Back to Journals » Patient Preference and Adherence » Volume 14
Experiences and Views of Medicine Information Among the General Public in Thailand
Authors Wongtaweepkij K , Krska J , Pongwecharak J, Jarernsiripornkul N
Received 8 April 2020
Accepted for publication 17 June 2020
Published 30 June 2020 Volume 2020:14 Pages 1073—1082
DOI https://doi.org/10.2147/PPA.S257454
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Kamonphat Wongtaweepkij,1 Janet Krska,2 Juraporn Pongwecharak,3 Narumol Jarernsiripornkul1
1Division of Clinical Pharmacy, Faculty of Pharmaceutical Sciences, Khon Kaen University, Khon Kaen, Thailand; 2Medway School of Pharmacy, Universities of Greenwich and Kent, Kent, UK; 3Pharmacy Practice and Management Research Unit, Division of Pharmaceutical Care, Faculty of Pharmacy, Rangsit Center, Thammasat University, Pathumthani, Thailand
Correspondence: Narumol Jarernsiripornkul
Department of Clinical Pharmacy, Faculty of Pharmaceutical Sciences, Khon Kaen University, Khon Kaen 40002, Thailand
Tel +66-4334-8353
Fax +66-4320-2379
Email [email protected]
Purpose: Written and electronic medicine information are important for improving patient knowledge and safe use of medicines. Written medicine information in Thailand is mostly in the form of printed package inserts (PIs), designed for health professionals, with few medicines having patient information leaflets (PILs). The aim of this study was to determine practices, needs and expectations of Thai general public about written and electronic medicine information and attitudes towards PILs.
Patients and Methods: Cross-sectional survey, using self-completed questionnaires, was distributed directly to members of the general public in a large city, during January to March 2019. It explored experiences of using information, expectations, needs and attitudes, the latter measured using a 10-item scale. Differences between sub-groups were assessed, applying the Bonferroni correction to determine statistical significance.
Results: Of the total 851 questionnaires distributed, 550 were returned (64.2%). The majority of respondents (88%) had received PIs, but only a quarter (26.2%) had received PILs. Most respondents (78.5%) had seen medicine information in online form. High educational level and income increased the likelihood of receiving PILs and electronic information. The majority of respondents (88.5%) perceived PILs as useful, but 70% considered they would still need information about medicines from health professionals. Indication, drug name and precautions were the most frequently read information in PIs and perceived as needed in PILs. Three-quarters of respondents would read electronic information if it were available, with more who had received a PIL having previously searched for such information compared to those who had not. All respondents had positive overall attitudes towards PILs.
Conclusion: Experiences of receiving PILs and electronic medicine information in Thailand are relatively limited. However, the general public considered PILs as a useful source of medicine information. Electronic medicine information was desired and should be developed to be an additional source of information for consumers.
Keywords: medicine information, general public, practices, attitudes, need and expectations
Introduction
Patients using medicines need information to enable them to maximize their safe and effective use.1 Information about both potential benefits and risks of medicines can improve patient knowledge and adherence.2–4 Presenting risk information to patients can also have an effect on their decisions about taking medicines,5 increasingly important for shared decision-making. In order to support this, patients require comprehensible information about side effects, interactions, precautions and benefits.1,6
However, studies in many countries have found that both patients and the general public have low level of knowledge and awareness about the risks of medicines they use.6,7 There may be several reasons for this limited knowledge. Some studies found limited provision of medicine information by health professionals (HPs) due to their concerns about its impact on the doctor–patient relationship, a lack of time for providing information, and complexity of the information.8 In addition, concerns have been expressed that providing such information may reduce adherence.9 A further key factor is the availability of suitable written information designed for patient use.
