Back to Journals » Open Access Rheumatology: Research and Reviews » Volume 15
Expert Opinion Guidance on the Detection of Early Connective Tissue Diseases in Interstitial Lung Disease
Authors Namas R, Elarabi M, Fayad F, Muhanna Ghanem AA, Al-Herz A , Hafiz W , Joshi A, Merashli M, Okais J, Uthman I , Essa KS, Omair MA
Received 16 December 2022
Accepted for publication 16 March 2023
Published 29 May 2023 Volume 2023:15 Pages 93—102
DOI https://doi.org/10.2147/OARRR.S401709
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Chuan-Ju Liu
Rajaie Namas,1 Mohamed Elarabi,1 Fouad Fayad,2 Aqeel A Muhanna Ghanem,3 Adeeba Al-Herz,4 Waleed Hafiz,5 Abhay Joshi,6 Mira Merashli,7 Jad Okais,2 Imad Uthman,7 Khuloud Saleh Essa,8 Mohammed A Omair9
1Department of Internal Medicine, Division of Rheumatology, Cleveland Clinic Abu Dhabi, Abu Dhabi, United Arab Emirates; 2Rheumatology Department, Hotel-Dieu de France Hospital, Lebanon and Saint Joseph University of Beirut-Beirut (Lebanon), Beirut, Lebanon; 3Rheumatology Unit, Mubarak Al Kabeer Hospital, Jabriya, Kuwait; 4Department of Rheumatology, Amiri Hospital, Kuwait City, Kuwait; 5Department of Medicine, College of Medicine, Umm Al-Qura University, Makkah, Saudi Arabia; 6Department of Rheumatology, Burjeel Day Surgery, Abu Dhabi, United Arab Emirates; 7Division of Rheumatology, Department of Internal Medicine, American University of Beirut, Beirut, Lebanon; 8Rheumatology Unit, Farwaniya Hospital, Kuwait City, Kuwait; 9Department of Medicine, King Saud University, Riyadh, Saudi
Correspondence: Rajaie Namas, Division of Rheumatology, Department of Internal Medicine, Cleveland Clinic Abu Dhabi, Abu Dhabi, United Arab Emirates, Tel +971559271578, Fax +734-763-4151, Email [email protected]; [email protected]
Abstract: There is a significant variation in symptoms and clinical presentation of connective tissue disorders (CTD) associated with interstitial lung disease (ILD) (CTD-ILD). This presents difficulties in the diagnosis and treatment of CTD-ILD. Early detection and treatment of CTD-ILD using a multidisciplinary approach have been shown to enhance patient outcomes. This exercise aims to explore clinical components to develop a screening tool for pulmonologists for early detection of CTD in ILD and to provide a framework for a multidisciplinary approach in managing CTD-ILD. This in turn will lead to early treatment of CTD-ILD in collaboration with rheumatologists. A panel of 12 leading rheumatologists from the Middle East and North Africa (MENA) region met virtually to select the most relevant clinical findings to aid in identifying CTD-ILD. Twelve panellists opted to investigate seven of the most common inflammatory autoimmune disorders. The panel discussed how to improve the early detection of CTD-ILD. Clinical characteristics were categorized, and a nine-item questionnaire was created. A biphasic algorithm was developed to guide early referral to a rheumatologist based on the presence of one of nine clinical features of CTD (Phase 1) or the presence of CTD-specific antibodies (Phase 2). A brief questionnaire has been developed to serve as a simple and practical screening tool for CTD-ILD detection. Additional research is needed to validate and evaluate the tool in longitudinal cohorts.
