Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 12
Exosomes from β-cells alleviated hyperglycemia and enhanced angiogenesis in islets of streptozotocin-induced diabetic mice
Authors Sun Y, Mao Q, Shen C, Wang C , Jia W
Received 25 April 2019
Accepted for publication 29 August 2019
Published 8 October 2019 Volume 2019:12 Pages 2053—2064
DOI https://doi.org/10.2147/DMSO.S213400
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Antonio Brunetti
Yun Sun, Qianyun Mao, Chao Shen, Chen Wang, Weiping Jia
Shanghai Key Laboratory of Diabetes Mellitus, Shanghai Diabetes Institute, Department of Endocrinology and Metabolism, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai 200233, People’s Republic of China
Correspondence: Chen Wang
Shanghai Key Laboratory of Diabetes Mellitus, Shanghai Diabetes Institute, Department of Endocrinology and Metabolism, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Yishan Road, Shanghai 200233, People’s Republic of China
Tel +86 212 405 8657
Fax +86 216 436 8031
Email [email protected]
Purpose: Exosomes are small nanoscale vesicles secreted from cells. Exosome-based therapeutic approaches have been evaluated in treating ischemic diseases. In the present study, we explored the effect of exosomes on streptozotozin (STZ)-induced diabetic mouse and its underlying mechanisms.
Methods: Exosomes were isolated from MIN6 cells. Transmission electron microscopy, dynamic light scattering and Western blot were used to identify the exosomes. STZ was used to establish diabetic or abnormal glucose tolerance mouse model. Histology study and flow cytometry were applied to detect the changes in immune responses.
Results: Transplantation of the exosomes into diabetic mice resulted in a longer median survival time compared with the untreated diabetic mice (P<0.01). Transplantation of the exosomes improved glucose tolerance, increased insulin content and preserved the architectures of islets in mice with abnormal glucose tolerance. Moreover, exosome treatment enhanced the expression of CD31, a marker of endothelial cells, and tended to reduce macrophage infiltration in islets of STZ-treated mice.
Conclusion: Exosomes derived from β-cells play a role in preserving pancreatic islet architecture and its function, and in inducing islet angiogenesis, which implicates that exosome treatment could be a novel therapeutic strategy for diabetes.
Keywords: exosomes, diabetes, vascular regeneration, macrophage infiltration
Introduction
Diabetes is a major chronic disease and brings a heavy burden to individuals, families and societies. According to IDF reports, 1 in 11 adults has diabetes globally, with a total number of up to 425 million.1 The chronic complications jeopardize the quality of life and eventually lead to high morbidity and mortality of diabetic patients.2,3 The failure and dysfunction of pancreatic islet β-cells is the core pathogenesis of both type 1 diabetes and type 2 diabetes.4,5 Secretion of enough insulin from β-cells is essential for glucose homeostasis in body. Based on the finding from UKPDS, defects in function β-cell mass may have already occurred during normal glucose tolerance before the onset of type 2 diabetes,6 by apoptosis and de-differentiation.7,8 Therefore, how to preserve function β-cell mass and delay the onset of the disease more efficiently through early intervention, especially activation of its internal repairing mechanism before the complete loss of its functions, has become a new target in prevention and treatment of diabetes.
The application of pancreas and islet transplantation has provided an opportunity to cure diabetes.9,10 However, the lack of sources and immune rejection limit the widespread of this approach.11,12 Exosomes with diameters ranging 60–200 nm approximately are small nanoscale vesicles secreted by cells.13,14 It exhibits a characteristic “discoid” ellipse or disc-like vesicle structure under electron microscopy, and its bilayer lipid membrane is rich in cholesterol, lecithin and sphingomyelin. It contains proteins, mRNAs and miRNAs, which can transmit messages through a variety of ways to the recipient cells.15 The formation and secretion of exosome’s cystic structure occur through stimulation of internal environment and factors, and hold great potential as novel diagnostic and prognostic molecular biomarkers of certain diseases. In addition, studies have shown that exosomes secreted in the extracellular environment in physiological or disease states are capable of various functions including reducing oxidative stress reactions, inhibiting apoptosis, inducing cell proliferation and promoting angiogenesis.16 They also play an important role in the regulation of inflammatory responses, the repairing of tissue damages and other pathophysiological progresses.16,17 Exosome-based therapeutic approaches have been applied in treating neurological disorders,18,19 skeletal muscle regeneration20 and heart remodeling in myocardial infarction.21 Skeletal muscle is the major site for insulin action. Sarcopenia was associated with insulin resistance in both obese and non-obese individuals. Mesenchymal stem cells (MSCs)-derived exosome treatment promoted myogenesis and angiogenesis in C2C12, and muscle regeneration in muscle injury mice.20 Moreover, MSCs-derived exosomes were reported to participate in myocardial repair and ameliorate cardiac damage after myocardial infarction via activating S1P/SK1/S1PR1 signaling.22 However, less effectiveness in lowering blood glucose is observed in diabetic model.23 Thus, it is conceivable to expect that exosomes from β-cells containing elements needed for the cells might be able to protect pancreatic islet β-cells. The results of this study are expected to lay a foundation for new ways of treatment of diabetes.
