Back to Journals » Clinical Ophthalmology » Volume 16
Explantation of KAMRA Corneal Inlay: 10-Year Occurrence and Visual Outcome Analysis
Authors Moshirfar M , Lau CK , Chartrand NA , Parsons MT , Stapley S , Bundogji N, Ronquillo YC , Linn SH , Hoopes PC
Received 20 July 2022
Accepted for publication 16 September 2022
Published 10 October 2022 Volume 2022:16 Pages 3327—3337
DOI https://doi.org/10.2147/OPTH.S382544
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Majid Moshirfar,1– 3 Chap-Kay Lau,4 Nicholas A Chartrand,4 Mark T Parsons,4 Seth Stapley,5 Nour Bundogji,2 Yasmyne C Ronquillo,1 Steven H Linn,1 Phillip C Hoopes1
1Hoopes Vision Research Center, Hoopes Vision, Draper, UT, USA; 2John A. Moran Eye Center, University of Utah School of Medicine, Salt Lake City, UT, USA; 3Utah Lions Eye Bank, Murray, UT, USA; 4University of Arizona, College of Medicine-Phoenix, Phoenix, AZ, USA; 5Arizona College of Osteopathic Medicine, Midwestern University, Glendale, AZ, USA
Correspondence: Majid Moshirfar, Medical Director Hoopes Vision Research Center, Hoopes Vision Research Center, 11820 S. State St. #200, Draper, UT, 84020, USA, Tel +1 801-568-0200, Fax +1 801-563-0200, Email [email protected]
Purpose: To evaluate 10 years of KAMRA corneal inlay explantation and associated visual outcomes.
Patients and Methods: Single-site retrospective chart review of 22 cases of AcuFocus KAMRA Inlay (ACI7000PDT) explantation (range 1 week– 1 year). Uncorrected distance visual acuity (UDVA), uncorrected near visual acuity (UNVA), corrected distance visual acuity (CDVA), and manifest refraction at 1 day, 1 week, 1 month, 3 months, 6 months, and 1 year post-explantation were reviewed.
Results: The explantation rate was 8.2% across 10 years. All patients underwent KAMRA explantation due to dissatisfaction with their vision including blurry near vision, impaired night vision, decreased vision in dim lighting, streaks or halos, haze, and double vision. Mean UDVA pre-implant was − 0.01± 0.13 logMAR (logarithm of the minimal angle of resolution), 0.30± 0.22 logMAR pre-explant, and 0.16± 0.15 logMAR post-explant (n=20). Mean UNVA pre-implant was 0.37± 0.09 logMAR, 0.38± 0.13 logMAR pre-explant, and 0.42± 0.21 logMAR post-explant (n=20). Mean CDVA pre-implant was − 0.01± 0.04 logMAR and 0.05± 0.11 logMAR post-explant (n=17). Mean CDVA pre-explant was 0.04± 0.07 logMAR and 0.04± 0.11 logMAR post-explant (n=19). Significant differences were observed between pre-implant and post-explant UDVA (p=0.009), and between pre-explant and post-explant UDVA (p=0.02). All patients (100%) had 20/20 or better CDVA pre-implant but decreased to 73.7% post-explant. Sixty percent (12/20) of the patients lost UDVA Snellen acuity lines post-explant. MRSE was − 0.31± 0.29 D pre-implant and +0.26± 0.77 D post-explant (p=0.007) with note of a hyperopic shift. The hyperopic shift in 31.6% (6/19) of patients did not resolve after explantation. Post-explant residual corneal haze occurred in 72.7% (16/22) of patients.
Conclusion: Although the KAMRA corneal inlay is a removable device, patients may experience residual corneal haze, hyperopic shift, and deficits in UDVA after explantation compared to pre-implantation UDVA.