Written medicine information (WMI) for patients may be available in various forms such as patient information leaflets (PILs), medication factsheets, brochures, and booklets. WMI is effective in improving awareness, knowledge,10–12 recall of medicine information,13 behaviors related to medicine taking including both adherence and seeking other sources of medicine information.14 WMI may be provided routinely with medicines in the form of PILs or package inserts (PIs). More recently, online information about medicines has become a further major source of information used by patients especially on adverse effects, how to use the medicines, and drug interactions.15 Internet sources however vary in the reliability of information provided; hence, websites supported by local and national health authorities are an important source of trustworthy information. Multimedia education can improve patient knowledge about medication and skill acquisition.16
In Thailand, surveys have shown that HPs are major sources of medicine information, with written information being used less frequently. One key reason for this is the lack of availability of PILs.17 More recently, a survey of outpatients in Thailand found that almost all had seen a PI, but few had ever received a PIL.18 PIs are essentially the summary of product characteristics required by regulators designed to provide up-to-date information to health professionals. Regulations in many countries, including Thailand, require these to be enclosed in packages of every medicinal products.19 However, there are potentially problems associated with patients’ ability to comprehend the information in the PIs due to the technical language used, small font, thin paper, too dense texts, and unattractive design.20 In addition, many studies have found that some of these leaflets have incomplete information about drug safety.17,21,22 The PIL is written in simple language using a patient-friendly format to ensure patients’ understanding. These must be provided with every medicine in European countries, instead of a PI. However, in Thailand, PILs are only voluntarily provided by pharmaceutical companies and few are distributed.
Attitudes of Thai patients towards receiving WMI are positive,9 they report reading any form of WMI and perceive PILs to be important.18 A previous survey of the general public in Thailand conducted in 2014 found that, although health professionals were the most desirable sources of information, information leaflets were wanted by almost 50%.23 However, no work has determined the use of and attitudes towards such leaflets in the Thai general public, nor has any research into the use or desire for electronic medicine information been carried out in this population. This study therefore aimed to determine experiences, needs and expectations of written medicine information and attitudes towards PILs among the Thai general public.
Patients and Methods
Study Design and Setting
A cross-sectional study was conducted in Khon Kaen province, Thailand, during 5 January to 31 March 2019.
Participants
Eligible participants were aged 18 years and over, and were living in Mueang District, Khon Kaen province. All provided verbal informed consent to take part in the study. Sample size was calculated using Yamane’s equation with 5% margin of error, based on the 2017 census of inhabitants domiciled in Mueang District, Khon Kaen province, a previous study of the general public which achieved a 77% valid response rate,18 and the rate of refusal to participate in a previous study which was 5.83%.24 The total number of participants required was 550.
Questionnaire Development
A questionnaire for self-administration was developed by the research team using a previous study regarding expectation and needs of Thai patients towards PILs.18 The questionnaire consisted of four sections as follows:
- Demographic characteristics including age, gender, education level, and income
- Reading and use of written and electronic medicine information
- Needs and expectations of written and online medicine information
- Attitudes towards PILs consisted of 10 statements, using 5-point Likert-type Scale with responses ranging from strongly disagree (1) to strongly agree (5).
Questionnaire Testing
The questionnaire was validated by three experts (one hospital pharmacist, two clinical pharmacists) using index of item objective congruence (IOC) technique. All questions passed the content validity with IOC >0.5 of each item. All three experts were also asked to assess the questionnaire language and flow for ease of understanding. The questionnaire was then administered to15 people recruited from non-academic staff of the University. All were asked to complete the questionnaire and they were then asked to comment on each question individually, in terms of ease of understanding. The final questionnaire required only very minor modifications following recommendations from pilot and validation test.
Questionnaire Distribution
The final version of questionnaire was directly distributed to potential participants using convenience sampling at six types of public areas: university campus, public parks, temples, markets, bus station and community centers located in Khon Kaen province, Thailand. To ensure consistency throughout this process, the questionnaire was distributed and returned by one researcher. The researcher provided assistance by reading the questionnaire to respondents having visual problems but without providing further explanation. The differences between PILs and PIs were however explained to all participants to facilitate them in differentiating between these types of written medicine information.
Data Analysis
All questionnaire responses were entered into and the data analyzed using SPSS for Windows version 19.0. Simple frequencies were used to report demographic data, practices regarding written and electronic medicine information, needs and expectations of written and online medicine information. Attitude scores were calculated by first reverse scoring responses to negative questions and summing scores, then these were classified into three equal categories; low (10–22 points), moderate (23–36 points) and good attitude (37–50 points) based on previous studies.25 Mean and standard deviations of each attitude statement were also calculated. Internal consistency of the attitude scale was tested using Cronbach’s α coefficient. Pearson chi-square and Fisher’s exact test were used to compare demographic characteristics of respondents who had and had not ever read PIs, and those who had or had not ever seen electronic medicine information. P-value less than 0.05 with Bonferroni adjustment was accepted as indicating significant differences between sub-groups.
Ethical Approval
The study was approved by the Khon Kaen University Ethics Committee for Human Research (Number HE611500) which approved the process of obtaining verbal informed consent. All data were kept securely stored on University premises to protect participants’ confidential information.
