Back to Journals » Drug Design, Development and Therapy » Volume 16
Exosomes Derived from Hypoxic Glioma Cells Reduce the Sensitivity of Glioma Cells to Temozolomide Through Carrying miR-106a-5p
Authors Wu P, Guo J, Yang H, Yuan D, Wang C, Wang Z
Received 17 July 2022
Accepted for publication 17 September 2022
Published 13 October 2022 Volume 2022:16 Pages 3589—3598
DOI https://doi.org/10.2147/DDDT.S382690
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Tin Wui Wong
Peizhang Wu,1,2 Jun Guo,2 Hongwei Yang,2 Debin Yuan,2 Chaoxiang Wang,2 Zhong Wang1
1Department of Neurosurgery, The First Affiliated Hospital of Soochow University, Suzhou, 215006, People’s Republic of China; 2Department of Neurosurgery, Yancheng First People’s Hospital, Yancheng, 224000, People’s Republic of China
Correspondence: Zhong Wang, Department of Neurosurgery, The First Affiliated Hospital of Soochow University, No. 188 Shizi Street, Suzhou, 215006, People’s Republic of China, Email [email protected]
Background: Hypoxia is a frequent feature of solid tumors which significantly affects the efficacy of treatments such as chemotherapy. In addition, exosomes from hypoxic cancer cells could contribute to the chemoresistance of tumor cells through carrying miRNAs. It has been shown that miR-106-5p level was upregulated in glioma. However, whether exosomes derived from hypoxic glioma cells could affect temozolomide (TMZ) resistance in glioma through carrying miR-106a-5p remains unexplored.
Methods: Exosomes were isolated from glioma cells under normoxia or hypoxia condition. EdU staining and flow cytometry assays were used to assess the cell proliferation and cell apoptosis. The relation between miR-106a-5p and PTEN was investigated by dual luciferase assay.
Results: MiR-106a-5p was enriched in exosomes derived from hypoxic glioma cells compared to exosomes from cells under normoxia condition. Additionally, hypoxic glioma cells were able to transfer exosomes to glioma cells, resulting in a significant increase of miR-106a-5p level in cells. TMZ remarkably suppressed glioma cell proliferation and triggered cell apoptosis. However, hypoxic glioma cell-derived exosomes markedly promoted the proliferation and suppressed the apoptosis in TMZ-treated glioma cells, and miR-106a-5p inhibitor was able to abolish these phenomena. Meanwhile, PTEN was verified to be a direct target of miR-106a-5p. Furthermore, TMZ elevated PTEN and Bax level and reduced p-Akt level in glioma cells, whereas these changes were reversed by hypoxia glioma cell-derived exosomes. Furthermore, hypoxia glioma cell-derived exosomes reduced the sensitivity of glioma cells to TMZ in vivo via downregulating PTEN.
Conclusion: Collectively, exosomal miR-106a-5p derived from hypoxia glioma cells could reduce the sensitivity of glioma cells to TMZ through downregulating PTEN. Thus, our study might provide new strategies for improving the clinical efficacy of TMZ on glioma.
Keywords: cancer, hypoxia, miR-106a-5p, chemosensitivity
Introduction
Glioma is a primary malignant tumor in brains, which occurs with a median survival period. Most patients with glioma die in 2 years of diagnosis.1,2 The major therapies for glioma include TMZ chemotherapy and radiation, and the combination of TMZ and radiation are more successful to radiation alone.3,4 TMZ often promotes apoptosis by inducing the DNA damage.5,6 The prognosis of many patients is not ideal as the glioma cells could acquire chemoresistance.7,8 It has been revealed that low oxygen tension (hypoxia) established in growing tumor masses could induce the chemoresistance of tumor cells.9,10 The hypoxic microenvironment can induce chemoresistance and decrease the chemosensitivity during the tumor progression.10–12 Thus, it is essential to study the mechanisms of chemo-responsiveness in glioma cells for discovering novel therapeutic methods.