Keywords: presbyopia, cornea, small aperture inlay, KAMRA, explantation
Introduction
KAMRA (AcuFocus Inc., Irvine, CA, USA, recently acquired by CorneaGen, Seattle, WA) is a small aperture corneal inlay designed to correct emmetropic presbyopia inlay by increasing the depth of focus through the pinhole effect. Made of polyvinylidene difluoride (PVDF), this 3.8 mm annular inlay with a 1.6 mm aperture and a thickness of 5 μm is surgically implanted into the non-dominant eye within a femtosecond-created intrastromal pocket. It was first approved by the FDA in 2015 for use in patients aged 45–60 years and has been used in both natural and post-LASIK emmetropic eyes.1 By 2017, KAMRA had been implanted in over 20,000 eyes worldwide.1 However, CorneaGen discontinued the product in the United States in February 2022.
Studies have shown overall safe and effective outcomes, including improvement in near vision with minimal change in distance vision.2,3 Complications include corneal haze, hyperopic shift, and potential impairment of CDVA.4,5 While many patients in these studies reported positive results, a subset of patients requested that the inlay be removed. FDA clinical trials found that 7.1% had undergone explantation by 24 months.6 Some studies have investigated these cases of explantation, identifying corneal haze or other visual disturbances as reasons for removal.7,8 The objective of our paper is to discuss the visual outcomes of 22 cases of explanted KAMRA corneal inlay at a single site over the span of 10 years.
Methods
This is a retrospective, single-site study of patients who underwent KAMRA inlay explantation at Hoopes Vision (Draper, UT, USA) between 11/27/2012 and 4/13/2022. Third-generation KAMRA inlays (ACI7000PDT) were implanted as part of a Phase III sponsor-initiated clinical trial from 11/10/2009 to 12/9/2009, and after gaining US-FDA approval, from 5/19/2015 to 5/11/2021. KAMRA implant and explant surgeries were performed by two surgeons.
Charts were reviewed to obtain patient demographics and clinical information including past ocular history. Pre-implantation, pre-explantation, and post-explantation uncorrected distance visual acuity (UDVA), uncorrected near visual acuity (UNVA), corrected distance visual acuity (CDVA), and manifest refraction were collected. Pre-implantation and pre-explantation data were gathered at the time point closest to the surgery date. Additionally, UDVA, UNVA, CDVA, and manifest refraction at 1 day, 1 week, 1 month, 3 months, 6 months, and 1 year post-explantation were analyzed. Visual acuities were measured using Snellen Chart at 20 feet for UDVA and CDVA, Rosenbaun Near Card at 14 inches for UNVA, and in eight cases, ETDRS chart at 4 meters for UDVA and CDVA. Visual acuities recorded in Jaeger or ETDRS units were converted to Snellen Acuity. Patient-reported symptoms, slit-lamp corneal exam findings at each time point, average depth of implantation, and reason for explantation were also collected.
This retrospective study was approved by Biomedical Research Alliance of New York (BRANY, Lake Success, NY, IRB number: #A20‐12‐547‐823) IRB and all procedures conform to the Declaration of Helsinki. Patient data were de-identified prior to analysis.
Statistical Analysis
The initial statistical analyses were done on Microsoft Excel’s Data Analysis tool (Redmond, WA, USA 2022). Results were re-analyzed using GraphPad Prism (San Diego, CA, USA 2022). Prior to statistical analysis, visual acuity data were converted from Snellen Acuity or Jaeger units into logMAR units. Demographic and visual acuity data were analyzed and presented in mean, standard deviation, minimum, and maximum. D’Agostino–Pearson, Anderson–Darling, Shapiro–Wilk, and Kolmogorov–Smirnov tests were run to determine the normality of the data sets. Significant differences between visual acuity at pre-implantation and post-explantation, and at pre- and post-explantation were determined by performing Wilcoxon matched-pairs signed rank tests if one or both sample sets did not follow normal distribution, while paired t-tests were performed if both sample sets followed normal distribution. A p-value of less than 0.05 was considered statistically significant. Time points with the most complete data were used; patients were excluded from statistical analysis if data were missing or incomplete. Since the MRSE difference between pre-implant and post-explant was found to be significant, vector graphs were constructed using the Alpins method to verify refractive changes graphically (Figure 1).
 |
Figure 1 Double angle vector diagrams (DAVD) pre-implantation (A) and post-explantation (B) that show differences in the arithmetic mean of astigmatism and centroid of the axis. |
Results
Of the 267 patients who received KAMRA inlay, 22 (8.2%) underwent explantation (Appendix 1). The mean age at explantation was 55.86±5.20 years (range, 46–64 years), and the procedure was done mainly in the left eye (81.8%). Time between implant and explant ranged from 5 to 148 months, with an average of 44.3±37.7 months. The average depth of KAMRA implantation was 220.7±17.8 µm (range, 205–250 µm).