Results
Response Rate
A total of 851 questionnaires were distributed, of which 550 were fully completed and analyzed (response rate 64.2%). Reasons given for refusal to participate in the study were: not convenient (n=225, 26.4%), not living in Mueang district, Khon Kaen (n=46, 5.4%) and not specified (n=30, 3.5%. No further data were gathered from non-responders).
Demographic Data
The majority of respondents were female (n=404, 73.5%) and half were aged 18–44 years (Table 1). Almost two-fifths of respondents had Bachelor’s degree and higher education (n=214, 38.9%) and just over half had income more than 10,000 baht per month (n=304, 55.3%).
 |
Table 1 Demographic Characteristics and Practices of Respondents in Relation to Experiences of PILs and Online Medicine Information |
Use of Written and Electronic Medicine Information
Of the total 550 respondents, 484 (88.0%) had received PIs, but only 144 (26.2%) had received PILs. The majority of respondents (296, 61.1%) indicated that they always read any medicine leaflets they received and 335 (81.1%) read the leaflets at the first time of receiving the medicine. We found significant differences in educational level, income, and frequency of reading leaflets between those who had and never received PILs (p<0.001). The respondents who had received PILs had higher educational level and income compared to those who had not. The proportion of respondents who always read leaflets was higher among those who had received PILs than those who had not, whereas all respondents who never read any leaflets about their medicines (n=71, 12.9% of total respondents) had never received a PIL. As was found with PILs, we also found significant differences in educational level and income between respondents who had used electronic information compared to those who had never used it (p<0.001) (Table 1).
Respondents reported that medicine labels (54.8%), and booklets (32.4%) were other common sources of written medicine information, but many also used electronic information sources which were websites (55.1%), television (48.1%), and Facebook (38.0%) (Table 2).
 |
Table 2 Sources of Written and Electronic Medicine Information That Respondents Had Ever Received or Searched |
The contents of PIs that respondents usually read were indications (n=347, 84.2%), followed by generic name of the medicines (n=303, 73.5%) and precautions (n=292, 70.9%) (Table 3).
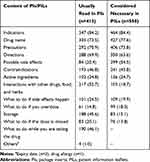 |
Table 3 Content of PIs Usually Read and Content of PILs Perceived as Necessary |
Needs and Expectations of Written and Electronic Medicine Information
More than half of the respondents (n=344, 62.5%) were not aware of PILs. Websites, mobile applications, and television or radio, were perceived to be the most needed sources of electronic medicine information. However, most respondents perceived PILs would be useful (n=487, 88.5%) with a higher proportion of those who had received PILs agreeing with this compared to those who had not (p=0.001) (Table 4). About half of the respondents (n=289, 52.5%) expected that PILs should be provided at every time of receiving a medicine. The majority of respondents (n=461, 83.8%) supported that production of PILs should be promoted by the Food and Drug Administration (FDA). Almost all respondents (n=527, 95.8%) also felt they needed advice from HPs and most (n=383, 69.6%) agreed that their need for this would be unchanged if PILs were available, but this was higher among respondents who had received PILs than among those who had not (81.3% versus 65.5%; p=0.001). The majority of respondents (n=433, 78.7%) reported that they would most likely read a PIL after receiving a medicine for the first time, as opposed to when they had questions (n=69; 12.6%) or when side effects occurred (n=48; 8.7%) and that they would read online PILs if they were available (n=412, 74.9%) (Table 4).
 |
Table 4 Expectations of Medicine Information in Relation to Receiving PILs and Accessing Electronic Medicine Information |
Respondents considered indications of medicines as the most important information to be included in PILs (n=464, 84.4%), followed by generic name of the medicines (n=427, 77.6%) and precautions (n=406, 73.8%) (Table 3).
