individual with a suboptimal response to weight loss and medical management, it may be important to establish the degree of venous sinus stenosis. One approach is to perform cerebral digital subtraction venography to establish if a stenotic venous sinus is present with a high trans-stenotic pressure gradient (e.g. ≥10 mmHg). If this is the case, treatment with venous sinus stenting may be appropriate. If stenosis is not present, or the trans-stenotic pressure gradient is sufficiently low to be clinically insignificant, one of the treatments to lower ICP may be more appropriate. One proposed set of criteria for dural venous sinus stenting in IIH is given in Table 242 and an overview of the current surgical technique is described by Cappuzzo et al.47
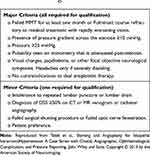 |
Table 2 Proposed criteria for dural venous sinus stenting in IIH adapted from Teleb et al42 |
Advantages Of Venous Sinus Stenting
There is growing evidence in the literature of the efficacy of venous sinus stenting. A recent systematic review and meta-analysis identified 473 patients from 24 studies concluded that in patients with refractory IIH and venous sinus stenosis with elevated pressure gradient, venous sinus stenting is associated with a reduction in pressure gradient and ICP, improvement in signs and symptoms of IIH, and acceptable stent survival rates.68 Venous sinus stenting appears to be equally effective as both a primary or secondary procedure, following CSF diversion.48
Another meta-analysis of 8 studies including 136 patients showed headache improvement in 83%, papilloedema improvement in 97%, and visual acuity improvement in 78%.69 Transient visual obscurations and diplopia, when present, resolved in 76% and 92%, respectively.70 The mean pressure gradient reduced from 20.6 mmHg (pre-stent) to 2.7 mmHg (post-stent).69 A systematic review comparing headache and visual outcomes in IIH following ONSF, lumboperitoneal shunt (LPS), ventriculoperitoneal shunt (VPS), or venous sinus stenting showed a similar improvement in visual outcomes across all modalities but a modest improvement in headache following CSF diversion in combination with venous sinus stenting.71 It is generally accepted however that improvement in headache is not a good proxy for improvement in ICP. There is evidence that venous sinus stenting in the appropriate patient is also associated with a trend towards shorter duration of medical treatment with acetazolamide.72 Longer term survival seems to compare favourably with CSF diversion. One study reported 120-day survival of 87.8% following venous sinus stenting.48 Even more convincing was a meta-analysis of 395 patients with a mean follow up of 18.9 months, in which stent survival in 84% was reported.68
High BMI has been correlated with worse outcomes in IIH, and there is also a positive correlation between BMI and intracranial venous pressure. Recent evidence suggests venous sinus stenting results in an even greater reduction in venous pressure for these “at risk” patients with higher BMI.60 There have been concerns that the effect of venous sinus stenting may not be fast enough to be useful in fulminant IIH. However, there is growing evidence of good efficacy even in patients with acute, severe loss of vision either following high-volume lumbar puncture46 or in conjunction with temporary CSF diversion.52 This is supported by the results of a review in which only 8% of patients with papilloedema at presentation developed optic atrophy, suggesting the speed of ICP normalisation was generally sufficient to prevent irreversible damage.70 Indeed, venous sinus stenting has been shown to result in immediate alteration of the dural venous sinus pressure measurements in patients with IIH.73
Comparing Venous Sinus Stenting To ONSF And CSF Diversion
The efficacy and safety profile of venous sinus stenting also seems to compare favourably to the other surgical treatment options in IIH, namely ONSF and CSF diversion. ONSF is typically used in patients with medically refractory IIH in whom severe visual loss rather than headache is the primary morbidity or where there is asymmetric papilloedema. The procedure involves creating slits in the optic nerve sheath which are thought to modulate CSF flow either directly via a dural fistula or indirectly by inducing fibrosis and blocking CSF flow between the subarachnoid space around the optic nerve and the intracranial optic nerve.74 However, major complications such as orbital