Materials and methods
Antibodies
Antibodies used in histological study were obtained from Abcam (Cambridge, UK) (guinea pig anti-mouse insulin antibody, goat anti-guinea pig antibody conjugated with CY3, goat anti-rabbit antibody conjugated with Alexa Fluor 488, donkey anti-goat antibody conjugated with CY3, and rabbit anti-F4/80 antibody), and Santa Cruz (California, USA) (rabbit anti-mouse glucagon antibody, goat anti-mouse CD31 antibody). Antibody used in the flow cytometry was purchased from BD Pharmingen (San Diego, USA) (anti-mouse F4/80-PE). Antibodies used in Western blotting include rabbit polyclonal anti-mouse CD9, rabbit anti-mouse CD63 and goat anti-rabbit IgG-HRP (Abcam, Cambridge, UK). Mouse anti-GAPDH monoclonal antibody was purchased from KangChen Bio-tech Inc. (Shanghai, China).
Extraction and identification of exosomes
MIN6 cells, a kind gift from Dr. F. Liu (Department of Cellular and Structural Biology, University of Texas Health Science Center at San Antonio, TX, USA), were purchased originally from American Type Culture Collection24 and cultured in high-glucose Dulbecco’s Modified Eagle Medium (Gibco, USA) supplemented with 15% exosome-free fetal bovine serum (FBS, Gibco, USA), 100μg/mL streptomycin, 100μg/mL penicillin, and 50μM β-mercaptoethanol. Exosomes were isolated from supernatant of MIN6 cells collected during a 48hrs period according to the previous reports.25,26 Cellular debris was firstly removed through centrifugation at 4°C (at 300g for 10 mins, at 2000g for 10 mins, and at 10,000g for 30 mins). The supernatant was then centrifuged at 100,000g for 70 mins at 4°C. The pellets primarily contained exosomes were collected and resuspended in PBS and stored at −80°C. Western blotting was used to identify the exosomes by the marker protein CD9 and CD63.25 The size distribution and concentration of the exosomes were measured by a ZetaSizer Nano ZS (Malvern Instruments, Worcestershire, UK). Accordingly, the samples were diluted in PBS, and tertiary measurements were performed at 25°C. Transmission electron microscopy (TEM) was used to observe the morphology of the exosomes.
Cell viability analysis
INS-1 (832/13) cells, a gift from Dr. Y. Liu (the Institute for Nutritional Sciences, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences), were derived from parental INS-1 cells via a transfection-selection strategy by Dr. Christopher B. Newgard and his colleagues,27 and were maintained in RPMI 1640 medium (Gibco, USA) supplemented with 10% FBS, 10mmol/L HEPES and 6.1 mmol/L glucose at 37°C and 5% CO2 in a humid atmosphere. The cells were seeded in 96-well plates at a density of 2.5×104/well. The cells were exposed to 0.4 mM palmitate in combination without or with different concentrations of exosomes (1.5×109 partials/mL, 4.5×109 partials/mL or 9×109 partials/mL). After 48hrs, the cells were tested for the viability using Methylthiazolyldiphenyl-tetrazolium Bromide (Beyotime, China) assay.
