Back to Journals » Research and Reports in Urology » Volume 11
Experience Of Medical Treatment With Desmopressin And Imipramine In Children With Severe Primary Nocturnal Enuresis In Taiwan
Authors Tai TT, Tai BT, Chang YJ, Huang KH
Received 1 July 2019
Accepted for publication 14 October 2019
Published 31 October 2019 Volume 2019:11 Pages 283—289
DOI https://doi.org/10.2147/RRU.S221443
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jan Colli
Thomson T Tai,1,2 Brent T Tai,2,3 Yu-Jun Chang,2 Kuo-Hsuan Huang4,5
1Loyola University Chicago, Stritch School of Medicine, Maywood, IL, USA; 2Department of Epidemiology and Biostatistics, Changhua Christian Hospital, Changhua, Taiwan; 3Burrell College of Osteopathic Medicine, Las Cruces, NM, USA; 4Department of Surgery, Erlin Christian Hospital, Changhua, Taiwan; 5Division of Urology, Changhua Christian Hospital, Changhua, Taiwan
Correspondence: Brent T Tai
Burrell College of Osteopathic Medicine, 3501 Arrowhead Dr, Las Cruces, NM 88001, USA
Tel +1 310 370 3757
Email [email protected]
Purpose: The aim of this study was to compare the efficacy and safety of desmopressin and imipramine in the treatment of severe primary nocturnal enuresis (NE) in Taiwan.
Patients and methods: This study was a retrospective chart review study conducted on children with primary monosymptomatic nocturnal enuresis (PMNE) or non-monosymptomatic nocturnal enuresis (PNMNE), referred to and treated by senior physicians in a Changhua medical center in Taiwan. After being screened, these children were treated with either desmopressin (n = 125) or imipramine (n = 71). All participants were treated for at least 3 months and followed afterward for at least 3 more months. The response and relapse rates were measured. Side effects were monitored. Age, gender, and severity of NE were recorded.
Results: After 3 months of treatment, 97 children treated with desmopressin were responsive (77.6%) while 58 children treated with imipramine were responsive (81.7%). Sixty-one children treated with desmopressin (48.8%) and 26 treated with imipramine (36.6%) relapsed during the 3-month post-treatment monitoring. The differences in responsive and relapse rates were not statistically significant. Four children treated with imipramine (5.6%) reported side effects while none was reported for children treated with desmopressin (P < 0.05). Age, gender, and the presence or absence of daytime enuresis did not influence the response rate to either drug (P < 0.05).
Conclusion: Currently, desmopressin is preferred over imipramine for treating NE due to the latter’s side effects. Our results demonstrated similar response rates for both drugs, with imipramine demonstrating minimal side effects. While health practitioners should pay attention to its side effects, concerns regarding imipramine toxicity in NE treatment are often overblown. Since imipramine is much cheaper than desmopressin, using imipramine to manage NE can allow health practitioners, especially in Taiwan, to treat the greatest number of children with NE.