Most patients had multiple reasons for explantation, but all reported dissatisfaction with their vision. Reasons for explantation included inadequate or blurry near vision (86.3%), impaired night vision (68.2%), decreased vision in dim lighting (45.5%), white streaks/halos (36.4%), haze (31.8%), dry eye (27.3%), double vision (18.2%) and headache (4.5%). Additionally, objective corneal haze findings were reported in 8/22 cases of pre-explantation. Fifteen of 22 cases had incomplete data at the one-year follow-up visit post-explantation. Of those with complete data, complaints included perpetual haze, being slow to focus, blurry vision, dry eye, sensation of monovision, and inadequate near vision.
In the 22 explant cases, 16 (72.7%) had post-explantation corneal haze findings (range 1 week to 1 year). One case (4.5%) had fibrosis at the KAMRA implantation site (post-operation 1 month), 3 (13.6%) had clear cornea (post-operation range 3 months to 1 year), and 2 (9.1%) did not have recorded findings. Figure 2 compares the corneal profile at different time points and Figure 3 displays changes in the optical coherence tomography (OCT) of stromal and epithelial maps.
Of the cohort that underwent KAMRA explantation, none of the eyes had simultaneous refractive surgery. Two eyes had a history of LASIK 9 and 13 years prior, and one eye had a history of PRK 20 years prior to KAMRA implantation. Two eyes had PRK enhancement between implantation and explantation for hyperopic shift (Figure 4). Only one eye required repositioning due to decentration and all other eyes had excellent centration. Notably, Case 1 received KAMRA implantation not for the indication of presbyopia, but to help improve the symptoms of a trauma-induced correctopia. Nearly 4 years after implantation, the same eye underwent pupiloplasty and cataract removal via phacoemulsification with an intraocular lens (IOL) implant. Five years later, KAMRA was removed due to dissatisfaction with both near and distance vision.
The comparison between pre- and post-explantation UDVA, UNVA, and CDVA is shown in Table 1. There was a statistically significant improvement in the mean UDVA from 0.30±0.22 logMAR to 0.16±0.15 logMAR (p=0.02), while the mean UNVA declined non-significantly from 0.38±0.13 logMAR to 0.42±0.21 logMAR (p=0.51), and mean CDVA had no significant changes from 0.04±0.07 logMAR to 0.04±0.11 logMAR (p=0.80). Two cases were excluded from UDVA and UNVA statistical analysis, and three cases were excluded from CDVA statistical analysis because data was incomplete. There were no statistically significant changes between pre- and post-explant sphere, absolute magnitude of cylinder, axis, and manifest refraction spherical equivalent (MRSE) (p=0.73, 0.99, 0.36, 0.66 respectively) (Table 1).
 |
Table 1 Comparisons Between KAMRA Pre-Explantation and Post-Explantation |
Pre-implantation and post-explantation UDVA, UNVA and CDVA are summarized in Table 2. There was a statistically significant decline in the mean UDVA from −0.01±0.13 logMAR to 0.16±0.15 logMAR (p=0.0009), while the mean UNVA improved non-significantly from 0.37±0.09 logMAR to 0.42±0.21 logMAR (p=0.36), and the mean CDVA declined non-significantly from −0.01±0.04 logMAR to 0.05±0.11 logMAR (p=0.09). Two cases were excluded from UDVA and UNVA statistical analysis, and five cases were excluded from CDVA statistical analysis because data was incomplete. From pre-implant to post-explant, the axis had no significant change. However, sphere increased significantly from −0.14±0.27 D to −0.57±0.82 D (p=0.003), absolute magnitude of cylinder increased significantly from −0.35±0.24 D to −0.61±0.41 D (p=0.02), and the MRSE showed a significant hyperopic shift from −0.31±0.29 D to +0.26±0.77 D (p=0.007). The double angle vector diagrams show that the arithmetic mean of astigmatism pre-implant was 0.34 D (Figure 1A) and 0.6 D post-implant (Figure 1B) (comparable to that in the table, using negative cylinder notation of −0.31 D and −0.6 D), a significant change. The vector diagrams also show a difference in the centroid with a pre-implantation value of 0.03 D × 105 and post-implantation value of 0.16 D × 104. The axis had no significant change.