Keywords: connective tissue disease-associated interstitial lung disease, CTD-ILD, rheumatologists, pulmonologists, screening, multidisciplinary team
Introduction
Connective tissue diseases (CTDs) refer to a group of systemic disorders that are characterized by autoimmune-mediated damage. The pathology of CTD is associated with circulating autoantibodies targeting various body system organs1–3 and exhibits varied pathology and clinical symptoms. Interstitial lung disease (ILD) is a collective term for over 200 types of parenchymal disorders characterized by lung injury, varying degrees of inflammation, and fibrosis.4,5 Clinically, connective tissue disease-associated interstitial lung disease (CTD ILD) is highly heterogenous and involves rheumatic immunity and respiratory complications affecting the airways, vessels, lung parenchyma, pleura, and respiratory muscles.6 There is significant variation in reported prevalence and incidence of CTDs primarily due to classification criteria used for diagnosis and geography in which the study has been conducted.6 By some estimates, ILD occurs in 15% of all CTDs7 and is one of the leading causes of morbidity and mortality in these patients.8
Although CTD-ILD management has advanced significantly in recent years, adequate medications and curative treatments are still needed.9 Historically, patients with ILD have been treated by a pulmonologist. The British thoracic society recommends that the treatment for ILD should follow a multidisciplinary team (MDT) model that includes a rheumatologist.10 Studies have shown that multidisciplinary in-hospital teams reduce the occurrence of adverse events and improve patient outcomes.11 In the Middle East and North Africa (MENA) region, due to practical and economic reasons, management of ILDs rarely follow the MDT model. Globally, ILD patients who do not exhibit classical symptoms associated with CTD at onset may receive a diagnosis of CTD years after being diagnosed with idiopathic ILD.1 For example, ILD associated with rheumatoid arthritis (RA-ILD) is a common manifestation of RA and may precede the joint inflammation thus confounding the diagnosis.12 Furthermore, no validated screening tools for detecting CTD signs and symptoms in ILD patients have been used.13
Considering that ILD may complicate the course of any CTD and that signs of CTD can be subtle, an underlying CTD should be ruled out in every ILD, even if the clinical suspicion is low or non-existent. The panellists reached the consensus that, given the limitations, the best strategy would be to support pulmonologists in making an informed decision on wisely referring patients with CTD-ILD to rheumatologists. This was due to the unique combination of a need for early diagnosis of CTD-ILD with a multidisciplinary approach to treatment and lack of MDT model in the MENA region.
As the illness group straddles two specialties, namely pulmonology and rheumatology, there is an unmet demand for tools for symptomatic diagnosis of CTD in patients with ILDs. This paper aims to give a scientifically sound and user-friendly solution.
As a result, the goal of this consensus paper is to investigate components for developing a screening tool for pulmonologists to employ, which will aid them in making early referrals to rheumatologists and diagnosing CTD in patients with ILDs. The tool is intended to be easy to use in daily practice having questions that will work as “red flags” for the referral. This approach will also initiate multidisciplinary collaboration in the treatment of CTD in patients with ILDs.
The objective was to come to a consensus on the symptoms, signs, and serological tests that should be used to diagnose CTD in patients who present with ILD to pulmonologists.
Methods
A panel of 12 rheumatologists gathered virtually to discuss the status of CTD-ILD management in the MENA region. Between June and August 2021, two virtual meetings took place. The panelists are all board-certified clinical rheumatologists with a focus on CTD-ILD who work in a range of settings, including government hospitals, tertiary care centers, research institutes, and private clinics. The panelists are from Kuwait, Lebanon, Saudi Arabia, and the United Arab Emirates. The panelists were able to present a comprehensive cross-section of rheumatology practice in the MENA region due to their expertise in CTD-ILD.
This panel attempted to develop a simple questionnaire for pulmonologists that includes the most prevalent signs and symptoms of CTD that should prompt a referral to a rheumatologist as soon as possible. In addition, the panel endeavored to present a list of suggested serological tests needed for detecting a CTD if a pulmonologist still has a high clinical concern for an emerging CTD in individuals without noticeable signs and symptoms.
The panel identified frequent signs and symptoms in patients with CTDs and CTD-ILDs based on their clinical experience and a review of the literature. The panel then filtered clinical signs and symptoms that might indicate a CTD that pulmonologists might see in patients with ILDs. In other words, the panel recognized symptoms that could be associated with ILDs but were also relevant to CTD-ILDs. The existence of CTDs in ILDs has been shown to have a significant effect on disease prognosis.14
The panelists considered the following questions for consensus (all answers are based primarily on the clinical experience of rheumatologists in the MENA region):
- What are the most prevalent CTDs in the MENA region?
- What are the most common signs and symptoms in:
- Rheumatoid arthritis
- Systemic Lupus Erythematosus
- Sjogren’s Syndrome
- Systemic Sclerosis/Scleroderma
- Sarcoidosis
- Inflammatory myositis
- What laboratory investigations are recommended in patients with CTD-ILD?