Keywords: Taiwan, severe nocturnal enuresis, cost-effective, retrospective analysis, adverse effects, side effects
Introduction
Nocturnal enuresis (NE) is a common developmental disorder among children. Epidemiological studies have shown that the overall prevalence of this disorder declines as age increases, dropping to 7% of children at the age of 10.1–3 In Taiwan, the overall prevalence of NE was estimated to be around 7–8%, as suggested by two separate studies.4,5
The adverse effects of NE are not to be underestimated since enuresis can carry a social stigma that can lead to behavioral problems and psychiatric disorders in children. Moreover, children above 10 years of age who suffer from NE may have an increased risk of social maladjustment. Because of its potential harm on their psychology, normal development, and social life, individualized and active treatment is often appropriate and necessary.6,7
Traditionally, when treating NE through pharmacological means, two types of medications are considered: tricyclic antidepressants or desmopressin. Desmopressin, in tablet or nasal form, is a synthetic ADH analog approved by the US Food and Drug Administration in 1990 for the treatment of NE. Its most notable side effect includes water intoxication, which patients should be warned about. However, desmopressin is generally considered safe and effective for treating NE in children. According to studies examining its efficacy, desmopressin has successfully treated an estimated 30% of children with NE as well as reducing an average of one wet night per week.8 Despite desmopressin’s efficacy in treating NE, only 10% of participants were continent at 12 months.9,10 Before desmopressin became the drug of choice in treating NE, imipramine, a tricyclic drug, has also shown its efficacy since the 1960s.11 Studies suggested that 10–75% of those patients with NE responded to imipramine.12,13 However, like those treated by desmopressin, children treated with imipramine often relapsed. Due to its anticholinergic activity, the side effect profile of imipramine is more extensive, including dry mouth, nausea, and tachycardia. In rare circumstances, the side effects may even cause death.14 Although desmopressin is often the drug of choice for treating NE in Taiwan, imipramine is still widely used, particularly for those with refractory NE.15 The objective of this study was twofold: 1) to compare the efficacy and safety of these two medications after a 12-week treatment period in a Taiwanese pediatric population, and 2) to compare their relapse rates during the 3-month post-treatment monitoring.
Materials And Methods
This study was a retrospective chart review study performed in a medical center in Changhua, Taiwan. All patients included in this study were investigated by senior urologists. Our initial screening criteria were as follows: 1) aged 5 or older, 2) presence of primary mono or non-monosymptomatic nocturnal enuresis (PMNE or PNMNE), 3) frequency of bedwetting ≥6 times per week, and 4) only one type of medication (desmopressin or imipramine) administered. We then separated these children into two groups, desmopressin (D group) and imipramine (I group), according to the medication they were taking. Patients were asked to stop any form of treatment they were on before beginning either imipramine or desmopressin and were excluded from the study if they had prior experiences with either medication. Children were also excluded from the study if they had any of the following problems: 1) neurogenic bladder (i.e., congenital spinal anomaly, spinal cord injury, or urogenital anomalies), 2) congenital systematic disease such as type I diabetes and cerebral palsy, 3) Tourette syndrome (TS) with attention deficit hyperactivity disorder (ADHD), and/or 4) secondary enuresis.
All the children were treated for at least 3 months and then monitored afterward for at least 3 more months. Participants in the D group were given one oral tablet of desmopressin acetate (0.1 mg, minirin, Ferring Pharmaceuticals Limited) an hour before bed for the first week of treatment. The dose was then increased up to four tablets depending on patient response. Participants in the I group were given imipramine according to their body weight (1 mg per kg body weight) for the first week. The dose was then increased from one tablet (25 mg) to a maximum of two tablets per night depending on patient response.
Treatment outcome was evaluated according to the criteria set by the International Continence Society (ICS) and the International Children’s Continence Society (ICCS). The criteria were as follows: 1) the frequency of bedwetting before and after treatment was documented, and 2) response to medication was assessed after 3 months of treatment (no response was defined as <50% reduction, partial response defined as a 50% to 99% reduction, and complete response defined as a 100% reduction in bedwetting frequency).16 Relapse was defined as an increase in bedwetting frequency during any single month of the 3-month post-treatment observation period as compared to the bedwetting frequency during the last month of the 3-month treatment period.
For analysis, demographic differences between groups were compared using the chi-square test or Fisher’s exact test when appropriate. In addition, the multivariate logistic regression analysis was used to compare the efficacy of the two drugs after controlling for age, gender, and the presence or absence of daytime enuresis or urgency. The difference in the frequency of adverse drug effects between the two drugs was also analyzed. All statistical analyses were performed using the IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp., Armonk, NY, USA), and P-value <0.05 was considered statistically significant.