MicroRNAs (miRNAs) have been found to be associated with chemoresistance in multiple types of cancers, including glioma.13–15 Li et al reported that miR-519a overexpression sensitized glioblastoma cells to TMZ treatment through targeting STAT3/Bcl2 signaling.14 MiR-182b could reduce chemoresistance to TMZ in glioma stem cells.16 Previous study revealed that miR-106a-5p was upregulated in glioma.17 Additionally, miR-106a-5p could facilitate 5-Fluorouracil resistance in colorectal cancer by targeting TGFβR2.18 However, the relationship between miR-106a-5p and TMZ resistance in glioma cells remain largely unclear.
Exosomes could participate in occurrence of glioma, which could induce the resistance during chemotherapy.19,20 It has been revealed that exosomal miRNAs dysregulation could affect the chemoresistance and chemosensitivity in cancers.21,22 Studies showed that exosomes derived from hypoxic tumor cells could contribute to TMZ resistance of tumor cells by transferring miRNAs.20,23 However, whether exosomes derived from hypoxic glioma cells could affect the response of glioma cells to TMZ through carrying miR-106a-5p remains unclear.
Based on the above backgrounds, we aimed to investigate the function of hypoxic exosomes from glioma cells in TMZ resistance during the progression of glioma. The study would supply a theoretical basis for discovering novel methods against glioma.
Materials and Methods
Cell Culture and Treatment
Glioma cells (U87MG, SHG44 and U251MG) and human brain glial cells (HEB) were bought from Shanghai cell bank of Chinese Academy of Sciences (Shanghai, China). Cells were cultured in DMEM medium (Thermo Fischer Scientific) with 10% fetal bovine serum (FBS), 1% penicillin and streptomycin at 37°C, 5% CO2.
Hypoxia Assay
SHG44 cells (5 x 103 cells for 96-well plate; 2×105 cells for 6-well plate) were plated and cultured for 24 h in the condition of 37°C and 5% CO2. Subsequently, cells were transferred into hypoxic chamber (Stemcell™ Technologies) with 1% O2 for 72 h.
Cell Transfection
SHG44 cells were transfected with mimics-ctrl (Invitrogen), miR-106a-5p mimics (Invitrogen) or miR-106a-5p inhibitor (Invitrogen) for 48 h by using lipofectamine 3000 (Invitrogen) in line with the protocol of manufacturer.
Exosome Extraction and Identification
Briefly, cell growth medium was replaced with medium (without FBS) when the confluence reached 80%. Next, the supernatant was centrifuged (10 min at 300 × g, 15 min at 2000 × g and 30 min at 10,000 × g). Ultracentrifugation (70 min at 120,000 × g twice) was then applied to collect the supernatant for exosome isolation, and transmission electron microscopy (TEM), nanoparticle tracking analysis (NTA) and Western blot were performed to identify the relevant exosomes.24 For TEM analysis, the morphology of exosomes was examined at 80 kV under a TEM (HT7700, HITACHI, Japan). For NTA, the size of exosomes was determined with a ZetaView analyzer (Particle Metrix, Germany).
Exosome Labeling and Uptake
SHG44 cells (2 x 105 cells) were plated onto a 6 well plate overnight. Next, PKH26-labeled exosomes (50 μg/mL) were co-incubated with SHG44 cells for 24 h before counterstaining with fluorescent phalloidin dye, which stains the cytoskeleton Images were then captured using a confocal laser scanning microscope (ZEISS LSM880, Germany). Magnification, x600. Cell nuclei was stained with DAPI.
CCK-8 Assay
SHG44 cells (5 x 103 cells) were seeded onto a 96-well plate overnight. After that, cells were exposed to TMZ (25 μM),25,26 TMZ + normoxic glioma cell-derived exosomes (N-exo, 50 μg/mL), TMZ + hypoxic glioma cell-derived exosomes (H-exo, 50 μg/mL) or TMZ + H-exo + miR-106a-5p inhibitor (inhibitor) for 48 h and then evaluated using CCK-8 (10 μL). The OD value was measured using a microplate reader (Thermo MULTISKAN MK3, Thermo Fischer Scientific, USA) at 450 nm.
Immunofluorescence
SHG44 cells (2 x 105 cells) were seeded onto a 6 well plate overnight. Next, the EdU-positive cells were evaluated by the Cell-Light EdU Apollo567 In Vitro Kit (RIBOBIO). Images were then visualized under a fluorescence microscope (OLYMPUS IX51, Japan). Magnification, x200.