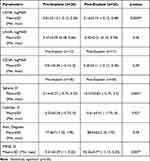 |
Table 2 Comparisons Between KAMRA Pre-Implantation and Post-Explantation |
Figure 5 displays visual acuity pre-implantation versus post-explantation.
UDVA: Pre-implantation, 59.1% were 20/20 and 4.5% were 20/40 or worse. Post-explantation 25% were 20/20, and 35% were 20/40 or worse (Figure 5A).
CDVA: Pre-implantation 77.3% were 20/20 and 0% were 20/40 or worse. Post-explantation 68.4% were 20/20 and 10.5% were 20/40 or worse (Figure 5B).
Cumulative UDVA and CDVA are defined as the total percentage of patients at a specified visual acuity or better than the specified visual acuity.
Cumulative UDVA: Pre-implantation 73% were 20/20 or better and 100% were 20/40 or better. Post-explanation 30% were 20/20 or better and 95% were 20/40 or better (Figure 5C).
Cumulative CDVA: Pre-implantation 100% of the cases were 20/20 or better. Post-explantation 73.7% were 20/20 or better and 100% were 20/40 or better (Figure 5D).
Of the 18 cases with recorded pre-implantation and post-explantation MRSE data, 8 out of 18 (44.4%) cases of pre-explantation experienced a hyperopic shift noted in MRSE, of which only two cases resolved post-explantation. There was also one case in 18 eyes (5.6%) of pre-explantation myopic shift that fully resolved post explantation. Mean MRSE was −0.31±0.29 pre-implantation and +0.26±0.77 post-explantation (Figure 6).
 |
Figure 6 Average MRSE for total patients from pre-implant to post-explant. Abbreviations: MRSE, manifest refraction spherical equivalent. |
Discussion
The FDA clinical trial rate of explantation was 7.1% over 24 months and 8.9% over 60 months. Our result of 8.2% over the course of 10 years is comparable to the rates described in the FDA clinical study.6 A literature search of five other KAMRA inlay implantation studies with sample sizes greater than 30 had an explantation range of 0% to 8.66% and a 6.51% explantation rate upon combining the total number of cases.9–13 In most cases, vision returned toward pre-implantation acuities following explantation.8 However, a notable minority of these patients did not regain pre-implantation corrected visual acuity, and most experienced the persistence of some stromal haze.
This study suggests that most patients can regain their pre-implantation UNVA and CDVA (Table 2) which is consistent with literature on KAMRA explantation reporting this procedure to be safe with no significant deficits.9,12,14 However, our study finds that most patients will have a statistically significant decrease in UDVA which has not been reported extensively in the literature. Additionally, while earlier studies found that pre-operative CDVA returns after explantation,9,10 our results show that a small subset will have worsened CDVA which is consistent with a few recent studies.8,15
Loss in Snellen visual acuity between pre-implantation and post-explantation may be attributed to a number of reasons including corneal haze or fibrosis, refractive changes, and/or loss of accommodation over time.8,12,15 Patients in this study had objective findings of corneal haze in both pre-explantation and post-explantation visits (Figure 7A and B). Notably, Figure 2 shows corneal haze in the corneal profile view 1-day post-explantation that remained at 2-years post-KAMRA explantation. The exact etiology of the corneal haze is unclear, but since the inlay is made of synthetic material, it can be hypothesized to be a host response to foreign body material. Ong et al conducted a histopathological examination of explanted KAMRA inlays from patients with postoperative corneal haze and found collagenous fibrotic membranes covering the inlay along with chronic inflammatory cells.7 Paley et al confirmed through immunostaining for keratocyte marker CD34 (negative) that the membranes were a result of scar-tissue formation reacting from the inlay, rather than normal stroma.16 These findings suggest that the corneal haze is caused by keratocyte-to-myofibroblast transdifferentiation secondary to the placement of the inlay.