Animal studies
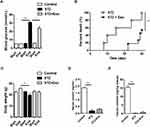
Male C57BL/6J mice were purchased from Shanghai Slaccas Company (Shanghai, China). All experimental procedures involving animals were approved by the Animal Ethics Committee of Shanghai Jiao Tong University Affiliated Six People’s Hospital in accordance with the Guidelines for the Care and Use of Laboratory Animals (DWSY2014-068/DWLL2019-0275). All animals were housed under a standard 12hrs/12hrs light-dark cycle at constant temperature of 23±1°C with free access to standard food and water. At 6 weeks of age, the mice were administered with either high-dose of streptozotocin (STZ) (H-STZ, 200mg/kg) or low-dose of STZ (L-STZ, 140mg/kg) by intraperitoneal injection once on day 0 (Figure 1). Transplantation of exosomes was performed according to the previous report.28 For H-STZ treated mice, 6×109 particles of exosomes in 0.2 mL PBS or only PBS as control were transplanted in situ pancreas after their fasting blood glucose levels reached at least 11.1 mmol/L. Non-fasting blood glucose levels and body weight were measured every week since the transplantation (Figure 1A). As to L-STZ treated mice, 6×109 particles of exosomes or PBS were transplanted in situ pancreas at day 10 after STZ injection, and 3×109 particles of exosomes 11 days after first transplantation through tail vein injection. An intraperitoneal glucose tolerance test (IPGTT) was performed at day 7, day 17 and day 35 with 2mg/kg glucose and 6hrs fasting (Figure 1B). Serum samples were collected before and 30mins after 2mg/kg glucose load for insulin measurement at day 17 and 28. At the end of the experiment, both H-STZ- and L-STZ-treated mice were sacrificed, blood samples were collected and pancreatic tissues were preserved for insulin extraction and histology study.
Histology study, Western blotting and insulin measurement
For immunofluorescence analysis, the pancreas was fixed in 4% formaldehyde and embedded in paraffin. Pancreatic sections were incubated at 4°C with guinea pig anti-insulin antibody (1:100 dilution), rabbit anti-glucagon antibody (1:100 dilution), or goat anti-CD31 antibody (1:50 dilution) overnight. Thereafter, the sections were stained with goat anti-guinea pig antibody conjugated with CY3 (1:300 dilution) for insulin, goat anti-rabbit antibody conjugated with Alexa Fluor 488 (1:400 dilution) for glucagon, and donkey anti-goat antibody conjugated to CY3 for CD31 (1:500 dilution). Pancreatic sections were also incubated with rabbit anti-F4/80 antibody (1:500 dilution) overnight for F4/80 staining. The digital images were acquired by a Zeiss Axio-Imager Standard Microscope (Carl Zeiss, Germany). Quantification of CD31 in islets was conducted by ZEN software (ZEN Version 2.0, Carl Zeiss Inc., Germany) from three nonconsecutive sections. Image was acquired using Zeiss 40× Objective. Data were expressed as the average ratio of CD31-positive density area in each islet over insulin-positive area of islets, as described.29
To determine CD9 and CD63 expression, MIN6 cells and exosomes extracted from the cells were loaded to 12% SDS-PAGE gels and followed by transferring onto nitrocellulose membranes. The signals were detected by enhanced chemiluminescence (Thermo Fisher, USA) and imaged by Image Quant LAS 4000 mini bio-molecular imager (GE Healthcare, Uppsala, Sweden). Insulin content in plasma and in acetic acid extracts from pancreatic tissues was measured by mouse insulin ELISA kits (Hong Kong University Antibody and Immunoassay Services, China).
Flow cytometry
Splenocytes were isolated from freshly obtained spleen from each L-STZ animals at day 35 after STZ. In brief, the spleens were taken out and the spleen cells were separated with a 100μm cell strainer. After removing red cells using Lysing Buffer (BD Pharmingen), single splenic cells were washed with PBS, incubated with the fluorescent-labeled F4/80 antibody (F4/80-PE) at 4°C in dark for 30mins, and washed with PBS twice before acquisition. 20–50×103 events were acquired by Aria II (Becton Dickinson, CA, USA) and analyzed by FlowJoV10.4.2 software (a Becton Dickinson, CA, USA).
Statistical analysis
Statistical analysis was performed with GraphPad Prism 5 for windows (GraphPad Software, San Diego, USA). The data in the study were showed as mean ± SEM, and the differences between/among groups were tested for significance with Student’s test or ANOVA and followed by Bonferroni’s multiple comparison test. Log-rank (Mantel-Cox) test was exploited to the survival curves comparison. P-values were two-tailed, and P<0.05 considered to be a significant difference.
Results
Characterization of exosomes
The size distribution of the vesicles in the supernatant derived from MIN6 cells was mainly in the range of 60–200 nm with an average size of 118.75±38.74nm (Figure 2A), which was consistent with the exosome size distribution from previous reports.30 Vesicles with size of about 100 nm could be observed clearly from the TEM image (Figure 2B) and the subcellular structures exhibited the characteristic spherical morphology of exosomes. Moreover, CD9 and CD63, typical markers of exosomes, were highly expressed in the vesicles (Figure 2C).
