Back to Journals » Drug Design, Development and Therapy » Volume 14
Exosomes Derived from Mesenchymal Stem Cells Protect the Myocardium Against Ischemia/Reperfusion Injury Through Inhibiting Pyroptosis
Authors Tang J , Jin L, Liu Y, Li L, Ma Y, Lu L, Ma J, Ding P , Yang X, Liu J, Yang J
Received 21 November 2019
Accepted for publication 25 July 2020
Published 16 September 2020 Volume 2020:14 Pages 3765—3775
DOI https://doi.org/10.2147/DDDT.S239546
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Jiayou Tang, 1,* Lu Jin, 2,* Yang Liu, 1 Lanlan Li, 1 Yanyan Ma, 1 Linhe Lu, 1 Jipeng Ma, 1 Peng Ding, 1 Xiuling Yang, 1 Jincheng Liu, 1 Jian Yang 1
1Department of Cardiovascular Surgery, Xijing Hospital, Air Force Military Medical University, Xi’an, Shaanxi Province, People’s Republic of China; 2State Key Laboratory of Military Stomatology & National Clinical Research Center for Oral Diseases & Shaanxi Clinical Research Center for Oral Diseases, Department of Maxillofacial Plastic Surgery, School of Stomatology, The Fourth Military Medical University, Xi’an, Shaanxi Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jian Yang
Department of Cardiovascular Surgery, Xijing Hospital, Air Force Medical University, No. 127 Changle West Road, Xi’an, Shaanxi Province 710032, People’s Republic of China
Tel +86-13892828016
Email [email protected]
Objective: Mesenchymal stem cells (MSCs) show unique advantages in cardiomyocyte repairment. Exosomes derived from MSCs can enhance the viability of myocardial cells after ischemia/reperfusion (I/R) injury and regulate inflammation response. The study was designed to ascertain whether MSCs-exo protect the myocardium against I/R injury through inhibiting pyroptosis, and the underlying mechanisms.
Methods and Results: Experiments were carried out in H/R and I/R model. Cell viability was inhibited and NLRP3 and caspase1 protein levels were upregulated in H/R model. However, MSCs could inhibit cell apoptosis and pyroptosis in H/R model. Moreover, we used MSCs-exo to treated H/R model, and flow cytometric analysis results showed the inhibition function of MSCs-exo on cell apoptosis, and Western blot data suggested that NLRP3 and Caspase-1 expressions were downregulated in H/R model. Furthermore, exosomal miR-320b targeted NLRP3 protein, and MSCs-exo OE could inhibit NLRP3 expression and pyroptosis in H/R. In addition, the inhibition function of MSCs-exo on pyroptosis also was found in I/R model, and HE and Tunel staining also got similar results.
Conclusion: Exosomes derived from mesenchymal stem cells could protect the myocardium against ischemia/reperfusion injury through inhibiting pyroptosis.
Keywords: exosome, mesenchymal stem cells, ischemia/reperfusion injury, pyroptosis, miR-320b
Introduction
Myocardial ischemia/reperfusion (I/R) injury refers to the phenomenon that metabolic dysfunction and structural damage are aggravated after myocardial ischemia restores blood supply. I/R injury is a clinically common disease, and its pathological process is closely related to postoperative complications such as coronary angioplasty, coronary revascularization and heart transplantation.1 Studies show that the pathogenesis of I/R injury is very complex. Kevin et al find2 that a small amount of oxygen free radical (OFR) could be observed in myocardial tissue after ischemia, while the rapid increase of OFR occurs several seconds to 1 min after reperfusion. Inflammation also exerts main roles in I/R injury. Researches suggested that when I/R injury occurs, vascular endothelial cells can release large amounts of inflammatory factors, such as TNF-α and IL-6.3 Furthermore, myocardial I/R intervention reduces the release of inflammatory factors and the expression of inflammatory cells,4 which significantly reduce the area of myocardial infarction, but clinical trials have not proved it.5 In addition, Calcium overload,6 mPTP opening,7 rapid recovery of physiological pH8 and apoptosis9 are also closely related to the development of I/R injury. Our study aimed to testify that inhibited pyroptosis has beneficial effects on I/R injury.
