Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
Expert Opinion on Diabetes Management Challenges and Role of Basal Insulin/GLP-1 RA Fixed-Ratio Combination in People with Type 2 Diabetes from Indonesia
Authors Suastika K , Eliana F, Kshanti IAM, Mardianto M, Mudjarnako SW , Natalia N , HS HN, Sibarani RP, Soewondo P, Soelistijo SA , Tarigan TJE, Zufry H
Received 29 March 2022
Accepted for publication 14 July 2022
Published 27 September 2022 Volume 2022:15 Pages 2977—2990
DOI https://doi.org/10.2147/DMSO.S367153
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ming-Hui Zou
Ketut Suastika,1 Fatimah Eliana,2 Ida Ayu Made Kshanti,3 Mardianto Mardianto,4 Sony Wibisono Mudjarnako,5 Nanny Natalia,6 Heri Nugrohom HS,7 Roy Panusunan Sibarani,8 Pradana Soewondo,9 Soebagijo Adi Soelistijo,5 Tri Juli Edi Tarigan,9 Hendra Zufry10
1Division of Endocrinology and Metabolism, Department of Internal Medicine, Faculty of Medicine, Udayana University, Sanglah Hospital Denpasar, Bali, Indonesia; 2Faculty of Medicine, Yarsi University, Jakarta, Indonesia; 3Division of Endocrinology, Metabolism and Diabetes, Department of Internal Medicine, Fatmawati General Hospital, Jakarta, Indonesia; 4Faculty of Medicine, University of Sumatra Utara/Adam Malik Central Hospital, Internal Medicine, Medan, Indonesia; 5Division of Endocrinology, Department of Internal Medicine, Faculty of Medicine Airlangga University, Dr. Soetomo General Academic Hospital, Surabaya, Indonesia; 6Faculty of Medicine, Padjadjaran University, Hasan Sadikin Hospital, Bandung, Indonesia; 7Faculty of Medicine, Diponegoro University, Central General Hospital Dr. Kariadi, Semarang, Indonesia; 8Endocrinology and Diabetes Center EMC Hospital Sentul City, Bogor, Indonesia; 9Division of Endocrinology, Department of Internal Medicine, Faculty of Medicine, University of Indonesia, Cipto Mangunkusumo Hospital, Jakarta, Indonesia; 10Division of Endocrinology, Metabolism and Diabetes, Department of Internal Medicine, School of Medicine Universitas Syiah Kuala/Dr. Zainoel Abidin General Teaching Hospital, Banda Aceh, Indonesia
Correspondence: Ketut Suastika, Division of Endocrinology and Metabolism, Department of Internal Medicine, Faculty of Medicine, Udayana University-Sanglah Hospital, Kamboja Street, Dangin Puri Kangin, No. 8, 80233, Denpasar, Bali, Indonesia, Tel +62 81 138 0916, Email [email protected]
Abstract: Indonesia is struggling with a rapidly growing burden of diabetes due to rapid socioeconomic transition. People with type 2 diabetes mellitus (T2DM) need appropriate treatment strategies to maintain glycemic control. New modalities with simplicity, such as fixed-ratio combination of basal insulin and glucagon-like peptide-1 receptor agonist (GLP-1 RA), further referred to as FRC, have proven to be an effective and practical therapeutic approach that may address this issue. In January 2021, a scientific expert meeting was held with the participation of endocrinologists from Indonesia to provide expert opinions regarding the optimal practical use of the FRC basal insulin/GLP1-RA. Topics discussed in the meeting included the challenges in diabetes management, clinical inertia with insulin therapy, local and international guideline positioning, initiation, titration, and switching of basal insulin and GLP-1 RA, including FRC, and the management of T2DM.
Keywords: fixed-ratio combination, basal insulin, GLP1 RA, type 2 diabetes mellitus
Key Summary Points
- A fixed-ratio combination regimen of basal insulin and glucagon-like peptide-1 receptor agonist (GLP-1 RA) has proven to be an effective and practical therapeutic approach in maintaining glycemic control and overcoming clinical therapeutic inertia in people with type 2 diabetes mellitus.
- Indonesian physicians face numerous practical questions related to the use of this new therapeutic class of FRC in clinical practice.
- A scientific expert meeting was held in January 2021 with the involvement of endocrinologists from Indonesia to provide expert insights regarding the optimal practical use of the FRC.
- The experts reached a consensus in their answers to all of the questions presented; these expert insights are elaborated in this paper.
Introduction
Globally, type 2 diabetes mellitus (T2DM) accounts for nearly 90–95% of all people with diabetes. This form of diabetes includes individuals who have relative (rather than absolute) insulin deficiency and peripheral insulin resistance.1,2 Over the past 30 years, the incidence of T2DM has risen dramatically, affecting 463 million people worldwide.2,3 In the Western Pacific (WP) region, 163 million people live with diabetes, which is predicted to rise to 212 million by 2045.3 Indonesia, one of the 39 countries and territories in the International Diabetes Federation (IDF) WP region, is striving hard to tackle this diabetes epidemic, affecting 6.2% of the total adult population (172,244,700).3
Several factors are attributable to this increasing trend of T2DM, one of them being lifestyle change.4 It is noteworthy that prediabetes is also one of the important determinants contributing to blood glucose intolerance, as one-third of prediabetes cases develop into diabetes.4 Therefore, eliminating unhealthy lifestyle activities (eg, smoking, excessive alcohol, and insufficient sleep) can be the most effective option for delaying the onset of diabetes as well as the management of T2DM and its related complication Clinician reluctance, patient injection fear, adherence issues due to a complex regimen, hypoglycemia, and weight gain may lead to clinical therapeutic inertia, which is a barrier to effective diabetes management, thereby causing patients to live with uncontrolled hyperglycemia and an increased risk of diabetes-related complications.