Attitudes Towards PILs
The overall mean attitude score was 38.87±4.68. Around a third of respondents (n=186, 33.8%) had moderate attitude (mean=33.53±2.12) and the remaining 364 (66.2%) respondents had good attitude (mean=41.60±4.75). None of the patients had a negative attitude toward PILs. Responses to the ten attitude statements are shown in Table 5.
 |
Table 5 Attitudes of Respondents Towards PILs |
A large majority of respondents agreed that PILs would help them use their medication more accurately and safely (n=544, 98.9% and n=514, 93.4%, respectively). PILs were considered as a source of information that could be easily accessed (n=528, 96.0%). However, PILs would not be their first choice of information source if they had questions about medicines (n=324, 58.9%). Around a third of respondents (n=177, 32.2%) agreed that reading PILs would make them feel worried or lacking confidence in using the medicine, and over half that they would still need advice from HPs (n=299, 54.4%). A large majority agreed that should be provided with all marketed medicines (n=507, 92.2%). Conversely, over two-thirds of respondents disagreed that reading PILs was a waste of time (n=378, 68.7%). Most respondents agreed that online medicine information should be provided as another source of medicine information (n=454, 82.5%) and that a QR code linked to an online PIL should be on medicine packages (n=428, 77.8%) (Table 5).
Discussion
The results of our study showed that a high proportion of the Thai general public have received some written medicine information, with PIs and medicine labels being the most common sources. Most participants read the leaflets inside medicine packages, which could be a PI or PIL, but only just over a quarter had received a PIL with a medicine. The findings are similar to those of a recent outpatient survey in which 91% had received a PI but only 24% had ever heard of PILs.18 This is most likely due to the current situation in Thailand, where PILs are not a legal requirement to accompany prescription medicines which constitute a large proportion of marketed medicines.19 This situation differs from practices in most high-income countries. For example in Australia, provision of consumer medicine information is obliged by the professional guideline produced by the Pharmaceutical Society of Australia.26 And more than 80% Australian consumers reported they received written information from pharmacists and inside the medicine boxes.27 In the UK, provision of information specific to patients with all medicines has been obliged by law since 199928 and 97% of patients were aware of having received one in 2006.29
Self-reported reading of leaflets provided with medicines shows considerable variation across studies; for example, 91% claimed to read PILs in a survey in Nigeria,30 while studies in Pakistan have reported that between 23% and 61% never read PIs.31 More than half of the respondents in our study (61%) claimed to always read the leaflets they received and 81% if it was the first time of use, which was slightly higher compared to the UK, where 71% of first-time medicine users read the PIL.29
The contents of PIs that our respondents usually read were the same as those considered necessary sections in the PILs, which were indication, drug name and precautions. These differed from the findings of some other studies in which side-effect section, dosage, when and how long to take it were the most commonly read or identified as important sections of medicine information leaflets.29,32,33 However, there were similar findings in a survey from Sri Lanka, where name, dose, indication and side effects were reported as the most desired sections in WMI.34 Indication was also the most desired item of information in surveys in Nigeria30 and Ghana,35 hence it is clear that these preferences vary across countries and may depend on information obtained by other means, such as verbally from health professionals. The safety information, such as side effects, precautions, contraindications, contained in PIs and PILs is particularly important for reducing adverse events in Thailand, since previous research has shown that patients rarely receive such information from health professionals.36
Our study found that younger people, those with higher educational levels and on higher incomes were more likely to have read information provided with medicines. This is in line with studies from high-income countries, where higher educational level is strongly associated with reading the package leaflets.37,38 Low-educated people or lack of literacy influenced information-seeking behavior about health issues.33 The healthcare insurance system in Thailand means that most people who have low income and lower education have less access to imported originator products where the PILs are mainly provided.17 Our study also found that more people who were younger, highly educated and receiving high-income have searched for electronic information about medicines. Access to computers at work could be one factor that could explain the high proportion in these groups who have searched for information on the internet.39 Studies elsewhere have also found that younger people are more likely to use the internet to search for both medical information generally and medicine information, due to the speed and ease of access it offers.15
A large majority of our respondents thought PILs would be useful and would read them, although expectations of PILs differed between those who had and had not experienced PILs. Receiving PILs increased perceptions of usefulness and awareness of PILs. While 92% agreed that PILs should be provided with all marketed medicines and 84% that the FDA should promote their use, only 53% thought they should be provided every time a medicine is dispensed, which is similar to the views of Thai outpatients.18 Although respondents who had received PILs were also more likely to search for online medicine information, online medicine information was viewed positively by over 80%, and 78% also agreed that medicine packages should have a QR code linked to an online PIL. Such findings are important for both regulators and manufacturers.