Results
This study included 196 patients (125 in the D group and 71 in the I group). Ninety-seven children in the D group (77.6%) and 58 children in the I group (81.7%) responded to treatment (Figure 1). Among them, 75 children in the D group (60%) and 49 children in the I group (69%) were fully responsive. Twenty-two children in the D group (22.4%) and 13 children in the I group (18.3%) were non-responsive. In addition, 61 children in the D group (48.8%) and 26 children in the I group (36.6%) relapsed during the 3-month post-treatment monitoring. There was no significant difference in either the response or relapse rate between the two groups (P < 0.05). Children with PMNE and PMNME in either group showed no difference in their responsiveness to desmopressin or imipramine. Four children in the I group (5.6%) reported adverse effects, while none were reported in the D group, showing a significant difference in the frequency of reported adverse effects (P = 0.016, Table 1). The side effects reported in the I group were dry mouth, constipation, or hand tremor. Despite the side effects, all completed the treatment course.
 |
Table 1 Patients Characteristics And Comparison Of Treatment Outcome |
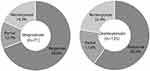 |
Figure 1 Comparison of the efficacy of imipramine and desmopressin in the treatment of primary nocturnal enuresis. |
We further compared the efficacy between the two drugs while examining age, gender, and the presence or absence of daytime enuresis or urgency as individual variables. We found no difference, suggesting that these variables did not affect the efficacy of the drugs (Table 2). A multivariate logistic regression analysis further demonstrated no significant difference in the efficacy of the two drugs while controlling for the above covariates. These results suggested that none of these variables affected the likelihood of a child responding to either desmopressin or imipramine (Table 3).
 |
Table 2 Stratification Analysis Of Treatment Outcome |
 |
Table 3 Logistic Regression Analysis Of The Efficacy Of Medical Treatment In Children With Primary Nocturnal Enuresis |
Discussion
Previous literature has compared the pharmacological effects of treating NE with either desmopressin or imipramine.17–19 Reported response rates were similar for both imipramine and desmopressin, but toxicity was often a concern when administering imipramine. Other studies yielded different conclusions. Monda and Husmann found that desmopressin worked better than imipramine for short-term treatment of NE, while both drugs showed similar response rates for long-term treatment.18 A study done in Korea by Lee, Suh, and Lee found more favorable response rates for desmopressin, as well as for a combination therapy of desmopressin and oxybutynin.19 Another study with a cross-over design concluded that intranasal desmopressin was more effective than imipramine in treating NE.20 In contrast, our study showed that there was no significant difference in response rates between oral desmopressin and imipramine after 3 months of treatment, suggesting that there was no difference in their efficacy. In addition, a 3-month post-treatment monitoring revealed no significant difference in the relapse rate between the two drugs. Interestingly, although the relapse rate for both drugs was high at 36.6% to 48.8%, it was still much lower than those reported by some clinical studies (up to 90%).21,22 This discrepancy can stem from the different definitions of relapse employed by different studies. Other possible factors included the age of the participants, the degree of severity of the participants’ bedwetting, and the duration of the treatment. Furthermore, because of the retrospective nature of our study, there may be differences in the participants’ demographics, besides the age, gender, and the presence of daytime symptoms, that affected the response and relapse rates of the participants.
In particular, all participants in our study had severe bedwetting (>6or more times per week). Yap et al in Singapore reported that short-term treatment of severe bedwetting with oral desmopressin yielded a response rate of 59%, with 26% of the children becoming completely dry.23 Our study showed a slightly higher response rate, which may be explained by our study’s longer treatment period and patient demographics. Our results also agreed with those found in Lottmann et al in which the authors concluded that long-term treatment of desmopressin in severe primary NE was effective and well tolerated in patients aged 5 or older.24 Meta-analysis of 18 randomized controlled studies found a reduction in the frequency of bedwetting ranging from 10% to 91%, but only about 25% of the treated children were completely dry during treatment.25 Furthermore, studies have demonstrated that 94.3% of patients relapsed after desmopressin was discontinued, indicating that its primary utility is symptomatic control and not cure.20
Age may also be a factor that influences the remission and relapse of NE after treatment. Previous studies have shown that age and severity of bedwetting were risk factors for remission of NE in children.15 Children with less severe NE achieved remission more quickly than did those with more severe NE at any given time. In addition, the likelihood of parents seeking medical attention increased with an older child or a child with more severe enuresis.15 Therefore, we suspected that age would influence treatment response to either desmopressin or imipramine. Contrary to expectations, our results showed that age did not mediate or modulate the response rate to desmopressin, and more research is necessary to address this issue.