 |
Figure 7 Corneal haze in the shape of the KAMRA inlay (A) 1.5 months post-explantation, and (B) 3 months post-explantation in the same patient (case 8). |
Refractive changes in the form of hyperopic or myopic shifts can also contribute to blurriness and lead to complaints about visual acuity. Hyperopic shift has been reported in the FDA clinical trial data as the main visual complaint for inlay removal and has been observed in other studies as well.7,9,17 In this study, 2 out of 22 patients underwent PRK enhancement between KAMRA implantation and explantation with the aim of correcting declining vision caused by hyperopic and astigmatic shifts (Figure 4). The hyperopic shift was somewhat reversible in both patients when treated with topical steroids; however, PRK enhancement was proposed to correct the hyperopic shift in these patients and avoid the clinical sequelae of long-term steroid use. Although PRK enhancement improved vision initially and reverted the MRSE to myopic values, these patients continued to experience hyperopic shifts that did not fully resolve post-explantation. Similarly, Fattah et al conducted a study on simultaneous KAMRA and LASIK treatment for hyperopic presbyopia and observed late-onset regression of hyperopic shift in 25% of the patients.4 Of the six patients who lost lines of CDVA, two patients who underwent PRK enhancement between KAMRA implantation and explantation were included. These findings, though inconclusive, indicate that excimer laser treatment to correct refractive shifts, while the KAMRA is still implanted, can potentially cause loss of CDVA.
Our cases are consistent with the hypothesized mechanism of hyperopic shift in KAMRA patients proposed by Amigo et al, that stromal thickening is secondary to an inflammatory origin caused by the inlay.18 The stromal thickening in the region anterior to the inlay then leads to flattening of the overlying epithelium causing refractive changes.19 In our study, KAMRA-shaped imprints developed in patients after implantation over time and appeared to normalize post-explantation in the pachymetry map (Figure 3A and B). Although this patient did not experience a hyperopic shift, slight corneal thickening of the epithelium where the inlay was implanted persisted 2 years after explanation (Figure 3C and D).
Loss of accommodation over time and presbyopia exacerbation is another consideration when examining the post-explantation loss of UNVA. Over time, the elasticity of the lens reduces along with deterioration in the tension of zonular fibers and activity of the ciliary muscles, resulting in decreased ability to accommodate from distance to near vision.20 In cases with line losses in UNVA (9 cases), the average time between implantation and explantation was 4.47 years, whereas cases with no line losses (11 cases) had an average of 2.50 years between procedures. Although the sample size is too small to do correlation studies, increased average time between implantation and explantation appears to have an increased chance of reduced UNVA. However, presbyopic exacerbation over time cannot account for the loss of CDVA or UDVA.
In the FDA clinical trial, the top three complaints at 36 months post-implantation were dryness (50%), night vision problems (38%), and blurry/fluctuating vision (36%).17 In our study, the top three subjective complaints included inadequate or blurry near vision (86.3%), difficulty with night vision (68.1%), and decreased vision in dim lighting (45.5%). Other explantation studies also reported patient dissatisfaction with visual outcomes,3,14 and a few studies noted explantation due to cosmetic reasons, stromal thinning, and other ocular complications.9,12 Our study includes seven cases of follow-up data from 1-year post-explantation, with subjective complaints including dry eye, blurry vision, haze, double vision, and sensation of monovision. The KAMRA inlay can be considered removable, but by no means can the implantation be considered a fully reversible procedure. These findings show that the cornea does not revert completely back to its pre-implantation state and that there is a risk of long-lasting visual effects even after KAMRA explantation.
It is to be emphasized that these complaints are patient-reported, and the exact definition may vary by individual. During visits prior to explantation, inlay placement and centration were reassessed. Only one patient in the explant group required surgical repositioning of the corneal inlay. This finding suggests that the change in visual acuity was not due to improper fitting or placement of the KAMRA inlay.