Apoptosis
SHG44 cells (2 x 105 cells) were seeded onto a 6-well plate overnight. Next, cells were centrifuged at 956 x g for 5 min and resuspended in 500 μL binding buffer. After that, cells were treated with Annexin V-FITC and PI (5 μL) and then subjected to a flow cytometer (BD AriaIII, BD Biosciences, USA). FlowJo software was applied for data quantification and analysis.
RT-qPCR
Total RNA was extracted using TRIzol® and reverse transcribed to cDNA using a PrimeScript kit. RT-qPCR was applied using a SYBR green (ELK Biotechnology) reaction mix, under the conditions: 2 min at 94°C, followed by 35 cycles (94°C for 30s and 55°C for 45s). The primers used were as follows: miR-106a-5p forward, 5’-TGGGTGCTTACAGTGCAGGTAG-3’ and reverse, 5’- CTCAACTGGTGTCGTGGAGTC-3’; U6 forward, 5’- CTCGCTTCGGCAGCACAT-3’ and reverse 5’-AACGCTTCACGAATTTGCGT-3’; PTEN forward, 5’- ATTCCCAGTCAGAGGCGCTAT-3’ and reverse, 5’- GAACTTGTCTTCCCGTCGTGT-3’; HIF-1α forward, 5’- CAGAATGAAGTGTACCCTAACTAGCC-3’ and reverse, 5’- ACAAATCAGCACCAAGCAGGT-3’; β-actin forward, 5’-GTCCACCGCAAATGCTTCTA-3’ and reverse, 5’-TGCTGTCACCTTCACCGTTC-3’. Relative quantification was completed using the 2−ΔΔt method and U6 and β-actin were used as the house keeping gene.
Western Blot
Total protein was obtained from each experimental sample using RIPA buffer and quantified using the BCA kit. Equal volumes of total protein (10 μg/lane) were separated by SDS-PAGE (10%) and transferred onto PVDF membranes. These membranes were incubated with primary antibodies targeting CD63 (1:1000; Abcam), CD81 (1:1000; Abcam), PTEN (1:1000; Abcam), Akt (1:1000; Abcam), p-Akt (1:3000; Proteintech), p53 (1:1000; Abcam), Bax (1:1000; Abcam), calnexin (1:1000; Abcam) and β-actin (1:10,000; Abcam). After that, membranes were incubated with the relevant secondary antibodies (HRP-conjugated, 1:10,000; Abcam). Blots were visualized with ECL reagent and IPP 6.0 was used to complete the densitometry analysis.
Dual Luciferase Assay
The 3’-UTR of PTEN, which contains the miR-106a-5p binding site, were purchased from GenePharma before being cloned into the pGL6-miR vectors to establish pGL6-miR PTEN 3‘-UTR wild-type (WT) or mutated (MT) plasmids. These plasmids were transfected into SHG44 cells together with miR-106a-5p mimics or mimics-ctrl using Lipofectamine 2000. Next, the luciferase activity was evaluated by the Dual Luciferase Reporter Assay System (Beyotime).
Animal Study
BALB/c female nude mice aged 6–8 weeks were housed in a room with a dedicated SPF facility. SHG44 cells (5 x 106 cells) were subcutaneously injected into the left flank of each mouse. When the tumor reached about 200 mm3, mice were divided into 3 groups (n = 4 per group) randomly: control, TMZ and TMZ + H-exo group. PBS or H-exo was injected intratumorally twice a week for 3 weeks. In addition, mice in the TMZ group were injected with 25 mg/kg TMZ intraperitoneally every day. The tumor volume was calculated once a week as per this equation: length × width2. Finally, the mice were sacrificed, and the tumors were weighed. All experiments were applied in accordance with the NIH Guide and Ethics Committee of The First Affiliated Hospital of Soochow University approved this research.