Despite the desire expressed for WMI in both paper and electronic form, it is clear that advice from the health professionals would still be needed, if WMI was more widely available. Although clearly viewed as easily accessible sources of information, PILs would not be the first choice of information source for people having questions about their medicines. Thus, pharmacists and physicians continue to have an important role providing both verbal and written information to patients.27 Previous work in Thailand has shown that a limited number of health professionals currently provide WMI to outpatients during the care process, despite viewing PILs as potentially useful.40 A study in Ghana found that if hospital pharmacists encouraged patients to read the PIL, this resulted in higher reading rates.41 Thus, if PILs were more widely available in Thailand, as is clearly desired by patients, the public and health professionals,36,40 advice to read it should also be provided when prescribing or supplying medicines. Concerns about the provision of medicine information causing anxiety and reducing adherence have been expressed, both among Thai health professionals and elsewhere.42,43 This study showed that 32% of the population felt they may be worried by receiving a PIL, which is less than the 49% was found in an earlier study in Thai outpatients.36
Implications for Practice and Policy
Greater provision of written information about medicines needs to be supported by the Thai FDA in terms of both quantity and quality, in a variety of formats. While this is especially important for those starting a medicine for the first time to make sure that consumers understand about benefits and risks enabling shared decision-making, the only way to assure this is for information to be available with all medicines and online. While PIs are now widespread in Thailand, PILs are seen as desirable by patients and the public. Hence, as well as providing verbal information, pharmacists and doctors should endeavor to provide PILs routinely and advise patients to read them. Moreover, electronic medicine information should be freely available on websites regarded as trustworthy to enable easy and quick access.
Strengths and Limitations
Our study is the first survey of written medicine information use and need involving the Thai general public and their attitudes towards PILs. We made sure that participants in our study understood the difference between PIs and PILs giving samples of both, since this was crucial to the aim of the survey. However, we used convenience sampling in only one area of northeast Thailand; hence, the results might not be representative of other regions. Furthermore, the views obtained in our study may differ from those of the general public in other countries, where provision of medicine information differs in terms of practices and policies and there may be variable access to electronic sources of information.
Conclusion
This study showed that the views of the general public in Thailand towards the availability of written medicine information concur with those previously found in outpatients. Although most Thai people had received a PI with a medicine, they considered PILs desirable and useful, hence there is a need for more widespread availability of the latter. Online medicine information is used by the public and should also be further developed. Electronic PILs which can be accessed via a QR code on medicine packages would be a further useful development.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Raynor DK, Savage I, Knapp P, Henley J. We are the experts: people with asthma talk about their medicine information needs. Patient Educ Couns. 2004;53(2):167–174. doi:10.1016/S0738-3991(03)00126-5
2. Al-Saffar N, Deshmukh AA, Carter P, Adib SM. Effect of information leaflets and counselling on antidepressant adherence: open randomised controlled trial in a psychiatric hospital in Kuwait. Int J Pharm Pract. 2005;13(2):123–132. doi:10.1211/0022357056181
3. Hamrosi K, Dickinson R, Knapp P, et al. It’s for your benefit: exploring patients’ opinions about the inclusion of textual and numerical benefit information in medicine leaflets. Int J Pharm Pract. 2013;21(4):216–225. doi:10.1111/j.2042-7174.2012.00253.x
4. Schmitt MR, Miller MJ, Harrison DL, et al. Communicating non-steroidal anti-inflammatory drug risks: verbal counseling, written medicine information, and patients’ risk awareness. Patient Educ Couns. 2011;83(3):391–397. doi:10.1016/j.pec.2010.10.032
5. Knapp P, Raynor DK, Berry DC. Comparison of two methods of presenting risk information to patients about the side effects of medicines. Qual Saf Heal Care. 2004;13(3):176–180. doi:10.1136/qshc.2003.009076
6. Maclennan K, Brounéus F, Parkin L. Public knowledge and desire for knowledge about drug safety issues: a survey of the general public in New Zealand. Pharmaceut Med. 2016;30:339–348.
7. Phueanpinit P, Pongwecharak J, Krska J, Jarernsiripornkul N. Knowledge and perceptions of the risks of non-steroidal anti-inflammatory drugs among orthopaedic patients in Thailand. Int J Clin Pharm. 2016;38(5):1269–1276. doi:10.1007/s11096-016-0363-9
8. Hamrosi KK, Raynor DK, Aslani P. Pharmacist and general practitioner ambivalence about providing written medicine information to patients – a qualitative study. Res Soc Adm Pharm. 2013;9(5):517–530. doi:10.1016/j.sapharm.2013.02.006
9. Jarernsiripornkul N, Phueanpinit P, Pongwecharak J, Krska J. Experiences of and attitudes towards receiving information about non-steroidal anti-inflammatory drugs: a cross-sectional survey of patients in Thailand. Expert Opin Drug Saf. 2016;338:1–10.