Differentiating PMNE from PNMNE in children may also be important in choosing the appropriate pharmacological therapy. Children with PNMNE may have underlying medical problems not found with questionnaires or basic diagnostic evaluations. As such, it is possible that children with PNMNE responded less favorably to pharmacological intervention than do children with PMNE. For such children, a urodynamic study must be considered.26 In Taiwan, imipramine is often used for this purpose. The exact mechanism of its action is unclear. It is believed to increase bladder capacity through a weak anticholinergic effect, which may decrease detrusor muscle contractions.27 However, our study found no significant difference in the response rate to either desmopressin or imipramine between children with PMNE and those with PNMNE, suggesting that the efficacy of both drugs was similar in either condition.
Despite the similar response rates to desmopressin and imipramine and the increased risk of imipramine toxicity, imipramine is still useful for refractory NE. A retrospective study done by Gepertz and Neveus reported that in children with NE who did not respond to desmopressin, alarm therapy, and anticholinergic treatment, imipramine was still useful when standard treatment has failed, especially among older children.28 They suggested that imipramine may have to be continued for three to 48 months before the child can achieve dryness. However, long-term treatment with imipramine may increase the risk of adverse effects. In their study, adverse effects, mostly nausea, were experienced, with weight gain, increased sweating, depressed mood, and muscular cramps reported sporadically. No severe or irreversible effects were found. Similarly, a study done by Glazener reported that when treated with desmopressin or imipramine, 5% and 17% of children with NE experienced side effects, respectively.29 In contrast, less than 6% of the children treated with imipramine in our study reported side effects. Furthermore, the side effects experienced were minimal, with the most common being either dry mouth, constipation, or slight hand tremors. A possible explanation of this discrepancy would be the duration of the treatment, or perhaps the ethnicity of the participants. Further research should explore a potential combination of imipramine and desmopressin in treating refractory NE.
Due to the fear of cardiotoxicity and hepatoxicity in imipramine overdose, many health practitioners have recommended against the use of imipramine in managing enuresis.13,14 However, studies have suggested that these adverse effects were the result of treating depression with imipramine, and that when imipramine is used to treat enuresis, a much smaller dose is prescribed.30 Other researchers argued that its adverse effects were minimal if imipramine was only used for less than 3 months.30 Our results supported their arguments. Our study found that not only was the frequency of reported side effects low, the effects reported were minimal. These results suggested that the concerns regarding imipramine toxicity in managing NE were often overblown. The uncertainty of imipramine’s side effect profile in these cases demonstrated the difficulty in prescribing imipramine for off-label use since the dosage required for treating bedwetting differed significantly than that required for treating anxiety or depression.
In Taiwan, treatment guidelines for enuresis outline two mainstays of treatment: 1) behavioral modification with an alarm system and 2) pharmacotherapy with desmopressin or imipramine.31 Desmopressin is often considered as the first-line treatment, with alarm therapy as an alternative since it is seldom used, possibly due to cultural practices. This guideline differs from treatment recommendations in other countries in that imipramine remains one of the main treatment options because of its relative safety in Taiwan. For instance, a recent review done by Jain S and Bhatt GC recommended desmopressin as the first-line drug, with imipramine recommended in resistant cases due to its adverse effects.32 However, our study failed to find side effects as harmful as previous studies have described, such as cardiotoxicity and hepatoxicity. From this perspective, our results supported the treatment guidelines for NE in Taiwan.
Another important consideration in healthcare decisions is cost. In our study, all participants were given similar number of tablets. We obtained similar results in terms of efficacy and safety. According to the Taiwan’s healthcare database, desmopressin (Minirin) 0.1 mg tablet costs the government 48.7 NTD while imipramine (Tofranil) 25 mg tablet costs 2.99 NTD. This means that more than 16 children with NE can be treated through imipramine before one child through desmopressin. Therefore, using imipramine instead of desmopressin allows health practitioners, especially in Taiwan’s single-payer healthcare system, to treat the greatest number of children with NE in a cost-effective manner.