Immunohistochemical (IHC) Staining
The samples were deparaffinized, rehydrated, and heated in a microwave. Then, sections were washed, incubated in H2O2 (3%) for 25 min and washed with PBS. Afterwards, goat serum was applied to block and incubate samples for 30 min. Later on, a primary antibody (anti-PTEN) was used to stain the samples overnight. Then, the secondary antibody (HRP-labeled) was applied to stain the samples for 30 min. All antibodies were obtained from Abcam. The result was observed by a microscope (OLYMPUS CX31, Japan). Magnification, x200.
TUNEL Staining
Paraffin sections were washed and permeabilized. Subsequently, TUNEL mixtures (50 μL) were used to incubate the sections for at 37°C with no light for 1.5 h. Next, cell nuclei were stained with DAPI. Finally, the result was observed by using a fluorescence microscope (Nikon Eclipse Ci-L, Japan). Magnification, x200.
Statistical Analysis
Mean ± SD was applied to express all data. One-way analysis of variance (ANOVA) followed by Tukey’s test or Student’s t-test was used for analyzing the differences between experimental groups and P < 0.05 suggested a significant change.
Results
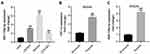
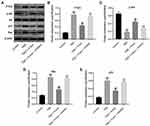
MiR-106a-5p Was Upregulated in Hypoxic Glioma Cells
To study the role of miR-106a-5p in glioma, RT-qPCR was performed. As indicated in Figure 1A, miR-106a-5p level was significantly higher in glioma cells, compared with HEB cells. In addition, miR-106a-5p level in SHG44 cells was much higher than that in other glioma cells (Figure 1A). Thus, SHG44 cells were selected of use in subsequent experiments. Meanwhile, the level of miR-106a-5p and HIF-1α were significantly upregulated in glioma cells under hypoxic condition compared to cells under normoxia condition (Figure 1B and 1C). Taken together, miR-106a-5p was upregulated in hypoxic glioma cells.
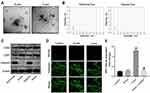
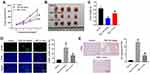
MiR-106a-5p Could Be Transferred from Hypoxic Glioma Cells to Glioma Cells via Exosomes
Hypoxic cancer cell-derived exosomes are believed to play several crucial roles in chemoresistance during the tumor progression.27,28 Given this, we then isolated exosomes from normoxic (H-exo) or hypoxic (N-exo) glioma cells. Our initial extraction data showed that these extracts were rounded particles (30–150 nm in diameter) (Figure 2B), and our subsequent evaluations revealed a high proportion of CD63 and CD81 expression in these extracts (Figure 2C). However, these extracts were negative for the endoplasmic reticulum calnexin protein (Figure 2C). These results showed that exosomes were isolated successfully.
Next, we investigated whether these exosomes can be transmitted into glioma cells by incubating glioma cells with PKH26-labeled H-exo or PKH26-labeled N-exo. After 24 h of incubation, PKH26 red dye was observed in glioma cells both in H-exo and N-exo groups (Figure 2D). In addition, compared to N-exo, H-exo sharply increased miR-106a-5p level in glioma cells, whereas that change was obviously reversed by miR-106a-5p inhibitor (Figure 2E). Collectively, miR-106a-5p was enriched in hypoxic exosomes derived from glioma cells and can be transferred from hypoxic glioma cells to glioma cells via exosomes.
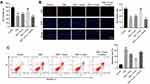
Exosomes Derived from Hypoxic Glioma Cells Reduced TMZ Sensitivity in Glioma Cells Through Carrying miR-106a-5p
To detect the role of exosomal miR-106a-5p in TMZ sensitivity in glioma cells, CCK8, EdU staining and flow cytometry assays were performed. As shown in Figure 3A, TMZ significantly inhibited the viability of glioma cells, and exhibited about 50% growth inhibition. In addition, TMZ markedly reduced the proliferation and induced the apoptosis of glioma cells (Figure 3B and C). However, H-exo notably abolished the anti-tumor effect of TMZ in glioma cells, while these phenomena were reversed in the presence of miR-106a-5p inhibitor (Figure 3A–C). To sum up, hypoxic exosomes derived from glioma cells could reduce the sensitivity of glioma cells to TMZ through carrying miR-106a-5p.