The panel reviewed numerous initial variables seen in patients with CTD-ILD and selected nine clinical variables and seven serological tests based on their discriminatory potential to guide the diagnosis of CTD-ILDs after careful consideration of the above questions. The consensus reached in answering the aforementioned questions inspired the development of a questionnaire for pulmonologists to use in deciding whether to consult a rheumatologist for CTD-ILD therapy.
A literature search was performed using the following databases: PubMed, Cochrane and Embase; to assess for the presence of any systematic screening tool for ILD in CTD patients in the MENA region. Results revealed no previous screening tools. Therefore, we utilized the pertinent publications on descriptive aspects of CTD-ILD.
Results
The panelists established a broad consensus on the clinical symptoms of the seven inflammatory autoimmune disorders discussed in this study. All further discussion is based on the reached consensus agreement.
The most common CTDs observed in clinical practice in the MENA region (in descending order) are:
- Rheumatoid Arthritis
- Systemic Lupus Erythematosus
- Sjogren’s Syndrome
- Systemic Sclerosis/Scleroderma
- Mixed Connective Tissue Disease
- Inflammatory Myositis
- Sarcoidosis
Corollary to the previous question, which was answered based on the panelists’ clinical experience, was: which CTD poses most concerns for ILD? According to the panel, the following CTDs are observed frequently among patients with ILDs.
- Systemic Sclerosis
- Sjogren’s Syndrome
- Inflammatory Myositis
- Rheumatoid Arthritis
- Mixed Connective Tissue Disease
- Systemic Lupus Erythematosus
- Sarcoidosis
The panel acknowledged that the list of CTDs that raise the most concern for ILD described above may differ from that reported in other regions and that this prevalence is unique to the MENA region. There was disagreement over whether pulmonologists should refer patients with sarcoidosis to rheumatologists. The most prevalent symptom of sarcoidosis is hilar lymphadenopathy, which may lead pulmonologists to diagnose sarcoidosis as ILD rather than CTD.
Even though CTDs have a wide range of signs and symptoms, the panel chose the following symptoms (in descending order) to characterize the respective CTD more than any other CTD (Table 1).
 |
Table 1 Symptoms That Characterize the Corresponding CTD More Than Any Other CTD (in Descending Order) |
The panel concurred that if any of the following signs or symptoms were present in a patient with ILD, rheumatologists should be consulted:
- Raynaud’s Phenomena
- Puffy fingers
- Skin lesions such as heliotrope rash, Gottron’s papules, malar rash, erythema nodosum
- Mechanic’s hands
- Inflammatory joint pain
- Dry eyes and dry mouth
- Dysphagia
- Proximal muscle weakness
- Skin Tightness/sclerodactyly
In the absence of any of the symptoms, pulmonologists should perform the following lab tests to assess if patients with ILD should consult a rheumatologist:
- Immunofluorescence antinuclear antibody test (IF-ANA) (titter more than 1:160 is considered significant)
- Extractable nuclear antigen (ENA) screening test (anti-centromere antibody, Scl-70 [Anti-topoisomerase], Smith-Ab, Ro/SSA and La/SSB autoantigens, Anti-Jo1 Ab, anti-RNP Antibodies)
- Rheumatoid factor (RF)
- Anti-citrullinated protein antibodies (ACPA) (previously Anti-cyclic citrullinated peptide antibodies (Anti-CCP)
- Creatine kinase (CK)
- Erythrocyte sedimentation rate (ESR)
- C-reactive protein (CRP)
The goal of this exercise was to determine clinically relevant components for a user-friendly CTD-ILD diagnosis questionnaire for pulmonologists. After that, the questionnaire would be validated as the next step. Pulmonologists will assess the final questionnaire in a clinical environment. Later, a second paper will be released that covers the procedure of collecting data from pulmonologists and analyzing the data to validate the questionnaire.
The panel proposes the following decision algorithm to determine if an ILD patient should be referred to a rheumatologist (Figure 1).