or retrobulbar haematoma, orbital cellulitis and traumatic optic neuropathy can occur in 1.5% of patients.75 There is also some evidence of progressive visual decline despite ONSF.75 Regarding CSF diversion, a large meta-analysis showed 86% improvement in headache, 70% improvement in papilloedema, and more modest improvement in vision. However, in this meta-analysis, there were major complications in 7.6% of patient, with shunt infection, subdural haematoma, tonsillar herniation, and CSF fistula occurring.69 Overall, CSF diversion is associated with significant morbidity and high revision rate;27 and up to 43% of patients require additional interventions after CSF diversion, such as shunt revision.1
Disadvantages Of Venous Sinus Stenting
The most common adverse event reported in the literature was transient post-procedure headache, typically lasting days, ipsilateral to the side of stenting which is thought to be secondary to dural stretch. These headaches have been reported in one review to occur in around 30% of patients.70 The rate of serious complications (such as intracranial haemorrhage and venous sinus thrombosis) has been reported as less than 2% in a number of systematic reviews68,76 and around 5% in a large case series.48 Serious complications following venous sinus stenting include subdural haemorrhage,34 intracranial dural arteriovenous fistula formation,77,78 occlusion of the contralateral transverse sinus,79 severe cerebellar haemorrhage.80 Impaired drainage of the ipsilateral vein of Labbé (VOL) has a reported incidence of 13% in one study.81 This is thought to occur when the stent is placed across the transverse and sigmoid sinuses, covering the VOL ostium with potentially severe consequences including cerebral oedema, venous cerebral ischaemia, and cerebral haemorrhage. Procedure-related complications include femoral pseudo-aneurysm, contrast extravasation, and anaphylaxis. In relation to potential thrombotic complications of venous sinus stenting, there is considerable variation in the use of antiplatelets and anticoagulants before and after venous sinus stenting. Most commonly, dual anti-platelet therapy (with aspirin and clopidogrel) is started as a pre-procedure and continued for at least 6 months to allow for epithelialisation of the stent to occur. Further research in this area will hopefully lead to a more standardised approach, and a reduction in the rate of thrombotic complications. In one review, the overall rate of serious complication following venous sinus stenting (2.9%) was higher than following ONSF (1.5%) but significantly lower than following CSF diversion (7.6%).69
The most common cause of failure is re-stenosis, which can occur in or adjacent to an existing stent. In-stent thrombosis may occur secondary to suboptimal placement of the original stent with residual thrombosis proximal to the stent.49 The mechanism of stent-adjacent stenosis (SAS) is poorly understood, but it is recognised as the main reason for stent revision. Postulated mechanisms are raised ICP or pressure gradient, floppy sinuses, uncontrolled endothelialisation, or ongoing extrinsic compression beyond the stented area which continues to incite the pathological positive feedback loop.68 This last point is supported by evidence from Ahmed et al34 where 5 of the 6 patients who required re-stenting had the long tapering stenosis and all of these had SAS. The pattern of change in the trans-stenosis venous pressure gradient may be predictive of SAS.82 The rate of re-stenosis varies in the literature. A meta-analysis of 395 patients with a mean follow-up of 18.9 months estimated the rate of SAS to be 14%.68 Asif et al48 reported the re-stenosis rate to be at least 20% at 120 days. Satti et al69 reported the rate of repeat procedures for re-stenosis to be 10.3%, with conversion to CSF diversion in 2.2%.
Research into the potential predictors for re-treatment has shown possible evidence that raised BMI and African-American race is associated with higher retreatment rates.83 Haemodynamic failure was strongly associated with female gender and pure extrinsic compression of the transverse-sigmoid junction.84 Patient presenting with highly raised opening pressures and those with persisting papilloedema post-procedure are at increased risk of stent failure.85 It is thought there may be a subgroup of IIH patients who are refractory to venous sinus stenting, in whom permanent CSF diversion is still required for disease control.