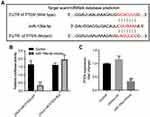
PTEN Was Identified to Be the Downstream Target of miR-106a-5p
To explore the downstream mRNA of miR-106a-5p, TargetScan was used. As revealed in Figure 4A, PTEN was predicted to be the downstream mRNA of miR-106a-5p. Additionally, the relative luciferase activity in WT-PTEN was significantly inhibited by upregulation of miR-106a-5p (Figure 4B). In addition, the level of PTEN in glioma cells was notably inhibited by miR-106a-5p mimics (Figure 4C). In summary, PTEN was a downstream target of miR-106a-5p.
Exosomes Derived from Hypoxic Glioma Cells Reduced TMZ Sensitivity in Glioma Cells Through Modulating PTEN/Akt Signaling
To further explore the mechanism by which exosomes from hypoxic glioma cells reduce TMZ sensitivity in glioma cells, Western blot was used. The data showed that TMZ significantly elevated the expressions of PTEN, Bax, p53 and decreased the expression of p-Akt in glioma cells (Figure 5A–E). However, H-exo notably reduced the expressions of PTEN, Bax, p53 and increased the expression of p-Akt in TMZ-treated glioma cells compared to TMZ treatment group, whereas these phenomena were reversed by miR-106a-5p inhibitor (Figure 5A–E). Taken together, hypoxic exosomes derived from glioma cells could reduce the sensitivity of glioma cells to TMZ through modulating PTEN/Akt signaling.
Exosomes Derived from Hypoxic Glioma Cells Reduced TMZ Sensitivity in Glioma Cells in vivo
Next, we further explored the role of exosomal miR-106a-5p in TMZ sensitivity in vivo. As illustrated in Figure 6A–C, the tumor volume and weight in mice were obviously decreased by TMZ treatment, while the anti-tumor effect of TMZ was partially abolished by H-exo. In addition, TMZ-induced cell apoptosis in tumor tissues was markedly reduced by H-exo (Figure 6D). Furthermore, the level of PTEN in tumor tissues was greatly upregulated by TMZ, whereas that change was obviously reversed by H-exo (Figure 6E). To sum up, hypoxic exosomes derived from glioma cells could reduce the sensitivity of glioma cells to TMZ in vivo.
Discussion
It has been reported that hypoxic exosomes derived from tumor cells could be involved in chemoresistance during the tumor progression. For example, Zeng et al found hypoxic exosomal circZNF91 could promote chemoresistance of normoxic pancreatic cancer cells via enhancing glycolysis;29 Dong et al indicated that hypoxic exosomes from non-small-cell lung cancer cells could induce the resistance of cancer cells to cisplatin through carrying miR-21.30 In this research, we found that exosomes derived from hypoxic glioma cells could reduce the sensitivity of glioma cells to TMZ.
Exosomes could deliver miRNAs to tumor cells, which could affect the response of tumor cells to drugs. For instance, Zhu et al suggested that exosomes could deliver miR-223 to ovarian cancer cells to elicit a chemoresistant phenotype.31 Qian et al found that hypoxic glioma cell-derived exosomal miR-1246 and miR-10b-5p could facilitate the migration and invasion of normoxic glioma cells.32 Yue et al showed that exosomal miR-301a derived from hypoxic glioma cells could promote radiation resistance.33 In this study, we found hypoxic glioma cell-derived exosomes could reduce the sensitivity of glioma cells to TMZ through carrying miR-106a-5p. Thus, this research firstly explored the relationship between hypoxic tumor cell-derived exosomal miR-106a-5p and TMZ sensitivity in glioma. On the other hand, miRNAs could be delivered from tumor cells to macrophages, and this phenomenon could enhance the malignant behavior of tumor cells.34,35 Therefore, the role of miR-106a-5p in tumor microenvironment needs to be further explored.