Pyroptosis is a new form of programmed cell death associated with inflammatory response, mainly mediated by Caspase-110 and involved in the development of various diseases such as rheumatic diseases,11 neurodegenerative diseases,12 infectious diseases,13 tumor, atherosclerosis.14 The main signaling pathway of pyroptosis is NLRP3 inflammatory pathway. Previous studies have shown that activated NLRP3 binds to ASC and procaspase-1 to form NLRP3 inflammasome, and then procaspase-1 is cleaved to mature Caspase-1, which activates interleukin 1 interleukin 18, then initiates and amplifies downstream signaling pathways, promotes inflammatory responses, eventually induce more cell damage.15,16 It is found that NLRP3 activation is time-dependent, involves in inflammatory response and promotes injury aggravation in the mouse I/R injury model.17 During early reperfusion, activation of Caspase-1 can induce pyroptosis of cardiomyocytes,18 and knockdown of Caspase-1 significantly decreases myocardial infarct size.19 So, the reduction in pyroptosis is a key for curing I/R injury. We found MSCs-exo could obviously decreased pyroptosis.
Exosomes are small vesicles of 30~100 nm in size that contain RNA and proteins.20 Valadi et al also find that exosomes are multifunctional vesicles that regulate the physiological functions of target cells by carrying functional mRNA non-coding RNA.21 Exosomes are produced and released in many tissues of human body, such as fat stem cells, mesenchymal stem cells (MSCs), tumor cells, macrophages and dendritic cells. It is reported that MSC-derived exosomes can perform physiological functions similar to stem cells, which protect kidney damage, reduce myocardial I/R injury and regulate immune system function.22 Feng et al23 find that MSCs-exo in myocardial infarction of mice model after blood deficit pretreatment can reduce myocardial cell apoptosis and reduce the degree of cardiac fibrosis. In addition, MSCs-exo significantly reduces the degree of cardiac fibrosis and repairs cardiac systolic function in the rat model of myocardial infarction with anterior descending coronary artery.24
MSCs-exosomes contain a large number of bioactive genetic materials, mainly miRNA and mRNA, which involve in physiological and pathological processes such as organism development, epigenetic regulation, immune regulation, tumorigenesis and progression.25 For example, miR-16, a miRNA enriched in MSCs-derived exosomes, targets vascular endothelial growth factor (VEGF) in vivo and in vitro tumors and down-regulates its expression to inhibit angiogenesis and tumor progression.26 In addition, exosomes have a role in inhibiting pyroptosis.27 We found that miR-320b was significantly increased in MSCs-exo, and could target NLRP3 protein to inhibit pyroptosis to prevent I/R injury.
Materials and Methods
Animals
Seven-week-old male SD rats were obtained from Charles River Laboratory Animal Technology Co. Ltd (Beijing, China). The rats were anesthetized by intraperitoneally injecting 1% pentobarbital sodium (40 mg/kg) to establish I/R injury model. All procedures were conformed to the Guide for the Care and Use of Laboratory Animal, and all rats were housed and cared for according to International Animal Care and Use Committee guidelines (IACUC) of the Air Force Medical University. The rats were housed in 12-h light/dark cycle in a temperature-controlled room with a free chow and tap water. All experiments in this study were approved by the Animal Care Committee of the Air Force Medical University (The Fourth Military Medical University).
Isolation of Cardiac Cells and Cell Culture
Primary cardiomyocytes were isolated and prepared from the heart of SD rats. Briefly, the hearts were excised and rinsed in Ringer’s solution consisting of 146 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 11 mM glucose, and 10 mM HEPES. After mincing and incubation in the same solution with the addition of 1 mg/mL collagenase IA and 0.12% trypsin at 37°C for 30 min, the suspensions thus obtained were left to rest without further stirring for 2–3 min to precipitate the undissociated tissue fragments. The supernatant was centrifuged at 400 × g for 10 min for the enrichment of viable cells. The cells were transferred to DMEM supplemented with 10% fetal calf serum, 50 U/mL penicillin, and 50 μg/mL streptomycin followed by a one-hour preincubation in Petri dishes for at least partial purification from the non-myocytic cells.
Primary cardiomyocytes were treated to establish Hypoxia/Reoxygenation (H/R) model. Incubate primary cardiomyocytes in an airtight and hypoxic jar fitted with a catalyst to scavenge free oxygen and induce hypoxia for 18 h. Human MSCs (hMSCs, American Type Culture Collection, Rockville, MD, USA) were cultured as previously reported.28 Briefly, MSCs were maintained in DMEM medium containing 10% fetal bovine serum, at 37°C with 5% CO2 in a humidified cell incubator. Primary cardiomyocytes between passages 3 and 5 were used for the experiments, the passages 3 and 10 of MSCs were used for the experiments.