Combination of insulin and a GLP-1 analogue in a fixed-ratio has been a solution that solved those problems, such as flexibility, a single injection, peak-less than the prior generation, decreasing or sustained weight, and less hypoglycaemic and less gastrointestinal effect.3 GLP-1 has cardiovascular protection as a new paradigm and concern.5
Endocrinologists from the Semarang city of Central Java as well as eastern (Denpasar and Surabaya) and western regions (Jakarta, Aceh, Bandung, and Medan) of Indonesia were invited to discuss and reach a consensus on the concerns in context and their possible solutions. This involved two main steps:
- First, the experts were asked to share the list of the questions of diabetes management challenges and the role of FRC, which is available on the market and relevant to their clinical practices. Then, validation and insight collection were performed within individual expert interviews.
- The experts then congregated for further discussion; the listed questions were grouped into nine main categories: (1) the position of FRC vs other treatment approaches, (2) treatment preferences of OAD regimen for T2DM management, (3) initiation of FRC, (4) deintensification from basal plus, premix insulin BD, and basal bolus, (5) intensification from FRC to basal plus/basal bolus, (6) titration of FRC, (7) intensification from GLP-1 RA to FRC, (8) the use of FRC in Ramadan fasting. The meeting consensus was summarized and approved by all expert participants.
Pathogenesis of T2DM from the Indonesian perspective.
In East Asian countries, T2DM is primarily characterized by β-cell dysfunction, lesser obesity, and younger age of onset compared to Caucasians.6 The deficit of functional β-cells is the core concern among Asian people with T2DM. Recent studies suggest that Asians have less β-cell functional capacity when compared with Caucasians.7 Therefore, the early preservation or recovery of functional β-cell mass seems to be pivotal for Asians.7
In addition, the β-cell regenerative capacity may be limited in Asians, when compared with Caucasians. The genetic and environmental factors could be the possible attributable factors for this limited β-cell regenerative capacity in Asians.7 This limited capacity may induce excess β-cell workload and thereby lead to β-cell failure, subsequently resulting in diabetes-related complications.7,8 Therefore, treatment strategies that preserve β-cell function in the early course of diabetes are crucial for the Indonesian population to prevent the onset of diabetes-associated complications.
Glucagon-Like Peptide-1: A Major Driver in T2DM Pathogenesis
Glucagon-like peptide-1 (GLP-1) is an incretin hormone that induces insulin secretion and reduces glucagon secretion, thereby regulating glucose metabolism.9 A case–control study conducted among 40 Indonesian patients—case group (subjects with T2DM, n = 20) and control group (subjects with normal glucose tolerance [NGT], n = 20)—clearly depicted that GLP-1 levels (both fasting and post-prandial) were lower in people with T2DM compared to those with NGT (2.06 ± 0.43 vs 2.87 ± 0.67 pg/L, p < 0.01; and 2.49 ± 0.60 vs 3.42 ± 0.85 pg/L, p = 0.02; respectively).9 Overall, this study confirmed that an important risk factor for T2DM in the Indonesian population is intact GLP-1 levels.9 The study findings of Yabe et al confirmed that there is a little enhancement of meal-induced secretion of GLP-1 in the Japanese,10 while Vollmer et al concluded that GLP-1 secretion induced due to 75-g oral glucose tolerance test is lower in the Japanese compared with that in Caucasians.11 This concludes that GLP-1 deficiency, in addition to β-cell dysfunction, plays a key role in the pathogenesis of T2DM in Indonesia, compared with that in Caucasians,9 elucidating the importance of incretin-based drugs, such as GLP-1 RAs and dipeptidyl peptidase-4 (DPP-4) inhibitors in East Asian patients.12
Contribution of Postprandial Glucose (PPG) to Hyperglycemia
Asians show higher glycemic excursion compared to Caucasians, to the same blood glucose load indicating the need for an effective PPG control in Asian people with T2DM.13 A continuous glucose monitoring conducted by Wang et al among 121 Asian people with T2DM, who were allocated to five groups based on quintiles of hemoglobin A1c (HbA1c) (ranging from 5.7% to 12.7%), also depicted that PPG contribution to either 24-h hyperglycemia or 4-h hyperglycemia after meals was significantly higher than fasting glucose and preprandial glucose in the lowest quintile of HbA1c (both p < 0.001) elucidating that PPG 24 and 4 h after meals was an important contributor to excess hyperglycemia.14 Even, the findings of the Diabetes Epidemiology: Collaborative Analysis of Diagnostic Criteria in Asia (DECODA) study and the Diabetes Epidemiology: Collaborative Analysis of Diagnostic Criteria in Europe (DECODE) study concluded that postprandial hyperglycemia is predominant in Asians compared to Caucasians Prevalence noted in Asians vs Caucasians: 37% vs 28%).15,16 Genetically, as most Indonesian people are close to other East and Southeast Asians (Native Indonesians), the scenario existing with Asians with respect to glycemic excursion would closely reflect in Indonesians. Therefore, PPG control is a significant component of T2DM management in Asia, including Indonesia.
Diabetes Risk Factors and Associated Complications
Type 2 diabetes mellitus is the sixth highest cause of death in Indonesia; hence, appropriate management is a prerequisite. A retrospective cohort study conducted among 14,517 Indonesian people using secondary data from the Indonesian Family Life Survey (IFLS) from 2007 to 2014 concluded that individuals who have a record of hypertension are 1.7 times more likely to have T2DM than those who do not have a record of hypertension. This study further depicted that individuals with greater body mass index (BMI) values are at greater risk of developing T2DM, ie, when compared with low BMI (Underweight BMI range: <18.5 kg/m2), a normal BMI (Normal BMI range: 18.5–24.9 kg/m2) increased the risk by 2.2 times, a higher BMI (overweight BMI range: 25–29.9 kg/m2) at 5.5 times, and a BMI value indicating obesity (Obesity BMI range: ≥30 kg/m2) was 7 times greater the risk of having T2DM. Therefore, controlling blood pressure, maintaining a normal BMI, and increasing physical activity since adolescence can prevent T2DM.17 Besides, an appropriate combination of antidiabetic drugs with different modes of action is necessary to maintain long-term metabolic control.