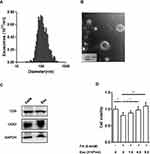 |
Figure 2 Characterization of exosomes derived from MIN6 cells. Notes: (A) Size distribution of exosomes evaluated by a ZetaSizer Nano ZS. (B) Transmission electron microscopy image of the exosomes, scale bar: 100nm. (C) Western blot analysis of exosome marker CD9 and CD63. (D) Cell viability in INS-1(832/13) (INS-1) cells. INS-1 cells were exposed to palmitate (PA, 0.4 mmol/L) and different concentrations of exosomes (Exo, 0–1.5-4.5–9.0X109/mL) for 48 hrs. Data were presented as fold-change of INS-1 cell viability over cells treated without palmitate and exosomes. * P<0.05, ** P<0.01, between indicated groups (n=4). Abbreviations: PA, palmitate; Exo, exosomes. |
To know if the exosomes derived from β-cells have any protective effect, we, then, co-cultured INS-1(832/13) cells exposed to palmitate (0.4mmol/L) together with the exosomes at indicated concentrations for 48hrs. As expected, exposure of palmitate significantly induced cell death (P<0.05) (Figure 2D). Addition of exosomes dose dependently improved the cell viability induced by palmitate (P<0.01, Figure 2D).
Exosome treatment prolonged the survival time of H-STZ-treated diabetic mice
Three to four days after STZ injection, non-fasting blood glucose concentrations in H-STZ-treated mice increased significantly (before STZ injection: 6.01±0.82 mmol/L vs after STZ injection: 21.01±5.11 mmol/L, P<0.001). Transplantation of the exosomes significantly decreased the non-fasting glucose levels despite its failure to reach normal levels (P<0.05, Figure 3A), and prolonged the median survival time (15 vs 30 days) (P<0.01, Figure 3B). No significant differences in the body weight, serum and pancreatic insulin content could be viewed between exosome treated and non-treated diabetic mice (Figure 3C–E).
Exosome treatment improved glucose metabolism, islet morphology and increased insulin content in L-STZ-treated mice
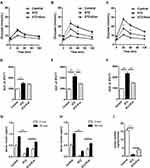
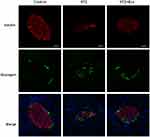
L-STZ-treated mice exhibited progressively impaired glucose tolerance at day 7, 17 and 35, respectively (Figure 4A–F) (P<0.05). At day 17 and 28, glucose-induced insulin secretion and pancreatic insulin content at the end of experiments decreased dramatically compared with normal controls (P<0.001, Figure 4g-i). In consistency, there was a disruption in islet architecture and decrease in insulin staining in these mice (Figure 5). However, compared to mice with only STZ injection, the exosome-treated mice exhibited an improvement of the glucose tolerance (P<0.05, Figure 4B and C) and a decrease in the area under curve (AUC) of IPGTT (P<0.01, Figure 4E and F). Consistently, the exosome treatment significantly elevated glucose-induced insulin secretion and pancreas insulin content (P<0.05, Figure 4G–I), and maintained the insulin staining and islet architectures (Figure 5). These results together suggested that the exosomes had a protective effect on β-cells through promoting the cell survival, and preserving the insulin storage and islet morphology.
Exosome treatment enhanced CD31 expression in islets from L-STZ mice
Disruption of inner vascularization is associated with STZ-induced β-cell death. To further understand the mechanism of diabetic alleviation using the exosome treatment, a histological analysis was carried out in these animals. As shown in Figure 6, the expression of CD31, a marker for endothelial cells, was weaker after L-STZ injection compared with normal control. However, its expression was greatly enhanced after the exosomes, indicating an association of the exosomes and vascular density (Figure 6).
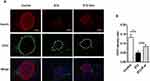
Exosome treatment tended to reduce infiltration of macrophages in islets from L-STZ-treated mice
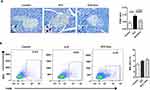
To decipher if exosomes play any role on inflammatory regulation, pancreas sections from mice at 35 days after L-STZ were stained with F4/80, a major macrophage marker. As shown in Figure 7A, F4/80-positive cells were increased in islets from STZ-treated mice. Exosome treatment tended to lower the expression of F4/80 in islet cells (Figure 7A). However, no changes could be observed in the relative frequency of macrophages in splenocytes isolated from STZ-treated alone or together with exosome-treated mice when compared with that of control mice (Figure 7B).