Conclusion
While desmopressin is often the drug of choice in treating primary NE worldwide and in Taiwan, we concluded that imipramine is a suitable alternative if desmopressin is not available, especially if cost-effectiveness is taken into consideration. While health practitioners should pay attention to the side effects of imipramine, concerns regarding its toxicity in NE treatment are often overblown. Furthermore, due to its cost-effectiveness, in cases of refractory NE requiring long-term management, imipramine could be considered as well.
Ethics Approval
The study procedures were approved by the institutional review board (IRB) of the Changhua Christian Hospital in Changhua. IRB approval number CCH: 050403. Patient consent to review their medical records was not required by the IRB of the Changhua Christian Hospital provided the patient data were deidentified and reasonable care was taken to ensure data confidentiality and security. Data analysis also had to be done on-site or through a remote connection to ensure data privacy. After the appropriate chart review was done on-site, the necessary patient information needed was downloaded and deidentified prior to analysis. Deidentified data were stored in a secure computer accessible only by the investigators. This study was performed in compliance with the Declaration of Helsinki.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Butler RJ, Golding J, Northstone K. Nocturnal enuresis at 7.5 years old: prevalence and analysis of clinical signs. BJU Int. 2005;96:404–410. doi:10.1111/bju.2005.96.issue-3
2. Safarinejad MR. Prevalence of nocturnal enuresis, risk factors, associated familial factors and urinary pathology among school children in Iran. J Pediatr Urol. 2007;3:443–452. doi:10.1016/j.jpurol.2007.06.001
3. Yeung CK. Nocturnal enuresis in Hong Kong: different Chinese phenotypes. Scand J Urol Nephrol Suppl. 1997;183:17–21.
4. Cher TW, Lin GJ, Hsu KH. Prevalence of nocturnal enuresis and associated familial factors in primary school children in Taiwan. J Urol. 2002;168:1142–1146. doi:10.1016/S0022-5347(05)64612-5
5. Tai HL, Chang YJ, Chang SC, et al. The epidemiology and factors associated with nocturnal enuresis and its severity in primary school children in Taiwan. Acta Paediatr. 2007;96:242–245. doi:10.1111/j.1651-2227.2007.00025.x
6. Tai TT, Tai BT, Chang YJ, et al. Parents have different perceptions of bed-wetting than children from six to 15 years of age. Acta Paediatr. 2015;104:e466–472. doi:10.1111/apa.13101
7. Tai TT, Tai BT, Chang YJ, et al. Parental perception and factors associated with treatment strategies for primary nocturnal enuresis. J Pediatr Urol. 2017;13(272):e1–e8.
8. Neveus T, Eggert P, Evans J, et al. Evaluation of and treatment for monosymptomatic enuresis: a standardization document from the International Children’s Continence Society. J Urol. 2010;183:441–447. doi:10.1016/j.juro.2009.10.043
9. Johnson M. Nocturnal enuresis. Urol Nurs. 1998;18:259–273.
10. Monda JM, Husmann DA. Primary nocturnal enuresis: a comparison among observation, imipramine, desmopressin acetate and bed-wetting alarm systems. J Urol. 1995;154(2):745–748. doi:10.1016/S0022-5347(01)67152-0
11. Maclean RE. Imipramine hydrochloride (Tofranil) and enuresis. Am J Psychiatry. 1960;117:551. doi:10.1176/ajp.117.6.551
12. Cendron M. Primary nocturnal enuresis: current. Am Fam Physician. 1999;59:1205–1214.
13. Glazener CMA, Grant AM, Wallace SA. Treating nocturnal enuresis in children. Eff Health Care Bull. 2003;8:1–8.
14. Glazener CM, Evans JH, Peto RE. Tricyclic and related drugs for nocturnal enuresis in children. Cochrane Database Syst Rev. 2003;CD002117.