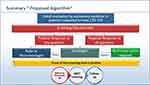 |
Figure 1 Decision tree to decide whether an CTD-ILD patient should be referred to a rheumatologist. |
Symptom heterogeneity in CTD-ILDs is a well-known phenomenon. Pulmonologists are often the first to see patients with ILD who solely have respiratory symptoms. Many patients with ILDs may have an undiagnosed CTD.15 Although a rheumatologist’s involvement is crucial for the early diagnosis and care of CTD-ILD patients, the MDT paradigm is not widely used in the MENA region. Confounding symptoms might also cause a CTD diagnosis to be delayed by several years. In clinical practice, there is an unmet demand for simple techniques to detect CTD in ILD patients. This predicament is unique to the Middle East and North Africa region, and it necessitates a unique solution. The development of a simple questionnaire that pulmonologists can use in their practice to diagnose ILD patients will allow for early referral to rheumatologists and early appropriate therapy, leading to lower morbidity and mortality for patients with CTD-ILDs.
Symptoms frequent in CTD patients were examined by the panel. They were then matched to symptoms of ILDs that are frequent in the Middle East and North Africa. The severity of the disease, the risk of disease progression, and the incidence of disease in the MENA region were all important criteria in determining which questions to include in the questionnaire. Only those indications and symptoms, as well as serology tests, that would serve as distinct “red flags” in the diagnosis of a CTD were included in the questionnaire.
Due to financial constraints, not all patients can undergo the necessary serology testing for CTD diagnosis. The panel chose baseline lab studies to be included in the questionnaire with this constraint in mind. We want to emphasize that there may be alternative tests that are more specific in diagnosing certain CTDs. However, these tests were not included in the diagnosis questionnaire if they did not match the twin criteria of simple availability and price.
We anticipate that pulmonologists would use the questionnaire regularly to detect CTDs in ILD patients early and treat them appropriately. The questionnaire should work in a similar way to how the DETECT algorithm detects pulmonary arterial hypertension.16
Because this questionnaire has not yet been validated and has not yet been evaluated in clinical practice, it is difficult to determine its clinical relevance at this time due to the lack of positive and negative predictive values. Also, the present study does not include the pulmonologists and radiologists.
Conclusion
The current work outlines the preliminary steps in the development of a diagnosis tool to differentiate patients with CTD-ILD from those with idiopathic or other ILDs. The panel concurred that inflammatory joint pain, early morning joint stiffness lasting more than 30 minutes, Raynaud’s phenomenon, puffy fingers, dermatological involvement, and proximal muscle weakness should be recognized as “red flags” for CTD and referred to a rheumatologist. The questionnaire will be reviewed by pulmonologists to lower the exercise’s limits. Pulmonologists should use the questionnaire and accompanying lab testing to help them make an informed decision about referring patients with ILD to rheumatologists. Under an MDT approach, early identification, and treatment of CTD- ILDs will begin with prudent referral to rheumatologists.
The proposed questionnaire is appended below:
- Raynaud’s phenomena:
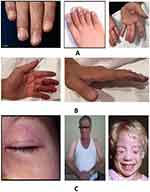
- a. Do the patient’s fingers turn pale or white? (Figure 2A)17
- b. Do they turn white or blue when they are exposed to cold, or when patient is stressed, or upset? and then turn red when the hands are warmed?
- c. Is there a similar pattern of symptoms in the patient’s ears, toes, nipples, knees, or nose, either separately or collectively?
- Has the patient had recurring puffy or swollen fingers that are not related to an exterior injury? (Figure 2B)18
- Do you notice the following skin lesions on visual inspection (or as part of the patient’s medical history)?
- a. Heliotrope rash: violet or bluish-purple rash on face, neck, knuckles, elbows, or chest (Figure 2C)19,20
- b. Gottron’s papules:
- (i) Does the patient have flat, reddish to violet papules and plaques?
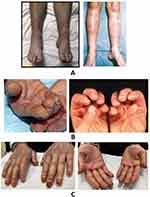
- (ii) Are the papules present over bony prominences especially over the dorsal aspects of the hand? (Figure 3A)21
- c. Malar rash: Is there or has there ever been a transitory rash on the patient’s face that is/was -
- (iii) Reddish and
- (iv) Symmetrical and
- (v) Spreading from the nose up to the cheeks BUT
- (vi) Not involving nasolabial folds
- (vii) Which the patient may describe as a “butterfly” rash (Figure 3B)22
- d. Erythema nodosum: Does the patient have or ever had (which resolved and left behind flat bruised appearance) an inflammation on a fatty layer of skin which is
- (viii) Reddish and
- (ix) Painful and
- (x) Tender
- (xi) Especially located in anterior part of the legs under the knees (Figure 4A)23,24
- Mechanic’s hands: On visual inspection, do you notice roughening and cracking of the skin of the tips and sides of the fingers, resulting in irregular, dirty-appearing lines that resemble those of a mechanic or manual labourer? (Figure 4B)25
- Inflammatory joint pain: Does the patient complain of:
- (i) Persistent, long-standing joint pain?