Conclusion
Our knowledge of IIH is advancing rapidly and a modern approach to the disease entity is emerging.7,9,86 Interventions for those who have precipitous visual loss needs to be investigated in a systematic way to provide a high level of confidence that vision will be preserved or improved. It seems apparent that venous sinus stenting does have a role in the management of IIH. However, clear study outcomes are required to systematically study its placement, and as such as validated trial outcomes are now required which should include both headache and visual outcomes. Although traditionally the primary surgical treatment of medically refractory IIH has been with CSF diversion, the efficacy and low complication rate associated with dural venous sinus stenting is appealing. As the evidence for venous sinus stenting is so far based on retrospective or uncontrolled prospective studies, there is a need for a more robust evidence base to inform practice in this area. Prospective head-to-head trials such as the VISION trial87 comparing venous sinus stenting versus CSF diversion procedure will be of great benefit. Despite good evidence of the effect of stenting on the venous sinus pressure gradient, the effect on CSF pressure is less clearly understood. Recent evidence however shows a significant decrease in CSF opening pressure that is maintained at 3 months post procedure, independent of weight loss or medical therapy.88 Unfortunately, CSF pressure post-stenting and at subsequent follow-up is often not reported in published studies. Further studies specifically measuring CSF pressure before and after stenting will improve our knowledge of this relationship. In order to compare the results from future studies, some authors have suggested angiography to confirm stenosis and pressure gradient.69 There needs to be standardised collection of data relating to patient characteristics, pre- and post-stenting headache metrics, neuro-ophthalmic visual examination, CSF studies, and quality of life measures. Long-term follow-up of clinical features and stent patency also need to be reported.
Disclosure
Dr Allan Thomas report personal fees from MicroVention, outside the submitted work; Miss Susan Mollan reports personal fees from Roche, Santen, Santhera, Allergan, Heidelberg Engineering, and Neurodiem, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Mollan SP, Ali F, Hassan-Smith G, et al. Evolving evidence in adult idiopathic intracranial hypertension: pathophysiology and management. J Neurol Neurosurg Psychiatry. 2016;87(9):982–992. doi:10.1136/jnnp-2015-311302
2. Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology. 2013;81:1159–1165. doi:10.1212/WNL.0b013e3182a55f17
3. Raoof N, Sharrack B, Pepper IM, Hickman SJ. The incidence and prevalence of idiopathic intracranial hypertension in Sheffield, UK. Eur J Neurol. 2011;18:1266–1268. doi:10.1111/ene.2011.18.issue-10
4. Mollan SP, Aguiar M, Evison F, et al. The expanding burden of idiopathic intracranial hypertension. Eye. 2018;81:1159.
5. Markey KA, Mollan SP, Jensen RH, Sinclair AJ. Understanding idiopathic intracranial hypertension: mechanisms, management, and future directions. Lancet Neurol. 2016;15(1):78–79. doi:10.1016/S1474-4422(15)00298-7
6. Hornby C, Botfield H, O’Reilly MW, et al. Evaluating the fat distribution in idiopathic intracranial hypertension using dual-energy X-ray absorptiometry scanning. Neuroophthalmology. 2017;42(2):99–104. doi:10.1080/01658107.2017.1334218
7. Hornby C, Mollan SP, Botfield H, et al. Metabolic concepts in idiopathic intracranial hypertension and their potential for therapeutic intervention. J Neuroophthalmol. 2018;38(4):522–530. doi:10.1097/WNO.0000000000000684
8. O’Reilly MW, Westgate CS, Hornby C, et al. A unique androgen excess signature in idiopathic intracranial hypertension is linked to cerebrospinal fluid dynamics. JCI Insight. 2019;4(6):125348. doi:10.1172/jci.insight.122686
9. Adderley NJ, Subramanian A, Nirantharakumar K, et al. Association between idiopathic intracranial hypertension and risk of cardiovascular diseases in women in the United Kingdom. JAMA Neurol. 2019;8:e191812.