Discussion
In the present study, we provide evidences demonstrating for the first time that the exosomes derived from β-cell lines exert a protective effect on STZ-induced glucose disturbance, in which the exosomes prolong the survival time in H-STZ-induced diabetic mice and alleviate glucose intolerance in L-STZ-treated mice. Most importantly, the exosome treatments confer resistance to insulin decrease in pancreas and serum, and maintain islet morphology in L-STZ mice. Furthermore, we find that these protective effects are associated with the increase of CD31 expression in pancreas and decrease of macrophage infiltration in islets suggesting a role of the exosomes on neovascularization and modulation of immune response within pancreatic islets, which might contribute to the protective effects of the exosomes on STZ-treated mice.
STZ, a highly selective pancreatic β-cells cytotoxic agent, has been widely applied in animal experiments to build diabetic model. In this study, we built two types of diabetic mouse model with different dosages of STZ injection. The H-STZ treatment destroyed pancreatic islet β-cells within a short time, resulting in rapid β-cells necrosis, significant hyperglycemia and diabetes in the mice. The L-STZ could not induce a rapid β-cell death within a short time, but induce abnormal glucose metabolism in the mice.31 Transplantation of the exosomes remarkably prolonged the survival time of H-STZ-treated mice, as well as improved glucose tolerance of the L-STZ-treated mice. Moreover, there were significant improvements in the serum insulin levels and pancreatic insulin content in L-STZ-treated mice. The islet morphology and insulin storage were preserved after exosome transplantation in these mouse models observed by the immunofluorescence analysis. Despite the limitation of exosomes failure to bring dysfunctional β-cells back to normal level, the above data indicated that the exosomes derived from β-cells played an essential role in the functional recovery of β-cell and internal glucose homeostasis maintenance.
Mechanisms involving the effects of the exosomes on diabetic alleviation might be related to the following aspects. Firstly, the exosomes exerted their above-mentioned effects through modulation of immune response. It is accepted that STZ exerts the cytotoxic effect in β-cells through excessive production of reactive oxygen species, activation of inflammation, and cell apoptosis.32 In the present study, the exosome treatment of L-STZ mice tended to decrease the infiltration of macrophages induced by STZ in islets. Macrophages play a crucial role in diabetes development through the induction of pancreatic inflammation33 and are featured by functional diversity and plasticity.
The ability of exosomes on immune regulation has been reported. However, the reported role of exosomes in immunity appears to be complex with both anti-inflammatory and pro-inflammatory properties probably depending on the parent cells they are derived from along with the contexts and environments.34–36 It has been demonstrated that exosomes from red blood cells are pro-inflammatory and are responsible for induction of TNF-α production by monocytes.37 Additionally, exosome-like vesicles released from adipose tissue differentiate monocytes into activated macrophages with increased secretion of TNF-α and IL-6. Injection of these exosomes led to insulin resistance in mice through TLR4 pathway.38 Similarly, exosomes from lipid-modified muscles are rich in lipid and can be incorporated into pancreas.39 It also regulated the adaptations of β-cell mass,40 suggesting a role of exosomes on inflammation and metabolism regulation. Yet, there are several studies supporting an immune suppressive role of the exosomes. MSCs-derived exosomes have been reported to suppress the secretion of pro-inflammatory factor TNF-α and IL-1β, but increase the concentration of anti-inflammatory factor TGF-β.41 Most interestingly, contrary to the findings, exosomes derived from adipose-derived stem cells attenuate high-fat diet-induced obesity and improve glucose tolerance and insulin sensitivity. Our data showing that exosomes protected islets against inflammation in islets through lowering the infiltration of macrophages are in line with the latter reports. The deep mechanisms involved in the regulatory effect of exosomes on macrophage need to be further elucidated.
Furthermore, we demonstrated in the present study that exosomes exert their ability on β-cell functional recovery through increasing endothelial cell proliferation and re-establishing vasculature in the islets. Islets are highly vascularized and its vasculature is essential for β-cell proliferation. Revascularization is one of the key factors for the improvement of islet function after transplantation. Endothelial cell CD31 is an important angiogenic and vascularizing factor,42 and has been commonly used as the marker for endothelial cell differentiation and angiogenesis.43,44 The finding in the present study that the expression of CD31 increased significantly in the pancreas from mice treated with the exosome compared with those treated with STZ only indicates a role of the exosomes on revascularization regulation in the pancreas. This notion is in agreement with the previous findings, in which exosomes derived from stem cells or cell lines (eg cardiomyocytes H9c2) promoted angiogenesis on would healing, ischemic stroke and myocardial ischemic injury through induction of expression of a number of growth factors.45–47 However, caution needs to be exercised in applying such angiogenesis-prone agents whenever potential risks in tumorigenicity exist.