Diabetes is a growing concern, and people with T2DM have a 3.2 times greater risk of having coronary artery disease and the risk of diabetes is 1.9 times greater for patients with heart diseases.17,18 A DISCOVER study, which is a three-year, prospective, observational study, conducted across six continents spanning 38 countries, including Indonesia, deduced that the burden of microvascular and macrovascular complications (prevalence: 18.8% and 12.7%, respectively) is substantial in people with T2DM.19,20 A cross-sectional study conducted among 155 patients at the Outpatient Diabetes Clinic of Cipto Mangunkusumo Hospital, Indonesia, depicted that the prevalence of diabetes-associated chronic complications was 69%, predominantly microvascular-related complications (56%), including nephropathy (2%), retinopathy (7%), and neuropathy (38%), and mixed complications (53%).21 Further, as per the results of a study by Andayani et al in patients with both microvascular and macrovascular complications, the total cost of management increased by up to 130% compared to those without complications. This study has deduced that the prevention of diabetic complications will clinically benefit patients as well as substantially reduce overall healthcare expenditure.22 Besides micro- and macrovascular complications, diabetic distress, which is a psychological condition associated with depression, stress, and anxiety, is also one of the important diabetes-associated complications in Indonesian patients. A cross-sectional study conducted on Java Island in three primary care (n = 108) and four tertiary care (n = 524) facilities demonstrated that psychological distress is common in patients with T2DM.23 As long-term diabetes is strongly associated with both microvascular and macrovascular complications, there is an urgent need for immediate and effective therapeutic approaches for the management of T2DM to alleviate diabetes-associated complications.
Indonesian Guideline Recommendations for Management of T2DM
The algorithm for T2DM management in Indonesia is shown in Figure 1,23 and the subsequent figure (Figure 2) explains the general strategy of insulin therapy for adult outpatients with T2DM in Indonesia.24,25
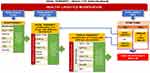 |
Figure 1 Algorithm of type 2 diabetes management in Indonesia. Abbreviations: AG-I, alpha-glucosidase inhibitors; DPP-4i, dipeptidyl peptidase-4 inhibitors; GLP-1 RA, glucagon-like peptide-1 receptor agonists; GN, repaglinide; HBA1c, glycated hemoglobin; SGLT-2i, sodium-glucose cotransporter-2 inhibitors; SU, sulfonylurea; TZD, thiazolidinediones. Notes: Adapted with permission from Indonesian Society of Endocrinology. Guideline of Management and Prevention of Type 2 Diabetes Mellitus in Indonesia 2021. Indonesian Society of Endocrinology Publisher 2021. Available from: https://pbperkeni.or.id/unduhan. Accessed July 25, 2022.24 |
 |
Figure 2 General strategy of insulin therapy in adult outpatients with T2DM. Abbreviations: BW, body weight; GLP1-RA, glucagon-like peptide-1 receptor agonist; HbA1c, glycated haemoglobin; IU, International units; kg, kilogram; SMBG, self-monitoring of blood glucose. Notes: Adapted with permission from Indonesian Society of Endocrinology. Practical Guideline of Insulin Therapy in Patients with Diabetes Mellitus 2021. Available from: https://pbperkeni.or.id/unduhan. Accessed July 25, 2022.23 |
The majority of the physicians and patients prefer to choose oral therapy before injectable basal insulin or a GLP-1 RA to achieve the individualized glycemic target of the patient.22 When glucose-lowering therapy fails to reach the glycemic target, insulin therapy is an appropriate choice for the treatment of T2DM, which can be used in combination with oral antidiabetic drugs (OADs), except for sulfonylurea, and GLP-1 analogs as fixed combinations. Timely and early insulin initiation can delay or reverse β-cell decreasing function or β-cell loss, respectively, because chronic hyperglycemia exerts harmful effects on β-cell function and regeneration and metabolic memory.24 In particular, many Asian patients require early insulin treatment because diabetes develops at a younger age, characterized by early β-cell dysfunction and insulin resistance.25 Besides, early β-cell dysfunction, East Asian people with T2DM have a higher risk of developing cardiovascular–renal complications than Europeans.26 Thus, there is a great need for the timely initiation of insulin, particularly in East Asian patients. It promotes better treatment to target glucose control with lower insulin dosage, lower rates of adverse events, and most importantly, is cheaper.24
Basal insulin therapy achieves glycemic target principally by decreasing nocturnal and fasting plasma glucose (FPG). However, PPG excursions cannot be controlled or considerably improved with basal insulin alone. Thus, the addition of PPG-lowering agents to basal insulin would benefit people with T2DM who are unable to achieve their recommended glycemic goals with basal insulin alone.26 The GLP-1 RAs would be the recommended PPG-lowering agents that induce the secretion of insulin postprandially and suppress glucagon release in a glucose-dependent fashion, and short-acting agents, such as exenatide and lixisenatide (Lixi), have a marked effect on delaying gastric emptying, leading to superior PPG-lowering efficacy of these agents.26 In particular, incretin-based drugs, including GLP-1 RAs and DPP-4 inhibitors, are more efficient in East Asian patients.13 The difference in the treatment responses to various glucose-lowering agents could be ascribed to different pathophysiological characteristics of T2DM, including lower insulin secretory function, lower body mass index, different genetic constitutions, preserved incretin hormone activity, and different dietary habits, in East Asians compared to other ethnic groups.6 A meta-analysis involving 15 randomized controlled trials with 4456 people with T2DM from East Asia (only China and Japan) depicted that there is a greater improvement in HbA1c-lowering efficacy with DPP-4 inhibitors, when compared to placebo, in Asians [- 0.75% (95% CI - 0.84 to - 0.65)] than in other ethnic populations, including White, African-American, Caucasians and others [- 0.61% (95% CI - 0.68 to - 0.54)] (P = 0.02).27 A recent meta-analysis demonstrated that GLP-1 RAs lower HbA1c more effectively in Asians when compared to non-Asians and are optimal medications for Asian people with T2DM with and without overweight/obesity.28 Therefore, incretin-based therapy plays a beneficial role in lowering HbA1c levels, possibly by repairing β-cell dysfunction through enhanced incretin activity in East Asian people with T2DM compared with that in Caucasians.