10. Mollan SP, Spitzer D, Nicholl DJ. Raised intracranial pressure in those presenting with headache. BMJ. 2018;363:k3252. doi:10.1136/bmj.k3252
11. Mollan SP, Hoffman J, Sinclair AJ. Advances in the understanding of headache in idiopathic intracranial hypertension. Curr Opin Neurol. 2019;32(1):92–98. doi:10.1097/WCO.0000000000000651
12. Mollan SP, Markey KA, Benzimra JD, et al. A practical approach to, diagnosis, assessment and management of idiopathic intracranial hypertension. Pract Neurol. 2014;14(6):380–390. doi:10.1136/practneurol-2014-000821
13. Corbett JJ, Savino PJ, Thompson HS, et al. Visual loss in pseudotumor cerebri. Follow-up of 57 patients from five to 41 years and a profile of 14 patients with permanent severe visual loss. Arch Neurol. 1982;39:461–474. doi:10.1001/archneur.1982.00510200003001
14. Mulla Y, Markey KA, Woolley RL, et al. Headache determines quality of life in idiopathic intracranial hypertension. J Headache Pain. 2015;16:521. doi:10.1186/s10194-015-0521-9
15. Scotton WJ, Mollan SP, Walters T, et al. Characterising the patient experience of diagnostic lumbar puncture in idiopathic intracranial hypertension: a cross-sectional online survey. BMJ Open. 2018;8(5):e020445. doi:10.1136/bmjopen-2017-020445
16. Mollan SP, Davies B, Silver NC, et al. Idiopathic intracranial hypertension: consensus guidelines on management. J Neurol Neurosurg Psychiatry. 2018;89(10):1088–1100. doi:10.1136/jnnp-2017-317440
17. Mollan SP, Hemmings K, Herd C, et al. What are the research priorities for idiopathic intracranial hypertension? A priority setting partnership between patients and health care professionals. BMJ Open. 2019;9(3):e026573. doi:10.1136/bmjopen-2018-026573
18. Piper R, Kalyvas AV, Young AMH, et al. Interventions for idiopathic intracranial hypertension. Cochrane Database Syst Rev. 2015;8:CD003434.
19. Ball AK, Howman A, Wheatley K, et al. A randomised controlled trial of treatment for idiopathic intracranial hypertension. J Neuro.l. 2011;258:874–881. doi:10.1007/s00415-010-5861-4
20. Wall M, McDermott MP, Kieburtz KD, et al. Effect of acetazolamide on visual function in patients with idiopathic intracranial hypertension: the idiopathic intracranial hypertension treatment trial. JAMA. 2014;311:1641–1651. doi:10.1001/jama.2014.3312
21. Sinclair AJ, Burdon MA, Nightingale PG, et al. Low energy diet and intracranial pressure in women with idiopathic intracranial hypertension: prospective cohort study. BMJ. 2010;341:c2701–c2701. doi:10.1136/bmj.c2701
22. Ottridge R, Mollan SP, Botfield H, et al. Randomised controlled trial of bariatric surgery versus a community weight loss programme for the sustained treatment of idiopathic intracranial hypertension: the Idiopathic Intracranial Hypertension Weight Trial (IIH:WT) protocol. BMJ Open. 2017;7(9):e017426. doi:10.1136/bmjopen-2017-017426
23. Çelebisoy N, Gökçay F, Şirin H, Akyürekli Ö. Treatment of idiopathic intracranial hypertension: topiramate vs acetazolamide, an open-label study. Acta Neurol Scand. 2007;116(5):322–327. doi:10.1111/ane.2007.116.issue-5
24. Scotton WJ, Botfield HF, Westgate CS, et al. Topiramate is more effective than acetazolamide at lowering intracranial pressure. Cephalalgia. 2019;39(2):209–218. doi:10.1177/0333102418776455
25. Markey K, Mitchell J, Botfield H, et al. 11β-hydroxysteroid dehydrogenase type 1 inhibition in idiopathic intracranial hypertension: a double-blind randomized controlled trial. Brain Commun. 2019. in progress.