15. Tai BT, Tai TT, Chang YJ, Huang KH. Factors associated with remission of primary nocturnal enuresis and changes of parental perception towards management strategies: a follow-up study. J Pediatr Urol. 2017;13(44):e1–e9.
16. Austin PF, Bauer SB, Bower W, et al. The standardization of terminology of lower urinary tract function in children and adolescents: update report from the standardization committee of the International Children’s Continence Society. J Urol. 2014;191:1863–1865 e13. doi:10.1016/j.juro.2014.01.110
17. Seyfhashemi M, Ghorbani R, Zolfaghari A. Desmopressin, imipramine, and oxybutynin in the treatment of primary nocturnal enuresis: a randomized clinical trial. Iran Red Crescent Med J. 2015;17(7). doi:10.5812/ircmj
18. Monda J, Douglas A. Primary nocturnal enuresis: a comparison among observation, imipramine, desmopressin acetate and bed-wetting alarm systems. J Urol. 1995;154(2):745–748. doi:10.1016/S0022-5347(01)67152-0
19. Lee T, Hong J, Hun J, et al. Comparison of effects of treatment of primary nocturnal enuresis with oxybutynin plus desmopressin, desmopressin alone or imipramine alone: a randomized controlled clinical trial. J Urol. 2005;174(3):1084–1087. doi:10.1097/01.ju.0000169160.84418.15
20. Vertucci P, Lanzi C, Capece G, et al. Desmopressin and imipramine in the management of nocturnal enuresis: a multicentre study. Br J Clin Pract. 1997;51:27–31.
21. Terho P. Desmopressin in nocturnal enuresis. J Urol. 1991;145:818–820. doi:10.1016/S0022-5347(17)38461-6
22. Caldwell PH, Sureshkumar P, Wong WC. Tricyclic and related drugs for nocturnal enuresis in children. Cochrane Database Syst Rev. 2016;CD002117.
23. Yap HK, Chao SM, Tan AY, et al. Efficacy and safety of oral desmopressin in the treatment of primary nocturnal enuresis in Asian children. J Paediatr Child Health. 1998;34:151–153. doi:10.1046/j.1440-1754.1998.00189.x
24. Lottmann H, Baydala L, Eggert P, et al. Long-term desmopressin response in primary nocturnal enuresis: open-label, multinational study. Int J Clin Pract. 2009;63:35–45. doi:10.1111/j.1742-1241.2008.01956.x
25. Moffatt ME, Harlos S, Kirshen AJ, et al. Desmopressin acetate and nocturnal enuresis: how much do we know? Pediatrics. 1993;92:420–425.
26. Kwak KW, Park KH.Clinical inconsistency of lower urinary tract symptoms between questionnaire and bladder diary in children with nocturnal enuresis. J Urol.2008;180:1085–1089. discussion 9–90. doi:10.1016/j.juro.2008.05.053
27. Schmitt BD. Nocturnal enuresis: finding the treatment that fits the child. Contemp Pediatrics. 1990;7:70–97.
28. Gepertz S, Neveus T.Imipramine for therapy resistant enuresis: a retrospective evaluation. J Urol.2004;171:2607–2610. discussion 9–10. doi:10.1097/01.ju.0000110613.51078.93
29. Glazener CM, Evans JH, Peto RE. Drugs for nocturnal enuresis in children (other than desmopressin and tricyclics). Cochrane Database Syst Rev. 2003;CD002238.
30. Hjalmas K, Arnold T, Bower W, et al. Nocturnal enuresis: an international evidence based management strategy. J Urol. 2004;171:2545–2561. doi:10.1097/01.ju.0000111504.85822.b2
31. Wang TM, Yang S, Tsai JD, et al. Management of nocturnal enuresis in Taiwan: consensus statements of the Taiwan enuresis expert committee. J Formos Med Assoc. 2018.
32. Jain S, Bhatt GC. Advances in the management of primary monosymptomatic nocturnal enuresis in children. Paediatr Int Child Health. 2016;36:7–14. doi:10.1179/2046905515Y.0000000023
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