Existing T2DM Challenges in Indonesia
Underdiagnosis and Achieving a Glycemic Target
Data from Basic Health Research, Ministry of Health, Indonesia, depicted that more than two-thirds of patients remain underdiagnosed, indicating that underdiagnosed diabetes is a significant issue in Indonesia.29 If underdiagnosed people with diabetes are left unaddressed, there will be a growing prevalence of diabetes in the country that poses a tremendous challenge to the Indonesian healthcare system. Essential steps to address this underdiagnosed issue would include placing diabetes as a high priority on the Government agenda and creating a national plan; identifying disparities and priority areas for Indonesia; and developing a framework for coordinated actions between all relevant stakeholders.29
Inadequate control of diabetes leads to long-term complications and higher death rates, emphasizing the importance of achieving glycemic targets. Glycemic and metabolic control remain unsatisfactory in people with T2DM in primary, secondary, and tertiary care settings in Indonesia.29,30 Only around one-third of patients achieved the American Diabetes Association (ADA)-recommended target for HbA1c, FPG, and PPG levels.30,31 The International Diabetes Management Practices Study (IDMPS) wave 2006, comprising 674 people with T2DM who received varied glucose-lowering treatments, depicted that only 37.4% reached the target value of HbA1c less than 7% (the average HbA1c in this study was 8.27%).32 This further indicated that the majority of patients did not attain the recommended glycemic target.33 According to the recent investigations of the REASON ASIA study, reasons for not achieving target HbA1c included poor diabetes education (50.7%), noncompliance to OADs (21.4%), and fear of hypoglycemia (19.7%).34 This indicates that achieving a glycemic target is the biggest challenge for healthcare professionals in this region.29
Recommended Mitigating Approaches
Based on extensive secondary data and primary research, the following eight approaches could help Indonesia address its diabetes challenge: (1) establishing a national diabetes strategy, (2) developing data systems and performance-management processes, (3) improving primary-care networks, (4) using new healthcare models to enhance screening and diagnosis approaches, particularly in inaccessible areas, (5) enhancing the skills of and giving incentives to healthcare professionals, (6) empowering patients to increase treatment adherence, (7) encouraging lifestyle changes with an increasing awareness of healthy living, and (8) establishing policies to induce healthier lifestyles.35
Considering the importance of disease management and improving the knowledge of healthcare professionals, including primary care physicians, support programs were conducted in Indonesia through the PDCI (Partnership for Diabetes Control in Indonesia), followed by DEEP (Diabetes Education Enhancement for Engaged Partnership) conducted by PERKENI (Indonesia Endocrinologist Association).
Optimal glycemic control is a key goal in people with T2DM and is crucial in reducing the risk of diabetes-related complications. As many patients do not achieve or maintain glycemic targets, there is a need for further therapies.33 Thus, there is a need for a large proportion of patients to be adjusted to more intensive pharmacotherapies and multidisciplinary approaches for management.29,36
Fixed-Ratio Combination: A Treat‐to–Success Approach to Address Diabetes Challenges in Indonesia
The American Diabetes Association–European Association for the Study of Diabetes (ADA-EASD) guidelines recommend the initiation of injectable therapy with a GLP-1 RA and/or basal insulin for people with T2DM inadequately controlled on one or more oral agents.36,37 Further intensification involving basal insulin and GLP-1 RA combination therapy is often required over time to maintain or improve glycemic control. However, real-world evidence (RWE) suggests that only 40–60% of people with T2DM achieve a glycemic target of <7% within 1 year of starting either GLP-1 RA or basal insulin.38
Due to a complex clinical presentation, T2DM requires individualized, patient-specific treatment. In comparison to single-agent therapy, the more effective option to manage this multifaceted pathophysiologic disorder is combining the complementary actions of each therapy to simultaneously target distinct physiological pathways. The DiabCare Asia 2008 study found basal insulin inhibits hepatic glucose production, reducing FPG levels, while GLP-1 RAs in conjunction with the meal improve glycemic control by stimulating insulin release and suppressing glucagon secretion. In particular, short-acting GLP-1 RAs delay gastric emptying, which has a pronounced PPG-lowering effect following administration. The other advantages of GLP-1 RAs include the prevention or reduction of weight gain and a low risk of hypoglycemia.37
Using a fixed-ratio combination of basal insulin and GLP-1 RA may provide a simplified, preferable combination regimen than separate administration of either component. Currently, two FRCs have been approved for the management of T2DM: iGlarLixi, a once-daily titratable FRC of basal insulin glargine 100 U/mL (iGlar) plus Lixisenatide (Lixi), and IDegLira, a once-daily titratable FRC of insulin degludec (IDeg) plus liraglutide (Lira). Currently, the only FRC available in the Indonesian market is iGlarLixi. Therapeutic inertia is a barrier to effective diabetes management. Based on the DISCOVER study, the average HbA1C level was still high in Indonesia.18 New therapeutic modalities of FRC with a simultaneous therapeutic approach may address this issue. However, Indonesian physicians are still facing numerous practical questions (Table 1) related to the use of this new therapeutic class of FRC in the clinical practice that cannot be answered by the recently published trial results, current guidelines, and summaries of product characteristics. Recognizing the importance of these questions and accepting the physicians’ need for additional information, Sanofi organized an expert meeting with the involvement of Indonesian endocrinologists to provide opinions on T2DM management in daily clinical practice and review this new therapeutic class. During the meeting, an expert member requested to publish the collected opinions to be more accessible for larger Indonesian physicians.
 |
Table 1 Practical Questions Regarding the Use of FRCs |
Accordingly, the objectives of the expert consensus meeting were as follows:
● To gather experts’ inputs regarding practical questions that arose from the use of currently available FRC, and
● To formulate responses that could be translated into local expert opinion to aid daily clinical practice.