26. Hoffmann J, Mollan SP, Paemeleire K, et al. European headache federation guideline on idiopathic intracranial hypertension. J Headache Pain. 2018;19(1):93. doi:10.1186/s10194-018-0919-2
27. Sinclair AJ, Kuruvath S, Sen D, et al. Is cerebrospinal fluid shunting in idiopathic intracranial hypertension worthwhile? A 10-year review. Cephalalgia. 2011;31:1627–1633. doi:10.1177/0333102411423305
28. Kalyvas AV, Hughes M, Koutsarnakis C, et al. Efficacy, complications and cost of surgical interventions for idiopathic intracranial hypertension: a systematic review of the literature. Acta Neurochir (Wien.). 2017;159(1):33–49. doi:10.1007/s00701-016-3010-2
29. Gilbert AL, Chwalisz B, Mallery R. Complications of optic nerve sheath fenestration as a treatment for idiopathic intracranial hypertension. Semin Ophthalmol Y. 2018;33(1):36–41. doi:10.1080/08820538.2017.1353810
30. Higgins JN, Owler BK, Cousins C, et al. Venous sinus stenting for refractory benign intracranial hypertension. Lancet. 2002;359:228–230. doi:10.1016/S0140-6736(02)07440-8
31. Owler BK, Parker G, Halmagyi GM, et al. Pseudotumor cerebri syndrome: venous sinus obstruction and its treatment with stent placement. J Neurosurg. 2003;98(5):1045–1055. doi:10.3171/jns.2003.98.5.1045
32. Donnet A, Metellus P, Levrier O, et al. Endovascular treatment of idiopathic intracranial hypertension: clinical and radiologic outcome of 10 consecutive patients. Neurology. 2008;70:641–647. doi:10.1212/01.wnl.0000299894.30700.d2
33. Bussière M, Falero R, Nicolle D, et al. Unilateral transverse sinus stenting of patients with idiopathic intracranial hypertension. Am J Neuroradiol. 2010;31:645–650. doi:10.3174/ajnr.A1890
34. Ahmed RM, Wilkinson M, Parker GD, et al. Transverse sinus stenting for idiopathic intracranial hypertension: a review of 52 patients and of model predictions. Am J Neuroradiol. 2011;32:1408–1414. doi:10.3174/ajnr.A2575
35. Albuquerque FC, Dashti SR, Hu YC, et al. Intracranial venous sinus stenting for benign intracranial hypertension: clinical indications, technique, and preliminary results. World Neurosurg. 2011;75:648–652. doi:10.1016/j.wneu.2010.11.012
36. Lazzaro MA, Darkhabani Z, Remler BF, et al. Venous sinus pulsatility and the potential role of dural incompetence in idiopathic intracranial hypertension. Neurosurgery. 2012;71(4):877–884. doi:10.1227/NEU.0b013e318267a8f9
37. He C-ZC, Ji X-MX, Wang L-JL, et al. Endovascular treatment for venous sinus stenosis in idiopathic intracranial hypertension. Zhonghua Yi Xue Za Zhi. 2012;92(11):748–751.