10. Rajesh R, Vidyasagar S, Varma DM, Guddattu V, Hameed A. Evaluating the impact of educational interventions on use of highly active antiretroviral therapy and adherence behavior in Indian human immunodeficiency virus positive patients: prospective randomized controlled study. J AIDS Clin Res. 2013;4:231.
11. Akour A, Bardaweel S, Awwad O, Al-Muhaissen S, Hussein R. Impact of a pharmacist-provided information booklet on knowledge and attitudes towards oral contraception among Jordanian women: an interventional study. Eur J Contracept Reprod Heal Care. 2017;22:459–464. doi:10.1080/13625187.2017.1412425
12. Mai A, Aslani P. Impact of Vietnamese written and verbal medicine information on Vietnamese-speaking Australians‘ knowledge and satisfaction. Br J Clin Pharmacol. 2007;64(4):527–535. doi:10.1111/j.1365-2125.2007.02968.x
13. Morrow DG, Weiner M, Young J, Steinley D. Improving medication knowledge among older adults with heart failure: a patient-centered approach to instruction design. Gerontologist. 2005;45(4):545–552. doi:10.1093/geront/45.4.545
14. Smith KG, Booth JL, Stewart D, Pfleger S, McIver L, Maclure K. Supporting shared decision-making and people’s understanding of medicines: an exploration of the acceptability and comprehensibility of patient information. Pharm Pract (Granada). 2017;15:1–7.
15. Abanmy NO, Al-quait NA, Alami AH, Al-juhani MH, Al-aqeel S. The utilization of Arabic online drug information among adults in Saudi Arabia. Saudi Pharm J. 2012;20(4):317–321. doi:10.1016/j.jsps.2012.07.001
16. Ciciriello S, Johnston RV, Osborne RH, et al. Multimedia educational interventions for consumers about prescribed and over-the-counter medications. Cochrane Database Syst Rev. 2013;30:CD008416.
17. Phueanpinit P, Pongwecharak J, Krska J, Jarernsiripornkul N. Medicine information leaflets for non-steroidal anti-inflammatory drugs in Thailand. Int J Clin Pharm. 2016;38(1):25–29. doi:10.1007/s11096-015-0220-2
18. Pongpunna S, Pratipanawatr T, Jarernsiripornkul N. Survey of outpatients’ use and needs of patient medicine information leaflets in Thailand. Int J Clin Pharm. 2018;41:141–150. doi:10.1007/s11096-018-0748-z
19. Division of Innovative Health Products and Services, Food and Drug Administration [homepage on the Internet]. Guideline for leaflet development for drug research and innovation; 2019. Available from: www.fda.moph.go.th/sites/oss/Shared%20Documents/SmPC-PIL_HPEP%20guideline_updated%20May2019.pdf.
20. Liu F, Abdul-Hussain S, Mahboob S, Rai V, Kostrzewski A. How useful are medication patient information leaflets to older adults? A content, readability and layout analysis. Int J Clin Pharm. 2014;36(4):827–834. doi:10.1007/s11096-014-9973-2
21. Al-Aqeel SA. Evaluation of medication package inserts in Saudi Arabia. Drug Healthc Patient Saf. 2012;4(1):33–38. doi:10.2147/DHPS.S29402
22. Ramdas D, Chakraborty A, Swaroop H, Faizan S, Praveen Kumar V, Srinivas BN. A study of package inserts in Southern India. J Clin Diagnostic Res. 2013;7(11):2475–2477.
23. Patsuree A, Krska J, Jarernsiripornkul N. Experiences relating to adverse drug reactions in the community: a cross-sectional survey among patients and the general public in Thailand. Expert Opin Drug Saf. 2016;338:1–9.
24. Samma K, Youngpattana W, Srinonghang S Experience of adverse reaction and behavior of taking herbal medicines and dietary supplement [Unpublished Report]. Khon Kaen: Khon Kaen University; 2017.