KAMRA inlay is not the only corneal inlay that has been on the market for the correction of presbyopia. Other alternatives include the Raindrop Near Vision Inlay (ReVision Optics, Lake Forest, CA), a permeable hydrogel-based inlay, and the Presbia Flexivue Microlens (Presbia, Irvine, CA) an acrylic-based inlay. The Raindrop inlay was discontinued in 2018 just 2 years after its FDA approval in 2016 due to corneal haze, while Flexivue Microlens is still awaiting FDA approval in the United States since 2019. Although these corneal inlays have been initially regarded as the solution to presbyopia, it has become evident that there are concerns regarding the long-term efficacy and reversibility of these devices. Nevertheless, synthetic corneal inlays have built the foundation for future innovations such as the IC-8 Small Aperture IOL (Acufocus, Irvine, CA)21 and allogenic corneal inlays that allow for greater biocompatibility.22,23
To our knowledge, this is the largest sample size of KAMRA corneal inlay explantation at a single-site location. However, the sample size is still considered relatively small and is one limitation of the study. Another limitation of this study is the variation in follow-up visits, measurements, and imaging collected at these time points. Due to variation in follow-up, some patients had longer follow-up times than other patients, which may affect the results. Additionally, different methods were used for visual acuity testing. To validate these findings, future studies that have larger sample sizes, consistent visual acuity testing, and linear long-term follow-up data can be conducted.
Conclusion
KAMRA inlay explantation compared to pre-implantation resulted in statistically significant differences in spherical, cylinder and MRSE power. Although UNVA and CDVA in a majority of patients returned close to their pre-implantation visual acuities, a slight reduction in UDVA was noted. Other effects after explantation such as hyperopic shift, corneal haze, and dry eye may be long-lasting in some patients. Since the KAMRA inlay is no longer manufactured in the United States, this study will aid ophthalmologists in managing current KAMRA inlay patients who are considering its removal.
Compliance with Ethics Guidelines
This study was approved by the Hoopes Vision Ethics Board and adhered to the tenets outlined in the Declaration of Helsinki. The study was HIPAA-compliant, with a routine surgical informed consent obtained from all patients involved. It was an IRB-approved study by the Biomedical Research Alliance of New York (BRANY, Lake Success, NY) in accordance with research standards and state law.
Acknowledgments
We thank the patients who were part of this retrospective study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No funding was received for the publication of this article.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Moarefi MA, Bafna S, Wiley W. A review of presbyopia treatment with corneal inlays. Ophthalmol Ther. 2017;6(1):55. doi:10.1007/s40123-017-0085-7
2. Dexl AK, Jell G, Strohmaier C, et al. Long-term outcomes after monocular corneal inlay implantation for the surgical compensation of presbyopia. J Cataract Refract Surg. 2015;41(3):566–575. doi:10.1016/j.jcrs.2014.05.051
3. Moshirfar M, Quist TS, Skanchy DF, Wallace RT, Linn SH, Hoopes PC. Six-month visual outcomes for the correction of presbyopia using a small-aperture corneal inlay: single-site experience. Clin Ophthalmol. 2016;10:2191. doi:10.2147/OPTH.S115798
4. Abdul Fattah M, Mehanna CJ, Antonios R, Abiad B, Jabbur NS, Awwad ST. Five-year results of combined small-aperture corneal inlay implantation and LASIK for the treatment of hyperopic presbyopic eyes. J Refract Surg. 2020;36(8):498–505. doi:10.3928/1081597X-20200618-01
5. Pluma-Jaramago I, Rocha-de-Lossada C, Rachwani-Anil R, Sánchez-González JM. Small-aperture intracorneal inlay implantation in emmetropic presbyopic patients: a systematic review. Eye. 2022;2022:1–7.
6. US Food & Drug Administration. KAMRA ® Inlay Professional Use Information. https://www.accessdata.fda.gov/cdrh_docs/pdf12/p120023d.pdf.
7. Ong HS, Chan AS, Yau CW, Mehta JS. Corneal inlays for presbyopia explanted due to corneal haze. J Refract Surg. 2018;34(5):357–360. doi:10.3928/1081597X-20180308-01
8. Moshirfar M, Skanchy DF, Rosen DB, et al. Visual prognosis after explantation of small-aperture corneal inlays in presbyopic eyes: a case series. Med Hypothesis Discov Innov Ophthalmol. 2019;8(3):129–133.