The expert opinion received on each of the nine categories has been detailed herein.
The position of FRC vs other treatment approaches.
Should the FRC Components Be Started Simultaneously or Sequentially?
According to the current ADA guidelines 2021,39 “if injectable therapy is needed to reduce HbA1c levels then consider GLP-1 RA in most patients before insulin. Add basal insulin if recommended target of HbA1c levels are not achieved”. Therefore, the treatment strategy would be a sequential approach, ie, GLP-1 RA followed by basal insulin. “For patients on GLP-1 RA and basal insulin combination, consider the use of FRC product (iGlarLixi or iDegLira)”. Overall, the sequential approach is the initial strategy after OAD failure. However, a simultaneous approach (FRC approach) is recommended when both the drugs are individually administered.
- In Indonesia, at baseline, patients had an average HbA1c level of 8.3% to 9.8%.22,31 All the experts agreed with the current ADA guidelines and concluded that the 9% HbA1c threshold is suitable to initiate the FRC. The FRC can also be considered when the difference between FPG and PPG is more than 55 mg/dL. The sequential approach is suggested when HbA1c levels are below 9%.
- Experts also agreed that a simultaneous approach would help in reducing the risk of comorbidities. Overall, as non-achievement of HbA1C target remains a burden across Indonesia, all experts unanimously agreed to prefer a simultaneous approach rather than a sequential approach in their routine practice.
- Injectable therapy, including FRC, GLP-1 RA, or basal insulin, is considered when the level of HbA1c improvement is required to achieve the individualized glycemic target. However, the following clinical characteristics of the patient should also be considered when deciding the most appropriate method of intensification: comorbidities, risk of hypoglycemia, level of obesity, significantly increased PPG values, and history of GI adverse events.
Is There an HbA1C Threshold Recommended to Start FRC for Indonesian Patients with T2DM?
- FRC is preferred as the first line if HbA1c levels are 2% above recommended glycemic target or levels of HbA1c are >9%.40 For patients with multiple OAD failure, with HbA1c levels >7.5% without metabolic decompensation, single-dose basal insulin is initiated. If the glycemic target has not been reached, multiple doses of insulins would be the option. The FRC offers a better option if the glycemic target is not achieved with basal insulin.
Can FRC Be Used as Treatment Alternatives for Selected Patients Treated with Basal-Bolus Therapy? Can FRC Be Used for Deintensification from Basal-Bolus (BB) Therapy?
The treatment deintensification or de-escalation in diabetes care is switching to a less-complicated alternative hypoglycemic treatment to reduce either the risk of hypoglycemia or treatment burden without affecting efficacy and safety. The deintensification treatment targets a less ambitious individualized glycemic goal due to aging and the development of significant comorbidities. In either of the cases, FRCs can be considered as alternative treatment options to the basal-bolus insulin regimen.41 However, the probability of successful deintensification could be markedly reduced if daily insulin dose and elevated HbA1c levels are taken into consideration during the management of T2DM.41 There are two modes of deintensification: (1) one-step deintensification can be initiated if the prior total daily insulin dose was ≤0.6 U/kg and (2) step-wise discontinuation of basal and/or bolus insulin would be needed if the previous total daily insulin dose was >0.6 U/kg.40,41
- The fasting C-peptide testing is highly recommended to evaluate residual β-cell function (normal range: 0.5 to 2.7 ng/mL), which is a prerequisite for safe deintensification.
- Regarding deintensification from multiple-daily insulin MDI injections, consider an FRC treatment in selected patients to decrease treatment burden/complexity or to reduce the risk of hypoglycemia or weight gain.
Should OADs Be Continued After Starting an FRC? If Not, How Should OADs Be Discontinued?
Consider the following aspects when discontinuing OADs.40
- Stop the usage of DPP-4 inhibitors, as they have no additional value to GLP-1 RAs and are not recommended in combination with a GLP-1 RA.
- Sulfonylureas should, as a general rule, be stopped (to decrease the risk of hypoglycemia and weight gain), or the substantial dose reduction indicated.
- Acarbose should be recommended only rarely, and it is better to stop it due to the increased risk of GI side effects.
- The continuation of glitazones is based on patient compliance. If safety concerns about combining them with insulin (eg, fluid retention and weight gain) exceed the expected beneficial effects of particularly targeting insulin resistance, then consider discontinuation.
- Sodium/glucose cotransporter 2 (SGLT2) inhibitors in combination with FRC are a potentially beneficial combination and are not associated with any other safety concerns.
- Metformin can be safely administered in combination with FRC.
Is There a Preferred Time of the Day to Use FRC? Before Breakfast, Lunch, or Dinner?
- The currently available FRC should be injected once a day within one hour before a meal.39 In the majority of the patients, the levels of PPG are typically higher after breakfast; therefore, experts agreed that the optimal time to use iGlarLixi is before breakfast.
- Further, they added that if two main meals are present assuming that there is no more than 4–5 h between the two meals, any time can be preferred.40
- However, pre-dinner injection is not preferable due to the risk of nocturnal hypoglycemia, and morning injection would be the best choice of administration. Also, in most of the randomized clinical trials, iGlarLixi was injected before breakfast.42
- Special situation: Fasting during Ramadan has several physiological effects on both homeostatic and endocrine processes. In people with diabetes, these changes and the type of medication being taken to treat diabetes can be associated with the development of complications, such as hypoglycemia and hyperglycemia. Pre-assessment and selecting a glucose-lowering therapy regimen will be important to reduce the risk of complications during Ramadan fasting. People with T2DM who were treated with iGlarLixi FRC were able to fast and iGlarLixi is given at Iftar.43 According to the wave 1 results of the SoliRam study, individuals with T2DM who received iGlarLixi were able to fast with a low incidence of hypoglycemia and favorable glycemic control, demonstrating that iGlarLixi can be a beneficial combination for individuals with T2DM during Ramadan.43
Should FRC Be Considered as Intensification Options After GLP-1 RAs Failure?