- (ii) Morning stiffness that lasts for up to 30 mins and is relieved by exercise?
- Does the patient complain of persistent dryness of eyes and mouth often described as sandy feeling in the eyes or of food sticking to the buccal surfaces?
- Does the patient complain of difficulty in swallowing?
- Is the patient complaining of proximal muscular weakness, which is commonly described as an inability to comb hair at the back of the head or difficulty rising from chairs?
- Is the patient experiencing tightness or tightening feeling on their skin (Figure 4C).26
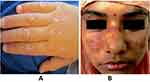 |
Figure 3 (A) Gottron’s papules on the dorsal aspect of the hand; (B) malar rash sparing nasolabial fold: notice the “butterfly” spread. Copyright (2022) ACR. |
Statement of Ethics and Consent
This consensus paper was developed in compliance with the Declaration of Helsinki.27 For research that uses publicly available data where identities of patients cannot readily be ascertained, US FDA regulation 45 CFR part 46.104 grants exemption from 1) IRB review and 2) taking patient consent.28 Regulations of UK, Australia and Netherlands too exempt research involving pre-existing nonidentifiable data in public domain from IRB review.29
To develop this guideline, research methodology was limited to review of existing literature available in public domain and the panelists’ own expertise and experience. No patient information was accessed or exposed. No multiple datasets were reviewed or cross-referenced so there is no risk of inadvertent patient identification. As no patient was contacted in any capacity, no informed consent was sought. As per the regulations quoted above, IRB review of development of this consensus and guideline was deemed unnecessary. No IRB review was conducted. The Figures 2A, 2C and 3B and 4A presented in the manuscript was taken from Rheumatology Image Library, American College of Rheumatology (ACR) image bank with prior permission.
Acknowledgments
Boehringer Ingelheim (BI) was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations. Writing, editorial support, and/or formatting assistance were provided by Cognizant technology solutions India Private Ltd, which was contracted and funded by Boehringer Ingelheim (BI).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Vij R, Strek ME. Diagnosis and treatment of connective tissue disease-associated interstitial lung disease. Chest. 2013;143(3):814–824. doi:10.1378/chest.12-0741
2. Fischer A, Du Bois R. Interstitial lung disease in connective tissue disorders. Lancet. 2012;380(9842):689–698. doi:10.1016/S0140-6736(12)61079-4
3. Solomon JJ, Fischer A. Connective tissue disease-associated interstitial lung disease: a focused review. J Intensive Care Med. 2015;30(7):392–400. doi:10.1177/0885066613516579
4. Mikolasch TA, Garthwaite HS, Porter JC. Update in diagnosis and management of interstitial lung disease. Clin Med. 2017;17(2):146–153. doi:10.7861/clinmedicine.17-2-146
5. Gaubitz M. Epidemiology of connective tissue disorders. Rheumatol. 2006;45(Suppl 3):iii3–iii4. doi:10.1093/rheumatology/kel282
6. Antoniou KM, Margaritopoulos G, Economidou F, et al. Pivotal clinical dilemmas in collagen vascular diseases associated with interstitial lung involvement. Eur Respir J. 2009;33(4):882–896. doi:10.1183/09031936.00152607
7. Shao T, Shi X, Yang S, et al. Interstitial lung disease in connective tissue disease: a common lesion with heterogeneous mechanisms and treatment considerations. Front Immunol. 2021;12:2092. doi:10.3389/fimmu.2021.684699
8. Castelino FV, Varga J. Interstitial lung disease in connective tissue diseases: evolving concepts of pathogenesis and management. Arthritis Res Ther. 2010;12(4):1. doi:10.1186/ar3097
9. Bradley B, Branley HM, Egan JJ, et al. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax. 2008;63(Suppl 5):v1–v58. doi:10.1136/thx.2008.101691