Cell Apoptosis Detection
MSCs cocultured with H/R model followed treated with GW4869 for 12 h. Cells were collected and resuspended in PBS or medium, centrifugated at 1000 rpm for 5 min. Next, 200 μL binding buffer resuspended the sediment and blended. Cells were incubated with 5μL Annexin V-FITC/PI for 10 min in dark, and centrifugated at 1000 rpm for 5 min. The sediment was resuspended with 200 μL binding buffer again followed by added 5 μL PI before detection. Samples were detected by Flow Cytometric Analysis (Beckman Coulter, CA, USA).
Western Blot
As previously reported,29 cell samples were cracked in RIPA lysis buffer plus PMSF on the ice, and total protein was measured with protein assay kit (BCA; Santa Cruz, California, USA). Equal amounts of total protein were separated in SDS-PAGE, and transferred into PVDF membranes (Millipore, USA) and incubated with prepared antibodies. In the end, the bands were visualized with enhanced chemiluminescence (ECL, ThermoFisher, MA, USA) and quantified by Image J. Antibodies against NLRP3, Caspase-1 and GAPDH were purchased from Abcam (Cambridge, MA, USA). Antibodies against CD9, CD81 and TSG101 were purchased from CST (Beverly, Massachusetts, USA).
qPCR
Sample’s total RNA was extracted using mirVana™ PARIS™ RNA and Native Protein Purification Kit (ThermoFisher, MA, USA) according to the directions. TaqMan™ Advanced miRNA cDNA Synthesis Kit was used to perform reverse transcriptase reactions, augment, and qPCR (ThermoFisher, MA, USA). First, the samples were performed the poly(A) tailing reaction, and then performed the adaptor ligation reaction. Next, the miR-Amp reaction was performed followed by the reverse transcription (RT) reaction carried out. Finally, real-time PCR was performed under the experiment settings and PCR thermal cycling conditions (Enzyme activation was at 95°C for 20 s, 1 cycle; Denature was at 95°C for 1 s and Anneal/Extend was at 60°C for 20 s, 40 cycles). The ΔΔCT method was used for quantitative analysis. The quantitative measures were normalized to U6 mRNA levels.
Isolation of Exosomes from MSCs
The exosomes were isolated from MSCs by ultracentrifugation method. Briefly, when MSCs achieved 70%-80% confluency, the culture medium was removed and the cells were washed twice with sterile PBS. Then, dilute the MSCs with PBS, centrifuge at 2, 000 × g for 30 min, and centrifuge at 12, 000 × g for 45 min to remove large cell debris and microvesicles. Then, the supernatant was ultracentrifuged at 110, 000 × g for 2 h at 4°C to form coarse exosome into pellets. The pellet was dissolved in PBS, washed twice with PBS and filtered with a 0.2 μm filter and then suspended in a defined amount of PBS for use.
Identification and Labeling of MSCs-exo
As previously reported,30 transmission electron microscope (TEM) could show purified-MSCs-exo double-layer capsule ultrastructure. The average diameter and concentration of exosome was measured by nanoparticle tracking analysis (NTA). In addition, MSCs-exo were labeled using a PKH67 green fluorescent labeling kit (Sigma-Aldrich, MO, USA).
HE Staining
Hearts were separated from sham, I/R, I/R+PBS and I/R+exosome group. The heart paraffin sections were dewaxed and hydrated; and then slices were incubated in hematoxylin solution for 15 min followed washed with PBS. Secondly, the slices were differentiated, stained by eosin solution for 15 s. In the end, the slices were dehydrated, transparentized and sealed, the images were captured under an optical microscope (Olympus, Tokyo, Japan).
Tunel Staining
The heart paraffin sections were dewaxed and hydrated; the endogenous peroxidase was inactivated by hydrogen peroxide. Next, Tunel test solution labeled sample biotin followed incubated with DAB for 20 min, and then the slices were incubated in hematoxylin solution for 15 min followed washed with PBS. Finally, the slices were dehydrated transparentized, sealed and observed under optical microscope (Olympus, Tokyo, Japan).