FRC in the Indonesian market can be considered as an intensification option because it achieves significantly better glycemic control than GLP-1 RAs alone in patients inadequately controlled by GLP-1 RA therapy.44 To overcome GLP-1 RA therapy-associated GI side effects, FRC treatment with smaller GLP-1 RA dose increments is recommended as this combination lowers the risk of such side effects.
How Should the Cardiovascular (CV) Benefits of GLP-1 RAs Be Taken into Account When Initiating an FRC?
If a patient has established CV disease:
● Based on the findings from the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial, liraglutide, a GLP-1 RA component of FRC, when administered at higher doses, has shown beneficial effect on major adverse cardiovascular events,45 iDegLira might be considered as the first choice as liraglutide is available as a component in this combination.
If a patient has not been diagnosed with CV disease:
● Glycemic control and achieving individual HbA1c target levels are the most important aspects in patients with no CV disease; and either of FRCs, ie, iDegLira or iGlarLixi, can be used, which is consistent with ADA–EASD recommendations.46–48
What Should Be Done When FRC is No Longer Sufficient to Provide Good Glycemic Control?
If FPG is controlled, but HbA1c is not in the target range, there is a need for treatment intensification. Options for intensifying FRC are summarized in Table 2
 |
Table 2 Option for Intensifying with FRC to Achieve Better Glycemic Control |
Treatment Preferences for Diabetes Management
Expert opinion on the preference of oral glucose-lowering therapy is listed in Table 3. 40,46,47 The responses of experts to other practical questions on treatment strategies for diabetes management in Indonesia are summarized in Table 4.
 |
Table 3 Expert Opinion on OAD Regimen and Initiation of FRC in Indonesian Perspective |
 |
Table 4 Expert Opinion on Other Practical Questions on the Clinical Situation and Treatment Strategies for Diabetes Management in Indonesia |
How to Minimize GI Side Effects of GLP-1 RA from FRC?
Glucagon-like peptide-1 receptor agonist is one of the components of FRC commonly associated with GI side effects including nausea and vomiting, due to decreased GI motility. Experts in this meeting agreed that FRC is a good option rather than GLP-1 RAs alone and have given some tips for patients to minimize GI side effects of GLP-1RA, such as eating small meals, avoiding spicy and high-fat food, and slow down when eating.
Titration of FRC
The titration recommendation of FRC is based on individual patient’s need for insulin. It is advised to optimize glycemic control via dose adjustment based on FPG. Close and continuous glucose monitoring is indicated during the transfer and the following weeks of dose titration.44 Table 5 summarizes the recommendations on insulin dose adjustment based on PERKENI 2021 guidelines.25 An initial dose of basal insulin is 10 units per day or 0.1–0.2 units/kg BW/day. Dose step titration of FRC will follow the strategy of dose adjustment associated with basal insulin. Slower titration of FRC is a part of the strategy to minimize GI side effects of the GLP-1 RA component.
 |
Table 5 PERKENI 2021 Guidelines—Recommendation on Insulin Dose Adjustment |
Fixed-Ratio Combination: New Era in Treatment of T2DM
An emerging FRC has been developed and marks a real paradigm shift in the treatment of T2DM. This FRC has several advantages, such as the delivery of multidrug components through a single-daily injection, improvement of FPG, reduction of PPG without increasing hypoglycemia risk, and mitigation of risk of weight gain with improved GI tolerability.26,46
People with T2DM who are unable to achieve their glycemic targets may benefit from the FRC complementary actions of basal insulin, which predominantly targets FPG, and GLP1RA, which predominantly targets PPG levels.47 Overall, the development of the FRC follows a patient-centric treatment approach since FRC offers many advantages over administering its component treatments individually. FRC provides simpler and more convenient treatment initiation, dosing schedules, and titration for clinicians and patients. This simplified regimen may improve patient adherence and provide compliance by reducing the number of injections required than administering the components individually.39
Conclusion
In particular, as Asians have less β-cell functional capacity compared with Caucasians, the importance of prevention and timely treatment strategy for T2DM aiming to preserve or recover functional β-cell mass should be emphasized in the Asian population. The development of an integrated approach with GLP-1 RAs and FRCs of them with basal insulin in addition to lifestyle modification is an alternative or an additional treatment option to overcome long-term complications associated with T2DM and prolonged hyperglycemia that may arise due to β-cell dysfunction/failure. Furthermore, FRC offers the benefit of two complementary medications delivered by a single daily injection, allowing a less complex and more convenient dosing schedule and easier titration. The recent ADA-EASD consensus report highlighted the advantages of FRC therapy over insulin-only regimens. All experts from Indonesia agreed to use the algorithm for the management of T2DM from renowned PERKENI guidelines. This algorithm has to be implemented in the titration for basal insulin and a GLP-1 RA. The experts reached a consensus for each of the presented questions. The expert insights will help healthcare professionals use FRC in their daily clinical practice.
Compliance with Ethics Guidelines
This article is based on previously conducted studies, published guidelines, and opinions of leading experts and does not contain any studies with human participants or animals performed by any of the authors.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, execution, acquisition of data, giving insight/feedback and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The Indonesian Society of Endocrinology provided financial support to the experts from the region across Indonesia who participated in the expert meeting and published the manuscript.
Disclosure
Ketut Suastika, Ida Ayu Made Kshanti, Mardianto, M, Sony Wibisono Mudjarnako, Nanny Natalia, Heri Nugroho HS, Roy Panusunan Sibarani, Pradana Soewondo, Soebagijo Adi Sulistijo, Tri Juli Edi Tarigan, and Hendra Zufry have no conflict of interest of this work.
References
1. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(Supplement_1):S14–S31. doi:10.2337/DC20-S002
2. Diabetes. Available from: https://www.who.int/health-topics/diabetes#tab=tab_1.
3. Western pacific members. Available from: https://www.idf.org/our-network/regions-members/western-pacific/members/western-pacific-members/104-indonesia.html.