25. Phueanpinit P, Jarernsiripornkul N, Pongwecharak J, Krska J. Hospital pharmacists’ roles and attitudes in providing information on the safety of non-steroidal anti-inflammatory drugs in Thailand. Int J Clin Pharm. 2014;36(6):1205–1212. doi:10.1007/s11096-014-0018-7
26. Jay E, Aslani P, Raynor D. User testing of consumer medicine information in Australia. Health Educ J. 2010;70:420–427. doi:10.1177/0017896910376131
27. Hamrosi KK, Raynor DK, Aslani P. Pharmacist, general practitioner and consumer use of written medicine information in Australia: are they on the same page? Res Soc Adm Pharm. 2014;10(4):656–668. doi:10.1016/j.sapharm.2013.10.002
28. The Medicines and Healthcare Products Regulatory Agency [homepage on the Internet]. Best practice guidance on patient information leaflets; 2012. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/328405/Best_practice_guidance_on_patient_information_leaflets.pdf.
29. Raynor D, Silcock J, Knapp P, Edmondson H. How do patients use medicine information leaflets in the UK? Int J Pharm Pract. 2007;15(3):209–218. doi:10.1211/ijpp.15.3.0008
30. Afolabi MO, Akinwale VO, Akinyemi OA, Irinoye AI. Patient use and perception of medicine information leaflets. Pharmacoepidemiol Drug Saf. 2012;21:110.
31. Rahim N, Rafiq K, Iffat W, Nesar S, Shakeel S. Patients comprehension of pharmaceutical package inserts information in Karachi, Pakistan. Trop J Pharm Res. 2015;14(12):2307–2311. doi:10.4314/tjpr.v14i12.22
32. Raynor DK, Blenkinsopp A, Knapp P, et al. A systematic review of quantitative and qualitative research on the role and effectiveness of written information available to patients about individual medicines. Health Technol Assess. 2007;11(5):1–160. doi:10.3310/hta11050
33. Hamrosi KK, Aslani P, Raynor DK. Beyond needs and expectations: identifying the barriers and facilitators to written medicine information provision and use in Australia. Heal Expect. 2014;17:220–231. doi:10.1111/j.1369-7625.2011.00753.x
34. Perera T, Ranasinghe P, Perera U, et al. Knowledge of prescribed medication information among patients with limited English proficiency in Sri Lanka. BMC Res Notes. 2012;5(1):658. doi:10.1186/1756-0500-5-658
35. Kyei S. Patients’ information leaflets: its’ influence on ophthalmic patient education and medication compliance. Br J Med Med Res. 2014;4(5):1217–1230. doi:10.9734/BJMMR/2014/6383
36. Jarernsiripornkul N, Phueanpinit P, Pongwecharak J, Krska J. Experiences of and attitudes towards receiving information about non-steroidal anti-inflammatory drugs: a cross-sectional survey of patients in Thailand. Expert Opin Drug Saf. 2016;15:417–426. doi:10.1517/14740338.2016.1139571
37. Koo MM, Krass I, Aslani P, Guzmàn WM, Le Duff M. Factors influencing consumer use of written drug information. Ann Pharmacother. 2003;37(2):259–267. doi:10.1177/106002800303700218
38. Vinker S, Eliyahu V, Yaphe J. The effect of drug information leaflets on patient behavior. Isr Med Assoc J. 2007;9(5):383–386.
39. Nangsangna RD, Da-costa Vroom F. Factors influencing online health information seeking behavior among patients in Kwahu West Municipal, Nkawkaw, Ghana. Online J Public Health Inform. 2019;11(2):e13.
40. Jarernsiripornkul N, Nakboon S, Anarj K, Wongtaweepkij K. Survey of healthcare professionals’ practices, expectations, and attitudes towards provision of patient information leaflets in Thailand. Int J Clin Pharm. 2020;42(2):539–548. doi:10.1007/s11096-020-00965-x
41. Ankrah DNA, Ofei CN. The effect of advice to read the medicine/patient information leaflet among patients in Ghana: a cross-sectional study. J Pharm Heal Serv Res. 2010;1:91–96. doi:10.1111/j.1759-8893.2010.00009.x
42. Phueanpinit P, Pongwecharak J, Krska J, Jarernsiripornkul N. Evaluation of community pharmacists’ roles in screening and communication of risks about non-steroidal anti-inflammatory drugs in Thailand. Prim Heal Care Res Dev. 2018;19:598–604. doi:10.1017/S1463423618000142
43. Phueanpinit P, Pongwecharak J, Sumanont S, Krska J, Jarernsiripornkul N. Physicians’ communication of risks from non-steroidal anti-inflammatory drugs and attitude towards providing adverse drug reaction information to patients. J Eval Clin Pract. 2017;23(6):1387–1394. doi:10.1111/jep.12806
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