Luciferase Reporter Assay
The putative binding sites of miR‐320b on the NLRP3 3′ UTR were predicted by TargetScan7.2 (http://www.targetscan.org/vert_72/). pMIR-NLRP3 3ʹ-UTR-WT and pMIR-NLRP3 3ʹ-UTR-Mut luciferase reporter plasmids were purchased by Hanbio (Shanghai, China). The sequences that could bind to miR-320b were partly mutated and inserted into the reporter plasmid in order to identify the binding specificity. The miR-320b mimic and its control were transfected into cells. After 48 h of transfection, the relative luciferase activities of cells were measured by the Dual-Glo Luciferase Assay System (Promega, Shanghai, China) in accordance with the manufacturer’s introductions. Renilla signals were used to normalize luciferase activity.
Cell Apoptosis Assay
Cells were collected, resuspended in cold PBS, centrifugated at 1000 rpm at room temperature for 5–10 min. Then, the supernatant was discarded and the sediment was resuspended with 300 μL 1×binding buffer in each sample. Cells were incubated with 5 μL Annexin V-FITC/PI for 15 min and then incubated with 5 μL PI before detection. Eventually, the samples were then detected using FACS (Beckman Coulter, CA, USA). Assays and FACS analysis were repeated three times.
Statistical Analysis
Data were expressed as mean ± SE. Student’s t-test was performed to determine the significance between two groups, one-way or two-way ANOVA with Bonferroni post-hoc tests for multiple groups, *p< 0.05; **p<0.01; ***p <0.001.
Results
Cell Viability Reduction and Pyroptosis Exacerbation in H/R Model
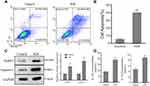
Hypoxia/reoxygenation (H/R) injury model of primary cardiomyocytes cultured in vitro is one of the main models for myocardial I/R injury.31 In this study, flow cytometric analysis data showed that cell apoptosis significantly increased in H/R model (Figure 1A and B). Pyroptosis is a programmed pro-inflammatory cell death caused by activation of NLRP3 inflammasomes, which is specific to Caspase-1 activation.32,33 As Figure 1C shown, NLRP3 and Caspase-1 levels were upregulated in H/R model. In addition, we also found the levels of IL-18 and IL-1β were increased in H/R model (Figure 1D).
MSCs Alleviate Pyroptosis in H/R Model
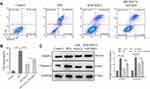
MSCs are highly proliferating, self-renewing cells, which exert important roles in the treatment of myocardial trauma.34 We cocultured H/R cells with MSCs. Flow cytometric analysis data confirmed MSCs could inhibit cell apoptosis in H/R model; however, its function was covered by GW4869 (Figure 2A and B). Furthermore, NLRP3 and Caspase-1 expression were decreased by MSCs in H/R model, but, GW4869 upregulated NLRP3 and Caspase-1 expression in MSCs cocultured H/R cells (Figure 2C). These results indicated that MSCs could alleviate pyroptosis in H/R model; however, its molecular mechanisms were unclear.
Exosomes Derived from MSCs Improve Pyroptosis in H/R Model
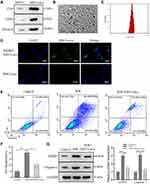
To further explore the specific molecular mechanisms of MSCs on pyroptosis, we extracted exosomes from MSCs to treat H/R model. Western blot data indicated CDC9, CD81 and TSG101 expression were significant upregulation in MSCs-exo (Figure 3A), and transmission electron microscope (TEM) showed exosome double-layer capsule ultrastructure (Figure 3B), Nanoparticle Tracking Analysis (NTA) indicated that the size of extracted particles is mostly around 100 nm, which is in line with the expected exosome characteristics (Figure 3C). In addition, PKH67 green fluorescent labeling got similar results (Figure 3D). Next, we treated H/R cells with MSCs-exo. Our study demonstrated MSCs-exo could obviously reduce cell apoptosis (Figure 3E and F), and the protein levels of NLRP3, Caspase-1 also were downregulated in H/R+ MSCs-exo (Figure 3G). These results demonstrated that MSCs-exo exerts a vital function in improving pyroptosis.