Since MIN6 cell line has been shown to be not a pure, but a mixed population of cells expressing pancreatic endocrine hormones and bioactive molecules such as insulin, glucagon, somatostatin and GPR109A,48,49 the exosome proteomics was conducted. A total of 1126 proteins were identified. Among them, 1029 proteins with gene ontology annotations were analyzed and shown in Figure S1. However, apart from insulin, the above-mentioned hormones and GPR109A were not found in the exosome, indicating that other factors might be involved in the effect of exosome on STZ-induced models. Further studies are needed to explore the mechanisms underlying the roles of exosomes on β-cell protection.
Conclusion
Our study demonstrated for the first time that exosomes derived from β-cells have the ability to maintain the homeostasis of glucose metabolism in STZ-induced diabetic mice, preserve islet architectures, and promote angiogenesis. These findings indicate a role of the exosomes on neovascularization regulation, which may provide a potential therapeutic strategy for diabetic treatment.
Ethical statement
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Abbreviations
AUC, Area Under Curve; FBS, fetal bovine serum; FFA, free fatty acid; IDF, International Diabetes Federation; IPGTT, intraperitoneal glucose tolerance test; STZ, streptozotocin; TEM, Transmission Electron Microscopy; TGF, transforming growth factor; TNF, Tumor Necrosis Factor.
Acknowledgments
The authors thank Xin Niu and Yang Wang from the Institute of Microsurgery on Extremities, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital for technique support. This study was funded by the Shanghai Nature Science Fund (16ZR1425800) and grants from the National Natural Science Foundation (81670707). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
Shanghai Sixth People’s Hospital has applied for a patent related to the methods described in this study, with Chen Wang, Yun Sun and Weiping Jia as co-inventors. The author reports no other conflicts of interest in this work.
References
1. International Diabetes Federation. IDF Diabetes Atlas.
2. Bannier K, Lichtenauer M, Franz M, et al. Impact of diabetes mellitus and its complications: survival and quality-of-life in critically ill patients. J Diabetes Complications. 2015;29(8):1130–1135. doi:10.1016/j.jdiacomp.2015.08.010
3. Wang L, Gao P, Zhang M, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA. 2017;317(24):2515–2523. doi:10.1001/jama.2017.7596
4. Chen C, Cohrs CM, Stertmann J, Bozsak R, Speier S. Human beta cell mass and function in diabetes: recent advances in knowledge and technologies to understand disease pathogenesis. Mol Metab. 2017;6(9):943–957. doi:10.1016/j.molmet.2017.06.019
5. Accili D, Ahren B, Boitard C, Cerasi E, Henquin JC, Seino S. What ails the beta-cell? Diabetes Obes Metab. 2010;12(Suppl 2):1–3. doi:10.1111/j.1463-1326.2010.01296.x
6. Meier JJ, Breuer TG, Bonadonna RC, et al. Pancreatic diabetes manifests when beta cell area declines by approximately 65% in humans. Diabetologia. 2012;55(5):1346–1354. doi:10.1007/s00125-012-2466-8
7. Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell. 2012;150(6):1223–1234. doi:10.1016/j.cell.2012.07.029
8. Accili D, Talchai SC, Kim-Muller JY, et al. When beta-cells fail: lessons from dedifferentiation. Diabetes Obes Metab. 2016;18(Suppl 1):117–122. doi:10.1111/dom.12723
9. Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–238. doi:10.1056/NEJM200007273430401
10. Bellin MD, Barton FB, Heitman A, et al. Potent induction immunotherapy promotes long-term insulin independence after islet transplantation in type 1 diabetes. Am J Transplant. 2012;12(6):1576–1583. doi:10.1111/j.1600-6143.2011.03977.x
11. Khosravi-Maharlooei M, Hajizadeh-Saffar E, Tahamtani Y, et al. Therapy Of Endocrine Disease: islet transplantation for type 1 diabetes: so close and yet so far away. Eur J Endocrinol. 2015;173(5):R165–R183. doi:10.1530/EJE-15-0094
12. Vendrame F, Hopfner YY, Diamantopoulos S, et al. Risk factors for type 1 diabetes recurrence in immunosuppressed recipients of simultaneous pancreas-kidney transplants. Am J Transplant. 2016;16(1):235–245. doi:10.1111/ajt.13426