4. Dany F, Dewi RM, Tjandrarini DH, et al. Urban-rural distinction of potential determinants for prediabetes in Indonesian population aged ≥15 years: a cross-sectional analysis of Indonesian Basic Health Research 2018 among normoglycemic and prediabetic individuals. BMC Public Health. 2020;20(1):1–9. doi:10.1186/S12889-020-09592-7/PEER-REVIEW
5. Yabe D, Seino Y, Fukushima M, Seino S. β cell dysfunction versus insulin resistance in the pathogenesis of type 2 diabetes in East Asians. Curr Diab Rep. 2015;15(6). doi:10.1007/S11892-015-0602-9
6. Inaishi J, Saisho Y. Ethnic similarities and differences in the relationship between beta cell mass and diabetes. J Clin Med. 2017;6(12):113. doi:10.3390/JCM6120113
7. Yu Pan C, Han P, Liu X, et al. Lixisenatide treatment improves glycaemic control in Asian patients with type 2 diabetes mellitus inadequately controlled on metformin with or without sulfonylurea: a randomized, double-blind, placebo-controlled, 24-week trial (GetGoal-M-Asia). Diabetes Metab Res Rev. 2014;30(8):726–735. doi:10.1002/DMRR.2541
8. Lastya A, Saraswati MR, Suastika K. The low level of glucagon-like peptide-1 (glp-1) is a risk factor of type 2 diabetes mellitus. BMC Res Notes. 2014;7(1). doi:10.1186/1756-0500-7-849
9. Yabe D, Kuroe A, Lee S, et al. Little enhancement of meal-induced glucagon-like peptide 1 secretion in Japanese: comparison of type 2 diabetes patients and healthy controls. J Diabetes Investig. 2010;1(1–2):56–59. doi:10.1111/J.2040-1124.2010.00010.X
10. Vollmer K, Hoist JJ, Bailer B, et al. Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes. 2008;57(3):678–687. doi:10.2337/DB07-1124
11. Seino Y, Kuwata H, Yabe D. Incretin-based drugs for type 2 diabetes: focus on East Asian perspectives. J Diabetes Investig. 2016;7(Suppl 1):102–109. doi:10.1111/JDI.12490
12. Chan JCN, Yeung R, Luk A. The Asian diabetes phenotypes: challenges and opportunities. Diabetes Res Clin Pract. 2014;105(1):135–139. doi:10.1016/J.DIABRES.2014.05.011
13. Wang JS, Tu ST, Lee IT, et al. Contribution of postprandial glucose to excess hyperglycaemia in Asian type 2 diabetic patients using continuous glucose monitoring. Diabetes Metab Res Rev. 2011;27(1):79–84. doi:10.1002/DMRR.1149
14. Tuomilehto J, Borch-Johnsen K, Tajima N, Cockram CS, Nakagami T. Cardiovascular risk profile assessment in glucose-intolerant Asian individuals – an evaluation of the World Health Organization two-step strategy: the DECODA Study (Diabetes Epidemiology: collaborative Analysis of Diagnostic Criteria in Asia). Diabetic Med. 2002;19(7):549–557. doi:10.1046/J.1464-5491.2002.00735.X
15. DECODE Study Group on behalf of the European Diabetes Epidemiology Study Group. Will new diagnostic criteria for diabetes mellitus change phenotype of patients with diabetes? Reanalysis of European epidemiological data. BMJ. 1998;317(7155):371–375. doi:10.1136/BMJ.317.7155.371
16. Native Indonesians. Wikipedia. Available from: https://en.wikipedia.org/wiki/Native_Indonesians.
17. Simbolon D, Siregar A, Talib RA. Physiological factors and physical activity contribute to the incidence of type 2 diabetes mellitus in Indonesia. Kesmas. 2020;15(3):120–127. doi:10.21109/KESMAS.V15I3.3354
18. Cho YM. Characteristics of the pathophysiology of type 2 diabetes in Asians. Ann Laparoscopic Endoscopic Surg. 2017;2(2):14. doi:10.21037/3728
19. Kosiborod M, Gomes MB, Nicolucci A, et al. Vascular complications in patients with type 2 diabetes: prevalence and associated factors in 38 countries (the DISCOVER study program). Cardiovasc Diabetol. 2018;17(1). doi:10.1186/S12933-018-0787-8
20. Tarigan TJE, Yunir E, Subekti I, Pramono LA, Martina D. Profile and analysis of diabetes chronic complications in Outpatient Diabetes Clinic of Cipto Mangunkusumo Hospital, Jakarta. Med J Indonesia. 2015;24(3):156–162. doi:10.13181/MJI.V24I3.1249
21. Murti Andayani T, Izham Mohamed Ibrahim M, Asdie AH. Assessing the impact of complications on the direct medical costs of type 2 diabetes mellitus outpatients. 2022.
22. Arifin B, van Asselt ADI, Setiawan D, Atthobari J, Postma MJ, Cao Q. Diabetes distress in Indonesian patients with type 2 diabetes: a comparison between primary and tertiary care. BMC Health Serv Res. 2019;19(1):773. doi:10.1186/S12913-019-4515-1/TABLES/2
23. Indonesian Society of Endocrinology. Practical Guideline of Insulin Therapy in Patients with Diabetes Mellitus 2021. Available from: https://pbperkeni.or.id/unduhan. Accessed July 25, 2022.
24. Indonesian Society of Endocrinology. Guideline of Management and Prevention of Type 2 Diabetes Mellitus in Indonesia 2021. Indonesian Society of Endocrinology Publisher 2021.