NLRP3 is a Target Gene of miR-320b
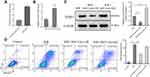
It has been reported that miRNAs involve in the occurrence and development of I/R injury, and play important roles in pyroptosis. To explore the molecular mechanism of exosomes on H/R injury, we screened miRNAs in H/R cells via PCR. Our data showed that miR-320b expression was inhibited in H/R model compared to control (Figure 4A). To confirm whether miR-320b regulates NLRP3 expression, we performed luciferase reporter assay. The luciferase activity significantly decreased following co-transfection with pMIR-NLRP3‑3ʹ‑UTR‑WT and miR-320b, compared with co-transfection with pMIR-NLRP3‑3ʹ‑UTR‑Mut and miR-320b, manifested that miR-320b specifically binds to the 3ʹ‑UTR of NLRP3, and regulates the NLRP3 expression negatively (Figure 4B and C).
Exosomal miR-320b Decreases Pyroptosis
In order to determine the relation of miR-320b and exosomes, we extracted exosomes from cardiomyocytes and MSCs. We found that miR-320b level was significant upregulation in MSCs-exo compared to cardiomyocytes-exo (Figure 5A), and MSCs-exo also induced miR-320b expression in H/R model (Figure 5B). Moreover, in H/R model, MSCs-exo OE could inhibit NLRP3 expression, but MSCs-exo KD got completely different results (Figure 5C). Likewise, MSCs-exo OE could decrease cell apoptosis, and MSCs-exo KD increased cell apoptosis in H/R model (Figure 5D). Taken together, we confirmed that exosomal miR-320b could rescue H/R though pyroptosis inhibition.
Exosomes Reduce Pyroptosis in I/R Injury Model
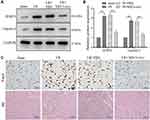
We next examined whether exosomes could make a difference for I/R injury and pyroptosis in vivo. Western blot results showed MSCs-exo inhibit pyroptosis via NLRP3 and Caspase-1 inhibition (Figure 6A and B). In addition, Tunel and HE staining also got similar results (Figure 6C). These results demonstrated exosomes could heal I/R injury through inhibiting pyroptosis in vivo.
Discussion
Our present study yielded several novel findings. First, our data indicated that exosomes derived from MSCs could produce anti-pyroptosis in H/R model. Secondly, exosomal miR-320b could inhibit pyroptosis by negative regulation of NLRP3 expression in H/R model. In addition, we found that MSCs-exo also could reduce pyroptosis and cure I/R injury in vivo. These results not only offer a new angle to understand the mechanisms underlying the protective effect of exosomes on I/R injury but also verify the function of exosomal miR-320b that serve as potential anti-pyroptosis in I/R injury model.
MSCs are a special type of adult stem cells with the potential for proliferation and differentiation, which exist in skin, fat, muscle, bone, bone marrow, umbilical cord, umbilical cord blood, placenta and amniotic fluid.35 Previous studies have found that MSCs have immunoregulation and anti-inflammatory, anti-apoptotic, wound-healing and tissue repair functions.36 MSCs are currently used as tools for the treatment of joint degenerative changes and the reconstruction of bone and cartilage for plastic surgery, aesthetic medicine, cardiovascular disease, endocrine and neurological diseases, and repairment of damaged musculoskeletal tissue.37 Studies have been found that MSC could repair I/R injury through multiple pathways such as inflammation. In addition, pyroptosis is a new kind of programmed cell death characterized by the release of inflammatory cytokines (NLRP3, Caspase-1), and involves in the development of I/R injury.17–19 Our study data suggested that though MSCs could inhibit cell apoptosis and the expression of NLRP3 and caspase1 protein in H/R injury model; however, the effect of MSCs was inhibited by GW4869. GW4869 is basically a recognized exosome inhibitor in the field and believed to inhibit exosome secretion. Could exosomes in MSCs play a role?
Recent researches demonstrated that MSCs-exo could promote the angiogenesis of damaged tissues,26 cell proliferation38 and immune regulation.39 And it is reported that MSCs-exo could reduce myocardial I/R injury22,23 and inhibit inflammatory cytokines expression.40 Recently studies suggested that MSCs-exo attenuate myocardial I/R injury through miR-182-regulated macrophage polarization.41 Then, we extracted exosomes from MSCs to treat H/R model. Sure enough, MSCs-exo could significantly decrease cell apoptosis and expression of pyroptosis-related proteins NLRP3 and Caspase-1. Since exosomes are complexes that contain proteins, mRNA and miRNA, we did not know exactly what is at work. So, based on previous studies, we boldly hypothesized that exosomal miRNAs play a role in inhibiting pyroptosis and repairing I/R injury.