13. Lakkaraju A, Rodriguez-Boulan E. Itinerant exosomes: emerging roles in cell and tissue polarity. Trends Cell Biol. 2008;18(5):199–209. doi:10.1016/j.tcb.2008.03.002
14. Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi:10.1146/annurev-cellbio-101512-122326
15. Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi:10.1038/ncb1596
16. Zhang J, Guan J, Niu X, et al. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;13:49. doi:10.1186/s12967-015-0417-0
17. Record M, Carayon K, Poirot M, Silvente-Poirot S. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim Biophys Acta. 2014;1841(1):108–120. doi:10.1016/j.bbalip.2013.10.004
18. Osier N, Motamedi V, Edwards K, et al. Exosomes in acquired neurological disorders: new insights into pathophysiology and treatment. Mol Neurobiol. 2018;55(12):9280–9293. doi:10.1007/s12035-018-1054-4
19. Zhang S, Eitan E, Wu TY, Mattson MP. Intercellular transfer of pathogenic alpha-synuclein by extracellular vesicles is induced by the lipid peroxidation product 4-hydroxynonenal. Neurobiol Aging. 2018;61:52–65. doi:10.1016/j.neurobiolaging.2017.09.016
20. Nakamura Y, Miyaki S, Ishitobi H, et al. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett. 2015;589(11):1257–1265. doi:10.1016/j.febslet.2015.03.031
21. Sahoo S, Losordo DW. Exosomes and cardiac repair after myocardial infarction. Circ Res. 2014;114(2):333–344. doi:10.1161/CIRCRESAHA.114.300639
22. Deng S, Zhou X, Ge Z, et al. Exosomes from adipose-derived mesenchymal stem cells ameliorate cardiac damage after myocardial infarction by activating S1P/SK1/S1PR1 signaling and promoting macrophage M2 polarization. Int J Biochem Cell Biol. 2019;114:105564. doi:10.1016/j.biocel.2019.105564
23. Jiang ZZ, Liu YM, Niu X, et al. Exosomes secreted by human urine-derived stem cells could prevent kidney complications from type I diabetes in rats. Stem Cell Res Ther. 2016;7:24. doi:10.1186/s13287-016-0287-2
24. Luo Y, He F, Hu L, et al. Transcription factor Ets1 regulates expression of thioredoxin-interacting protein and inhibits insulin secretion in pancreatic beta-cells. PLoS One. 2014;9(6):e99049. doi:10.1371/journal.pone.0099049
25. Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Current Protocols in Cell Biology. John Wiley and Sons;2006:3.22.1-3.22.29.
26. Lobb RJ, Becker M, Wen SW, et al. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell Vesicles. 2015;4:27031. doi:10.3402/jev.v4.27031
27. Ronnebaum SM, Ilkayeva O, Burgess SC, et al. A pyruvate cycling pathway involving cytosolic NADP-dependent isocitrate dehydrogenase regulates glucose-stimulated insulin secretion. J Biol Chem. 2006;281(41):30593–30602. doi:10.1074/jbc.M511908200
28. Zhao T, Luo D, Sun Y, et al. Human urine-derived stem cells play a novel role in the treatment of STZ-induced diabetic mice. J Mol Histol. 2018;49(4):419–428. doi:10.1007/s10735-018-9772-5
29. Su D, Zhang N, He J, et al. Angiopoietin-1 production in islets improves islet engraftment and protects islets from cytokine-induced apoptosis. Diabetes. 2007;56(9):2274–2283. doi:10.2337/db07-0371
30. van der Pol E, Coumans FA, Grootemaat AE, et al. Particle size distribution of exosomes and microvesicles determined by transmission electron microscopy, flow cytometry, nanoparticle tracking analysis, and resistive pulse sensing. J Thromb Haemost. 2014;12(7):1182–1192. doi:10.1111/jth.12602
31. Hayashi K, Kojima R, Ito M. Strain differences in the diabetogenic activity of streptozotocin in mice. Biol Pharm Bull. 2006;29(6):1110–1119. doi:10.1248/bpb.29.1110
32. Chandirasegaran G, Elanchezhiyan C, Ghosh K, Sethupathy S. Berberine chloride ameliorates oxidative stress, inflammation and apoptosis in the pancreas of Streptozotocin induced diabetic rats. Biomed Pharmacother. 2017;95:175–185. doi:10.1016/j.biopha.2017.08.040
33. Calderon B, Suri A, Pan XO, Mills JC, Unanue ER. IFN-gamma-dependent regulatory circuits in immune inflammation highlighted in diabetes. J Immunol. 2008;181(10):6964–6974. doi:10.4049/jimmunol.181.10.6964
34. Altevogt P, Bretz NP, Ridinger J, Utikal J, Umansky V. Novel insights into exosome-induced, tumor-associated inflammation and immunomodulation. Semin Cancer Biol. 2014;28:51–57. doi:10.1016/j.semcancer.2014.04.008
35. Chaput N, Thery C. Exosomes: immune properties and potential clinical implementations. Semin Immunopathol. 2011;33(5):419–440.