25. Rosenstock J, Aronson R, Grunberger G, et al. Benefits of LixiLan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide, versus insulin glargine and lixisenatide monocomponents in type 2 diabetes inadequately controlled on oral agents: the LixiLan-O randomized trial. Diabetes Care. 2016;39(11):2026–2035. doi:10.2337/dc16-0917
26. Cai Y, Zeng T, Wen Z, Chen L. Ethnic differences in efficacy and safety of alogliptin: a systematic review and meta-analysis. Diabetes Ther. 2018;9(1):177–191. doi:10.1007/S13300-017-0352-6
27. Hanefeld M, Fleischmann H, Siegmund T, Seufert J. Rationale for timely insulin therapy in type 2 diabetes within the framework of individualised treatment: 2020 update. Diabetes Ther. 2020;11(8):1645–1666. doi:10.1007/S13300-020-00855-5
28. Soewondo P, Ferrario A, Tahapary DL. Challenges in diabetes management in Indonesia: a literature review. Global Health. 2013;9(1):1–17. doi:10.1186/1744-8603-9-63/TABLES/5
29. Ma RCW, Chan JCN. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci. 2013;1281(1):64. doi:10.1111/NYAS.12098
30. Cholil AR, Lindarto D, Pemayun TGD, Wisnu W, Kumala P, Puteri HHS. DiabCare Asia 2012: diabetes management, control, and complications in patients with type 2 diabetes in Indonesia. Med J Indonesia. 2019;28(1):47–56. doi:10.13181/MJI.V28I1.2931
31. Universitas Indonesia. Current practice in the management of type 2 diabetes in Indonesia: results from the International Diabetes Management Practices Study (IDMPS). Available from: https://scholar.ui.ac.id/en/publications/current-practice-in-The-management-of-type-2-diabetes-in-indonesi.
32. Zhang F, Tang L, Zhang Y, Lü Q, Tong N. Glucagon-like peptide-1 mimetics, optimal for Asian type 2 diabetes patients with and without overweight/obesity: meta-analysis of randomized controlled trials. Sci Rep. 2017;7(1):1–11. doi:10.1038/s41598-017-16018-9
33. Vichayanrat A, Matawaran BJ, Wibudi A, et al. Assessment of baseline characteristics, glycemic control and oral antidiabetic treatment in Asian patients with diabetes: the Registry for Assessing OAD Usage in Diabetes Management (REASON) Asia study. J Diabetes. 2013;5(3):309–318. doi:10.1111/1753-0407.12038
34. McKinsey. Tackling Indonesia’s diabetes challenge: eight approaches from around the world. Available from: https://www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/tackling-indonesias-diabetes-challenge-eight-approaches-from-around-The-world.
35. Soewondo P, Soegondo S, Suastika K, Pranoto A, Soeatmadji DW, Tjokroprawiro A. The DiabCare Asia 2008 study – outcomes on control and complications of type 2 diabetic patients in Indonesia. Med J Indonesia. 2010;19(4):235–244. doi:10.13181/MJI.V19I4.412
36. Bailey CJ. The current drug treatment landscape for diabetes and perspectives for the future. Clin Pharmacol Ther. 2015;98(2):170–184. doi:10.1002/CPT.144
37. Hinnen D, Strong J. iGlarLixi: a new once-daily fixed-ratio combination of basal insulin glargine and lixisenatide for the management of type 2 diabetes. Diabetes Spectr. 2018;31(2):145–154. doi:10.2337/DS17-0014
38. Niemoeller E, Souhami E, Wu Y, Jensen KH. iGlarLixi reduces glycated hemoglobin to a greater extent than basal insulin regardless of levels at screening: post hoc analysis of LixiLan-L. Diabetes Ther. 2018;9(1):373–382. doi:10.1007/S13300-017-0336-6
39. Haluzík M, Flekač M, Lengyel C, et al. Expert opinion on the therapeutic use of the fixed-ratio combination of insulin glargine 100 U/mL and Lixisenatide: a Central/Eastern European perspective. Diabetes Ther. 2020;11(4):1029–1043. doi:10.1007/S13300-020-00777-2
40. Peng XV, McCrimmon RJ, Shepherd L, et al. Glycemic control following GLP-1 RA or basal insulin initiation in real-world practice: a retrospective, observational, longitudinal cohort study. Diabetes Ther. 2020;11(11):2629–2645. doi:10.1007/S13300-020-00905-Y
41. Morea N, Retnakaran R, Vidal J, et al. iGlarLixi effectively reduces residual hyperglycaemia in patients with type 2 diabetes on basal insulin: a post hoc analysis from the LixiLan‐L study. Diabetes Obes Metab. 2020;22(9):1683. doi:10.1111/DOM.14077
42. Hassanein M, Sahay RK, Malek R, et al. Real-world safety and effectiveness of iGlarLixi in people with type 2 diabetes who fast during ramadan: results from wave 1 of the SOLIRAM study. J Endocr Soc. 2021;5(Suppl 1):A334. doi:10.1210/JENDSO/BVAB048.681
43. Blonde L, Rosenstock J, Del Prato S, et al. Switching to iGlarLixi versus continuing daily or weekly GLP-1 RA in type 2 diabetes inadequately controlled by GLP-1 RA and oral antihyperglycemic therapy: the lixilan-G randomized clinical trial. Diabetes Care. 2019;42(11):2108–2116. doi:10.2337/DC19-1357
44. Marso SP, Daniels GH, Brown-Frandsen K. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322. doi:10.1056/nejmoa1603827
45. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S111–S124. doi:10.2337/DC21-S009
46. Skolnik N, Del Prato S, Blonde L, Galstyan G, Rosenstock J. Translating iGlarLixi evidence for the management of frequent clinical scenarios in type 2 diabetes. Adv Ther. 2021;38(4):1715. doi:10.1007/S12325-020-01614-5
47. PDB39 evaluation of the long-term clinical and economic impact of a 1% HbA1C reduction in patients with type 2 diabetes in Indonesia. Available from: https://www.infona.pl/resource/bwmeta1.element.elsevier-c3d25540-6dea-39b7-9f9d-5566c449a697.
48. Fda. SOLIQUA 100/33 (insulin glargine and lixisenatide injection); 2022. Available from: www.fda.gov/medwatch.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