In order to find the target gene of NLRP3 protein, we then performed bioinformatics analysis with miRDB and TargetScan. We found that miR-320b was a likely target gene of NLRP3. Some studies' results indicate that miR-320b exerts a role in numerous diseases’ pathological process. The latest research suggests that miR-320b protein was over-expression in Chronic obstructive pulmonary disease (COPD), which could attenuate the immune response and consequently tissue damage.42 Zhang et al also find that NR2F2‐AS1 influences cancer cell proliferation, invasion and apoptosis through regulating miR‐320b targeting.43 In addition, miR-320b is significantly down-regulated in several cancers including glioblastoma and gastric cancer, exerting a role in tumourigenesis. However, it was unclear about the relationship miR-320b and inflammation. Our study data indicated that miR-320b specifically binds to the 3ʹ‑UTR of NLRP3, and regulates the NLRP3 expression negatively. Furthermore, miR-320b was higher in MSCs-exo than cardiomyocytes-exo, and MSCs-exo also induced miR-320b expression in H/R model. And exo-OE could significantly inhibit pyroptosis. What is more, MSCs-exo also could reduce pyroptosis and repair I/R injury in vivo.
In summary, exosomal miR-320b protected the myocardium against I/R injury through inhibiting pyroptosis-related protein NLRP3 and Caspase-1. Our research results provided a new idea for the future treatment of I/R injury.
Disclosure
Jiayou Tang and Lu Jin are co-first authors for this study. The authors report no conflicts of interest for this work.
References
1. Castedo E, Segovia J, Escudero C, et al. [Ischemia-reperfusion injury during experimental heart transplantation. Evaluation of trimetazidine’s cytoprotective effect]. Rev Esp Cardiol. 2005;58(8):941–950. Spanish.
2. Kevin LG, Camara AK, Riess ML, et al. Ischemic preconditioning alters real-time measure of O2 radicals in intact hearts with ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2003;284(2):H566–574.
3. Arslan F, de Kleijn DP, Timmers L, et al. Bridging innate immunity and myocardial ischemia/reperfusion injury: the search for therapeutic targets. Curr Pharm Des. 2008;14(12):1205–1216.
4. Song JQ, Teng X, Cai Y, et al. Activation of Akt/GSK-3beta signaling pathway is involved in intermedin(1-53) protection against myocardial apoptosis induced by ischemia/reperfusion. Apoptosis. 2009;14(11):1299–1307.
5. Armstrong PW, Granger CB, Bett N, Brieger D, et al. Pexelizumab for acute ST-elevation myocardial infarction in patients undergoing primary percutaneous coronary intervention: a randomized controlled trial. JAMA. 2007;297(1):43–51.
6. Gomez L, Li B, Mewton N, et al. Inhibition of mitochondrial permeability transition pore opening: translation to patients. Cardiovasc Res. 2009;83(2):226–233.
7. Ruiz-Meana M, Inserte J, Fernandez-Sanz C, et al. The role of mitochondrial permeability transition in reperfusion-induced cardiomyocyte death depends on the duration of ischemia. Basic Res Cardiol. 2011;106(6):1259–1268.
8. Lemasters JJ, Bond JM, Chacon E, et al. The pH paradox in ischemia-reperfusion injury to cardiac myocytes. EXS. 1996;76:99–114.
9. Lee P, Sata M, Lefer DJ, et al. Fas pathway is a critical mediator of cardiac myocyte death and MI during ischemia-reperfusion in vivo. Am J Physiol Heart Circ Physiol. 2003;284(2):H456–463.
10. Chen Y, Smith MR, Thirumalai K, et al. A bacterial invasin induces macrophage apoptosis by binding directly to ICE. EMBO J. 1996;15(15):3853–3860.
11. Zhao C, Gu Y, Zeng X, et al. NLRP3 inflammasome regulates Th17 differentiation in rheumatoid arthritis. Clin Immunol. 2018;197:154–160.
12. Fricker M, Tolkovsky AM, Borutaite V, et al. Neuronal Cell Death. Physiol Rev. 2018;98(2):813–880.
13. Chen H, Lu Y, Cao Z, et al. Cadmium induces NLRP3 inflammasome-dependent pyroptosis in vascular endothelial cells. Toxicol Lett. 2016;246:7–16.
14. Duewell P, Kono H, Rayner KJ, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464(7293):1357–1361.
15. Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140(6):821–832.