38. Kumpe DA, Bennett JL, Seinfeld J, et al. Dural sinus stent placement for idiopathic intracranial hypertension. J Neurosurg. 2012;116:538–548. doi:10.3171/2011.10.JNS101410
39. Radvany MG, Solomon D, Nijjar S, et al. Visual and neurological outcomes following endovascular stenting for pseudotumor cerebri associated with transverse sinus stenosis. J Neuroophthalmol. 2013;33:117–122. doi:10.1097/WNO.0b013e31827f18eb
40. Fields JD, Javedani PP, Falardeau J, et al. Dural venous sinus angioplasty and stenting for the treatment of idiopathic intracranial hypertension. J Neurointerv Surg. 2013;5:62–68. doi:10.1136/neurintsurg-2011-010156
41. Ducruet AF, Crowley RW, McDougall CG, Albuquerque FC. Long-term patency of venous sinus stents for idiopathic intracranial hypertension. J Neurointerv Surg. 2014;6:238–242. doi:10.1136/neurintsurg-2013-010691
42. Teleb MS, Cziep ME, Issa M, et al. Stenting and angioplasty for idiopathic intracranial hypertension: a case series with clinical, angiographic, ophthalmological, complication, and pressure reporting. J Neuroimaging. 2015;25:72–80. doi:10.1111/jon.2015.25.issue-1
43. Aguilar-Perez M, Martinez-Moreno R, Kurre W, et al. Endovascular treatment of idiopathic intracranial hypertension: retrospective analysis of immediate and long-term results in 51 patients. Neuroradiology. 2017;59:277–287. doi:10.1007/s00234-017-1783-5
44. Liu KC, Starke RM, Durst CR, et al. Venous sinus stenting for reduction of intracranial pressure in IIH: a prospective pilot study. J Neurosurg. 2017;127:1126–1133. doi:10.3171/2016.8.JNS16879
45. Satti SR, Leishangthem L, Spiotta A, Chaudry MI. Dural venous sinus stenting for medically and surgically refractory idiopathic intracranial hypertension. Interv Neuroradiol. 2017;23:186–193. doi:10.1177/1591019916680110
46. Dinkin MJ, Patsalides A. Venous sinus stenting in idiopathic intracranial hypertension: results of a prospective trial. J Neuroophthalmol. 2017;37(2):113–121. doi:10.1097/WNO.0000000000000426
47. Cappuzzo JM, Hess RM, Morrison JF, et al. Transverse venous stenting for the treatment of idiopathic intracranial hypertension, or pseudotumor cerebri. Neurosurg Focus. 2018;45(1):E11. doi:10.3171/2018.5.FOCUS18102
48. Asif H, Craven CL, Siddiqui AH, et al. Idiopathic intracranial hypertension: 120-day clinical, radiological, and manometric outcomes after stent insertion into the dural venous sinus. J Neurosurg. 2018;129:723–731. doi:10.3171/2017.4.JNS162871
49. Shields LBE, Shields CB, Yao TL, et al. Endovascular treatment for venous sinus stenosis in idiopathic intracranial hypertension: an observational study of clinical indications, surgical technique, and long-term outcomes. World Neurosurg. 2019;121:e165–e171. doi:10.1016/j.wneu.2018.09.070
50. Farb RI, Vanek I, Scott JN, et al. Idiopathic intracranial hypertension: the prevalence and morphology of sinovenous stenosis. Neurology. 2003;60(9):1418–1424. doi:10.1212/01.WNL.0000066683.34093.E2
51. King JO, Mitchell PJ, Thomson KR, Tress BM. Cerebral venography and manometry in idiopathic intracranial hypertension. Neurology. 1995;45(12):2224–2228. doi:10.1212/WNL.45.12.2224
52. Elder BD, Rory Goodwin C, Kosztowski TA, et al. Venous sinus stenting is a valuable treatment for fulminant idiopathic intracranial hypertension. J Clin Neurosci. 2015;22:685–689. doi:10.1016/j.jocn.2014.10.012
53. Davson H, Hollingsworth M, Segal MB. The mechanism and drainage of the cerebrospinal fluid. Brain. 1973;93:665–678. doi:10.1093/brain/93.4.665
54. King JO, Mitchell PJ, Thompson KR, Tress BM. Manometry combined with cervical puncture in idiopathic intracranial hypertension. Neurology. 2002;58:26–30. doi:10.1212/WNL.58.1.26
55. Rohr A, Dörner L, Stingele R, et al. Reversibility of venous sinus obstruction in idiopathic intracranial hypertension. Am J Neuroradiol. 2007;28(4):656–659.