9. Ylmaz ÖF, Alagöz N, Pekel G, et al. Intracorneal inlay to correct presbyopia: long-term results. J Cataract Refract Surg. 2011;37(7):1275–1281. doi:10.1016/j.jcrs.2011.01.027
10. Dexl AK, Seyeddain O, Riha W, et al. One-year visual outcomes and patient satisfaction after surgical correction of presbyopia with an intracorneal inlay of a new design. J Cataract Refract Surg. 2012;38(2):262–269. doi:10.1016/j.jcrs.2011.08.031
11. Harb W, Chamoun N, Harb G. KAMRA inlay implantation for presbyopia compensation: a retrospective evaluation of patient satisfaction and subjective vision 12-month postoperative. Middle East Afr J Ophthalmol. 2019;26(2):65. doi:10.4103/meajo.MEAJO_159_16
12. Vukich JA, Durrie DS, Pepose JS, Thompson V, van de Pol C, Lin L. Evaluation of the small-aperture intracorneal inlay: three-year results from the cohort of the U.S. Food and Drug Administration clinical trial. J Cataract Refract Surg. 2018;44(5):541–556. doi:10.1016/j.jcrs.2018.02.023
13. Igras E, O’Caoimh R, O’Brien P, Power W. Long-term results of combined LASIK and monocular small-aperture corneal inlay implantation. J Refract Surg. 2016;32(6):379–384. doi:10.3928/1081597X-20160317-01
14. Alió JL, Abbouda A, Huseynli S, Knorz MC, Homs MEM, Durrie DS. Removability of a small aperture intracorneal inlay for presbyopia correction. J Refract Surg. 2013;29(8):550–556. doi:10.3928/1081597X-20130719-05
15. Romito N, Basli E, Goemaere I, Borderie V, Laroche L, Bouheraoua N. Persistent corneal fibrosis after explantation of a small-aperture corneal inlay. J Cataract Refract Surg. 2019;45(3):367–371. doi:10.1016/j.jcrs.2018.11.003
16. Paley GL, Harocopos GJ. Histopathologic analysis of explanted KAMRA corneal inlays demonstrating adherent fibroconnective tissue scar formation. Ocul Oncol Pathol. 2019;5(6):440. doi:10.1159/000498944
17. US Food & Drug Administration. PMA P120023: FDA Summary of Safety and Effectiveness Data (SSED). https://www.accessdata.fda.gov/cdrh_docs/pdf12/p120023b.pdf.
18. Amigó A, Martinez-Sorribes P, Recuerda M. Late-onset refractive shift after small-aperture corneal inlay implantation. J Cataract Refract Surg. 2018;44(5):658–664. doi:10.1016/j.jcrs.2018.03.029
19. Greenwood M, Bafna S, Thompson V. Surgical correction of presbyopia: lenticular, corneal, and scleral approaches. Int Ophthalmol Clin. 2016;56(3):149–166. doi:10.1097/IIO.0000000000000124
20. Lockhart TE, Shi W. Effects of age on dynamic accommodation. Ergonomics. 2010;53(7):892–903. doi:10.1080/00140139.2010.489968
21. Hooshmand J, Allen P, Huynh T, et al. Small aperture IC-8 intraocular lens in cataract patients: achieving extended depth of focus through small aperture optics. Eye. 2019;33(7):1096. doi:10.1038/s41433-019-0363-9
22. Liu YC, Teo EPW, Ang HP, et al. Biological corneal inlay for presbyopia derived from small incision lenticule extraction (SMILE). Sci Rep. 2018;8(1):1–10. doi:10.1038/s41598-017-17765-5
23. Jacob S, Kumar DA, Agarwal A, Agarwal A, Aravind R, Saijimol AI. Preliminary evidence of successful near vision enhancement with a new technique: prEsbyopic Allogenic Refractive Lenticule (PEARL) corneal inlay using a SMILE lenticule. J Refract Surg. 2017;33(4):224–229. doi:10.3928/1081597X-20170111-03
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