16. Lamkanfi M, Dixit VM. Manipulation of host cell death pathways during microbial infections. Cell Host Microbe. 2010;8(1):44–54.
17. Toldo S, Abbate A. The NLRP3 inflammasome in acute myocardial infarction. Nat Rev Cardiol. 2018;15(4):203–214.
18. Audia JP, Yang XM, Crockett ES, et al. Caspase-1 inhibition by VX-765 administered at reperfusion in P2Y12 receptor antagonist-treated rats provides long-term reduction in myocardial infarct size and preservation of ventricular function. Basic Res Cardiol. 2018;113(5):32.
19. Frantz S, Ducharme A, Sawyer D, et al. Targeted deletion of caspase-1 reduces early mortality and left ventricular dilatation following myocardial infarction. Mol Cell Cardiol. 2003;35(6):685–694.
20. Johnstone RM, Adam M, Hammond JR, et al. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262(19):9412–9420.
21. Valadi H, Ekström K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659.
22. Börger V, Bremer M, Görgens A, et al. Mesenchymal stem/stromal cell-derived extracellular vesicles as a new approach in stem cell therapy. ISBT Sci Ser. 2016;11(S1):228–234.
23. Feng Y, Huang W, Wani M, et al. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS One. 2014;9(2):e88685.
24. Zhao Y, Sun X, Cao W, et al. Exosomes derived from human umbilical cord mesenchymal stem cells relieve acute myocardial ischemic injury. Stem Cell Int. 2015.
25. Yu B, Zhang X, Li X. Exosomes derived from mesenchymal stem cells. Int J Mol Sci. 2014;15(3):4142–4157.
26. Lee JK, Park SR, Jung BK, et al. Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS One. 2013;8(12):e84256.
27. Tavakoli Dargani Z, Singla R, Johnson T, et al. Exosomes derived from embryonic stem cells inhibit doxorubicin and inflammation-induced pyroptosis in muscle cells. Can J Physiol Pharmacol. 2018;96(3):304–307.
28. Bai L, Lennon DP, Eaton V, et al. Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia. 2009;57(11):1192–1203.
29. Signore M, Manganelli V, Hodge A. Antibody validation by western blotting. Methods Mol Biol. 2017;1606:51–70.
30. Xu B, Zhang Y, Du XF, et al. Neurons secrete miR-132-containing exosomes to regulate brain vascular integrity. Cell Res. 2017;27(7):882–897.
31. Hu L, Zhou L, Wu X, et al. Hypoxic preconditioning protects cardiomyocytes against hypoxia/reoxygenation injury through AMPK/eNOS/PGC-1α signaling pathway. Int J Clin Exp Pathol. 2014;7(11):7378–7388.
32. Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157(5):1013–1022.
33. Henao-Mejia J, Elinav E, Thaiss CA, et al. Inflammasomes and metabolic disease. Annu Rev Physiol. 2014;76:57–78.
34. Tse HF, Kwong YL, Chan JK, et al. Angiogenesis in ischemic myocardium by intramyocardial autologous bone marrow mononuclear cell implantation. Lancet. 2003;361(9351):47–49.
35. da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119(Pt 11):2204–2213.
36. Takehara Y, Yabuuchi A, Ezoe K, et al. The restorative effects of adipose-derived mesenchymal stem cells on damaged ovarian function. Lab Invest. 2013;93(2):181–193.
37. Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45:e54.
38. Looze C1, Yui D, Leung L, et al. Proteomic profiling of human plasma exosomes identifies PPARgamma as an exosome-associated protein. Biochem Biophys Res Commun. 2009;378(3):433–438.
39. Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14(3):195–208.
40. Ji T, Xu C, Sun L, et al. Aryl hydrocarbon receptor activation down-regulates IL-7 and reduces inflammation in a mouse model of DSS-induced colitis. Dig Dis Sci. 2015;60(7):1958–1966.
41. Zhao J, Li X, Hu J, et al. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc Res. 2019;115(7):1205–1216.
42. Keller A1, Ludwig N, Fehlmann T. Low miR-150-5p and miR-320b expression predicts reduced survival of COPD patients. Cells. 2019;8(10):
43. Zhang S, Zhang X, Sun Q, et al. LncRNA NR2F2-AS1 promotes tumourigenesis through modulating BMI1 expression by targeting miR-320b in non-small cell lung cancer. J Cell Mol Med. 2019;23(3):2001–2011.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.