56. Buell TJ, Raper DMS, Pomeraniec IJ, et al. Transient resolution of venous sinus stenosis after high-volume lumbar puncture in a patient with idiopathic intracranial hypertension. J Neurosurg. 2018;129(1):153–156. doi:10.3171/2017.3.JNS163181
57. Bono F, Giliberto C, Mastrandrea C, et al. Transverse sinus stenoses persist after normalization of the CSF pressure in IIH. Neurology. 2005;65(7):1090–1093. doi:10.1212/01.wnl.0000178889.63571.e5
58. Kelly LP, Saindane AM, Bruce BB, et al. Does bilateral transverse cerebral venous sinus stenosis exist in patients without increased intracranial pressure? Clin Neurol Neurosurg. 2013;115(8):1215–1219. doi:10.1016/j.clineuro.2012.11.004
59. Stevens SA, Previte M, Lakin WD, et al. Idiopathic intracranial hypertension and transverse sinus stenosis: a modelling study. Math Med Biol. 2007;24:85–109. doi:10.1093/imammb/dql025
60. Raper DMS, Ding D, Buell TJ, et al. Effect of body mass index on venous sinus pressures in idiopathic intracranial hypertension patients before and after endovascular stenting. Neurosurgery. 2018;82(4):555–561. doi:10.1093/neuros/nyx186
61. Anconina R, Zur D, Kesler A, et al. Creating normograms of dural sinuses in healthy persons using computer-assisted detection for analysis and comparison of cross-section dural sinuses in the brain. J Clin Neurosci. 2017;40:190–194. doi:10.1016/j.jocn.2017.02.006
62. Boddu SR, Gobin P, Oliveira C, et al. Anatomic measurements of cerebral venous sinuses in idiopathic intracranial hypertension patients. PLoS One. 2018;13(6):e0196275. doi:10.1371/journal.pone.0196275
63. Karahalios DG, Rekate HL, Khayata MH, et al. Elevated intracranial venous pressure as a universal mechanism in pseudotumor cerebri of varying etiologies. Neurology. 1996;46:198–202. doi:10.1212/WNL.46.1.198
64. West JL, Greeneway GP, Garner RM, et al. Correlation between angiographic stenosis and physiologic venous sinus outflow obstruction in idiopathic intracranial hypertension. J Neurointerv Surg. 2019;11(1):90–94. doi:10.1136/neurintsurg-2018-014004
65. Esfahani DR, Stevenson M, Moss HE, et al. Quantitative magnetic resonance venography is correlated with intravenous pressures before and after venous sinus stenting. Neurosurgery. 2015;77:254–260. doi:10.1227/NEU.0000000000000771
66. Boddu SR, Gobin P, Oliveira C, et al. Contrast enhanced magnetic resonance venography in the follow-up evaluation of idiopathic intracranial hypertension patients with cerebral venous sinus stenting. Clin Imaging. 2018;50:330–335. doi:10.1016/j.clinimag.2018.04.016
67. Smith KA, Peterson JC, Arnold PM, et al. A case series of dural venous sinus stenting in idiopathic intracranial hypertension: association of outcomes with optical coherence tomography. Int J Neurosci. 2017;127:145–153. doi:10.3109/00207454.2016.1152967
68. Saber H, Lewis W, Sadeghi M, et al. Stent survival and stent-adjacent stenosis rates following venous sinus stenting for idiopathic intracranial hypertension: a systematic review and meta-analysis. Interv Neurol. 2018;7(6):490–500. doi:10.1159/000490578
69. Satti SR, Leishangthem L, Chaudry MI. Meta-analysis of CSF diversion procedures and dural venous sinus stenting in the setting of medically refractory idiopathic intracranial hypertension. Am J Neuroradiol. 2015;36(10):1899–1904. doi:10.3174/ajnr.A4377
70. Dinkin MJ, Patsalides A. Venous sinus stenting for idiopathic intracranial hypertension: where are we now? Neurol Clin. 2017;35:59–81. doi:10.1016/j.ncl.2016.08.006
71. Lai LT, Danesh-Meyer HV, Kaye AH. Visual outcomes and headache following interventions for idiopathic intracranial hypertension. J Clin Neurosci. 2014;21:1670–1678. doi:10.1016/j.jocn.2014.02.025
72. Shazly TA, Jadhav AP, Aghaebrahim A, et al. Venous sinus stenting shortens the duration of medical therapy for increased intracranial pressure secondary to venous sinus stenosis. J Neurointerv Surg. 2018;10:310–314. doi:10.1136/neurintsurg-2017-013103
73. Boddu SR, Gobin P, Oliveira C, Dinkin M, Patsalides A. Pressure variations in cerebral venous sinuses of idiopathic intracranial hypertension patients. J Vasc Interv Neurol. 2018;10(1):25–30.