36. Alexander M, Hu R, Runtsch MC, et al. Exosome-delivered microRNAs modulate the inflammatory response to endotoxin. Nat Commun. 2015;6:7321. doi:10.1038/ncomms8321
37. Danesh A, Inglis HC, Jackman RP, et al. Exosomes from red blood cell units bind to monocytes and induce proinflammatory cytokines, boosting T-cell responses in vitro. Blood. 2014;123(5):687–696. doi:10.1182/blood-2013-10-530469
38. Deng ZB, Poliakov A, Hardy RW, et al. Adipose tissue exosome-like vesicles mediate activation of macrophage-induced insulin resistance. Diabetes. 2009;58(11):2498–2505. doi:10.2337/db09-0216
39. Aswad H, Forterre A, Wiklander OP, et al. Exosomes participate in the alteration of muscle homeostasis during lipid-induced insulin resistance in mice. Diabetologia. 2014;57(10):2155–2164. doi:10.1007/s00125-014-3337-2
40. Jalabert A, Vial G, Guay C, et al. Exosome-like vesicles released from lipid-induced insulin-resistant muscles modulate gene expression and proliferation of beta recipient cells in mice. Diabetologia. 2016;59(5):1049–1058. doi:10.1007/s00125-016-3882-y
41. Chen W, Huang Y, Han J, et al. Immunomodulatory effects of mesenchymal stromal cells-derived exosome. Immunol Res. 2016;64(4):831–840. doi:10.1007/s12026-016-8798-6
42. El-Shemi AG, Kensara OA, Alsaegh A, Mukhtar MH. Pharmacotherapy with thymoquinone improved pancreatic beta-cell integrity and functional activity, enhanced islets revascularization, and alleviated metabolic and hepato-renal disturbances in streptozotocin-induced diabetes in rats. Pharmacology. 2018;101(1–2):9–21. doi:10.1159/000480018
43. Watanabe H, Sumi S, Urushihata T, et al. Immunohistochemical studies on vascular endothelial growth factor and platelet endothelial cell adhesion molecule-1/CD-31 in islet transplantation. Pancreas. 2000;21(2):165–173. doi:10.1097/00006676-200008000-00010
44. Narang AS, Cheng K, Henry J, et al. Vascular endothelial growth factor gene delivery for revascularization in transplanted human islets. Pharm Res. 2004;21(1):15–25. doi:10.1023/B:PHAM.0000012147.52900.b8
45. Shabbir A, Cox A, Rodriguez-Menocal L, Salgado M, Van Badiavas E. Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis in vitro. Stem Cells Dev. 2015;24(14):1635–1647. doi:10.1089/scd.2014.0316
46. Venkat P, Chopp M, Cell-Based CJ. Exosome therapy in diabetic stroke. Stem Cells Transl Med. 2018;7(6):451–455. doi:10.1002/sctm.18-0014
47. Ribeiro-Rodrigues TM, Laundos TL, Pereira-Carvalho R, et al. Exosomes secreted by cardiomyocytes subjected to ischaemia promote cardiac angiogenesis. Cardiovasc Res. 2017;113(11):1338–1350. doi:10.1093/cvr/cvx118
48. Nakashima K, Kanda Y, Hirokawa Y, Kawasaki F, Matsuki M, Kaku K. MIN6 is not a pure beta cell line but a mixed cell line with other pancreatic endocrine hormones. Endocr J. 2009;56(1):45–53. doi:10.1507/endocrj.K08E-172
49. Yang S, Li X, Wang N, et al. GPR109A expression in the murine min6 pancreatic beta cell line, and its relation with glucose metabolism and inflammation. Ann Clin Lab Sci. 2015;45(3):315–322.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.