10. Epstein NE. Multidisciplinary in-hospital teams improve patient outcomes: a review. Surg Neurol Int. 2014;5(Suppl 7):S295–S303. doi:10.4103/2152-7806.139612
11. Cano-Jiménez E, Rodríguez TV, Martín-Robles I, et al. Diagnostic delay of associated interstitial lung disease increases mortality in rheumatoid arthritis. Sci Rep. 2021;11(1):1. doi:10.1038/s41598-021-88734-2
12. Spinillo A, Beneventi F, Epis OM, et al. Prevalence of undiagnosed autoimmune rheumatic diseases in the first trimester of pregnancy. Results of a two‐steps strategy using a self‐administered questionnaire and autoantibody testing. BJOG. 2008;115(1):51–57. doi:10.1111/j.1471-0528.2007.01530.x
13. Yoo H, Hino T, Han J, et al. Connective tissue disease-related interstitial lung disease (CTD-ILD) and interstitial lung abnormality (ILA): evolving concept of CT findings, pathology and management. Eur J Radiol Open. 2021;1(8):100311. doi:10.1016/j.ejro.2020.100311
14. Mittoo S, Gelber AC, Christopher-Stine L, et al. Ascertainment of collagen vascular disease in patients presenting with interstitial lung disease. Respir Med. 2009;103(8):1152–1158. doi:10.1016/j.rmed.2009.02.009
15. Coghlan JG, Denton CP, Grünig E, et al. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis. 2014;73(7):1340–1349. doi:10.1136/annrheumdis-2013-203301
16. Castillo GD, Callejas-Moraga EL, García G, et al. High sensitivity and negative predictive value of the DETECT algorithm for an early diagnosis of pulmonary arterial hypertension in systemic sclerosis: application in a single center. Arthritis Res Ther. 2017;19(1):1.
17. Sen S, Sinhamahapatra P, Choudhury S, et al. Cutaneous manifestations of mixed connective tissue disease: study from a tertiary care hospital in eastern India. Ind J Dermatol. 2014;59(1):35–40. doi:10.4103/0019-5154.123491
18. Sukumaran S, Palmer T, Vijayan V. Heliotrope rash and gottron papules in a child with juvenile dermatomyositis. J Ped. 2016;1(171):318. doi:10.1016/j.jpeds.2015.12.077
19. Nevins E, Zayat AS, Browning AJ, et al. Renal cell carcinoma-associated adult dermatomyositis treated laparoscopic nephrectomy. Urol Annals. 2013;5(4):299–301. doi:10.4103/0974-7796.120302
20. Ricceri F, Prignano F. Gottron papules: a pathognomonic sign of dermatomyositis. Can Med Assoc J. 2013;185(2):148. doi:10.1503/cmaj.111791
21. Shrestha D, Dhakal AK, Shiva Raj KC, et al. Systemic lupus erythematosus and granulomatous lymphadenopathy. BMC Pediatr. 2013;13(1):1–6. doi:10.1186/1471-2431-13-179
22. Shepard Z, Rios M, Solis J, et al. Common dermatologic conditions in returning travelers. Cur Trop Med Rep. 2021;8(2):104–111. doi:10.1007/s40475-021-00231-8
23. Quin A, Kane S, Ulitsky O. A case of fistulizing Crohn’s disease and erythema nodosum managed with Adalimumab. Nat Clin Pract Gastroenterol Hepatol. 2008;5(5):278–281. doi:10.1038/ncpgasthep1099
24. Bishnoi A, Parsad D. Hyperkeratotic fissured plaques on both hands: mechanic’s hands. Cleve Clin J Med. 2018;85(4):268–269. doi:10.3949/ccjm.85a.17045
25. Huang H, Feng RE, Li S, et al. A case report: the efficacy of pirfenidone in a Chinese patient with progressive systemic sclerosis-associated interstitial lung disease: a CARE-compliant article. Med. 2016;95(27):e4113. doi:10.1097/MD.0000000000004113
26. Whitaker D, Henderson DW, Shilkin KB. The concept of mesothelioma in situ: implications for diagnosis and histogenesis. Semin Diagn Pathol. 1992;9(2):151–161.
27. WMA Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. WMA – The World Medical Association; 2022.
28. HHS.gov. Exemptions (2018 Requirements); 2022.
29. Scott AM, Kolstoe S, Ploem MC, et al. Exempting low-risk health and medical research from ethics reviews: comparing Australia, the United Kingdom, the United States and the Netherlands. Health Res Policy Sys. 2020;18(1):1–8. doi:10.1186/s12961-019-0520-4
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.