74. Banta JT, Farris BK. Pseudotumor cerebri and optic nerve sheath decompression. Ophthalmology. 2000;107:1907–1912. doi:10.1016/S0161-6420(00)00340-7
75. Uretsky S. Surgical interventions for idiopathic intracranial hypertension. Curr Opin Ophthalmol. 2009;20:451–455. doi:10.1097/ICU.0b013e3283313c1c
76. Nicholson P, Brinjikji W, Radovanovic I, et al. Venous sinus stenting for idiopathic intracranial hypertension: a systematic review and meta-analysis. J Neurointerv Surg. 2018. doi:10.1136/neurintsurg-2018-014172
77. Li K, Ren M, Meng R, Wang F, Ji X. Dural arteriovenous fistula formation complicated cerebral venous sinus stenosis after venous sinus stenting. World Neurosurg. 2018;120:400–402. doi:10.1016/j.wneu.2018.08.230
78. Buell TJ, Raper DM, Ding D, et al. Development of an intracranial dural arteriovenous fistula after venous sinus stenting for idiopathic intracranial hypertension. J Neurointerv Surg. 2018;10(7):e15. doi:10.1136/neurintsurg-2017-013282.rep
79. Coffman SA, Singh J, Wolfe S, Fargen KM. Unexpected occlusion of the contralateral transverse sinus after stenting for idiopathic intracranial hypertension. Interv Neuroradiol. 2018;24(6):718–721. doi:10.1177/1591019918787161
80. Lavoie P, Audet MÈ, Gariepy JL, et al. Severe cerebellar hemorrhage following transverse sinus stenting for idiopathic intracranial hypertension. Interv Neuroradiol. 2018;24(1):100–105. doi:10.1177/1591019917734389
81. Boddu SR, Gobin P, Dinkin M, et al. Impaired drainage of vein of Labbé following venous sinus stenting for idiopathic intracranial hypertension. J Neurointerv Surg. 2018;11:1–7.
82. Raper D, Buell TJ, Ding D, et al. Pattern of pressure gradient alterations after venous sinus stenting for idiopathic intracranial hypertension predicts stent-adjacent stenosis: a proposed classification system. J Neurointerv Surg. 2018;10(4):391–395. doi:10.1136/neurintsurg-2017-013135
83. El Mekabaty A, Obuchowski NA, Luciano MG, et al. Predictors for venous sinus stent retreatment in patients with idiopathic intracranial hypertension. J Neurointerv Surg. 2017;9(12):1228–1232.
84. Kumpe DA, Seinfeld J, Huang X, et al. Dural sinus stenting for idiopathic intracranial hypertension: factors associated with hemodynamic failure and management with extended stenting. J Neurointerv Surg. 2016;9:867–874. doi:10.1136/neurintsurg-2016-012810
85. Goodwin CR, Elder BD, Ward A, et al. Risk factors for failed transverse sinus stenting in pseudotumor cerebri patients. Clin Neurol Neurosurg. 2014;127:75–78. doi:10.1016/j.clineuro.2014.09.015
86. Mitchell J, Mollan SP, Vivek V, Sinclair AJ. Novel advances in monitoring and therapeutic approaches in IIH. Curr Opin Neurol. 2019;32(3):422–443. doi:10.1097/WCO.0000000000000690
87. Chandran A, Pulhorn H, McMahon C. Idiopathic intracranial hypertension VISION (Venous intervention versus shunting in IIH for optic nerve swelling) trial: patient perspective questionnaire. Br J Neurosurg. 2017;21:1–5.
88. Patsalides A, Oliveira C, Wilcox J, et al. Venous sinus stenting lowers the intracranial pressure in patients with idiopathic intracranial hypertension. J Neurointerv Surg. 2018. doi:10.1136/neurintsurg-2018-014032
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.