This study revealed PTEN was the downstream target of miR-106a-5p. PTEN was known to be an inhibitor of cell growth, which could suppress tumor progression.36,37 In addition, PTEN was confirmed to inhibit the cell growth via inactivation of Akt signaling.38,39 Consistently, this research found exosomes derived from hypoxic glioma cells could reduce the expression of PTEN and upregulate the level of p-Akt in TMZ-treated glioma cells. Meanwhile, Zhu et al found miR-106a-5p could promote the tumorigenesis of gastric cancer through targeting Smad7,40 and our study was similar to this previous research. Smad7 was known to be an inhibitor of cancer cell invasion, and its overexpression could inhibit the malignant behavior of cancer cells.41,42 Hence, the similar function between PTEN and Smad7 might result in the similarity between our study and Zhu et al.
Indeed, there are some shortcomings in this research. First, in this study, we explored the role of hypoxic glioma cell-derived exosomal miR-106-5p in the regulation of TMZ resistance of glioma. However, whether other miRNAs (eg, miR-1246, miR-10b-5p and miR-301a) are involved in the regulation of TMZ resistance of glioma need to be investigated in the future. Second, more downstream targets of miR-106a-5p in glioma remain unexplored. Third, more signaling pathways involved in hypoxic exosomes-mediated chemoresistance are needed to be further discovered. Thus, more investigations are essential in the coming future.
In summary, exosomes derived from hypoxic glioma cells could reduce the sensitivity of glioma cells to TMZ through carrying miR-106a-5p. Hence, our research might provide a theoretical basis for exploring new strategies against glioma.
Disclosure
The authors declared no competing interests in this research.
References
1. Chen X, Niu W, Fan X, et al. Oct4A palmitoylation modulates tumorigenicity and stemness in human glioblastoma cells. Neuro Oncol. 2022. doi:10.1093/neuonc/noac157
2. Pan E, Mitchell SB, Tsai JS. A retrospective study of the safety of BCNU wafers with concurrent temozolomide and radiotherapy and adjuvant temozolomide for newly diagnosed glioblastoma patients. J Neurooncol. 2008;88(3):353–357. doi:10.1007/s11060-008-9576-7
3. McMahon DJ, Gleeson JP, O’Reilly S, Bambury RM. Management of newly diagnosed glioblastoma multiforme: current state of the art and emerging therapeutic approaches. Med Oncol. 2022;39(9):129. doi:10.1007/s12032-022-01708-w
4. Li B, Wang J, Liu F, et al. A novel pseudogene methylation signature to predict temozolomide outcome in Non-G-CIMP glioblastomas. J Oncol. 2022;2022:6345160. doi:10.1155/2022/6345160
5. Chen M, Huang B, Zhu L, et al. DNA damage response evaluation provides novel insights for personalized immunotherapy in glioma. Front Immunol. 2022;13:875648. doi:10.3389/fimmu.2022.875648
6. Wang YH, Gu J, Yu JH, et al. Diffuse midline glioma with H3-K27M mutation: a rare case with GFAP-positive anucleate whorled patterns. Medicine. 2022;101(24):e29448. doi:10.1097/MD.0000000000029448
7. Zhou J, Xu N, Liu B, et al. lncRNA XLOC013218 promotes cell proliferation and TMZ resistance by targeting the PIK3R2-mediated PI3K/AKT pathway in glioma. Cancer Sci. 2022;113:2681–2692. doi:10.1111/cas.15387
8. Zhou Y, Chen L, Ding D, et al. Cyanidin-3-O-glucoside inhibits the beta-catenin/MGMT pathway by upregulating miR-214-5p to reverse chemotherapy resistance in glioma cells. Sci Rep. 2022;12(1):7773. doi:10.1038/s41598-022-11757-w
9. Zichittella C, Barreca MM, Cordaro A, et al. Mir-675-5p supports hypoxia-induced drug resistance in colorectal cancer cells. BMC Cancer. 2022;22(1):567. doi:10.1186/s12885-022-09666-2
10. Jiang S, Li X, Zhang F, et al. Manganese dioxide-based nanocarrier delivers paclitaxel to enhance chemotherapy against orthotopic glioma through hypoxia relief. Small Methods. 2022;6:e2101531. doi:10.1002/smtd.202101531
11. Assidicky R, Tokat UM, Tarman IO, et al. Targeting HIF1-alpha/miR-326/ITGA5 axis potentiates chemotherapy response in triple-negative breast cancer. Breast Cancer Res Treat. 2022;193(2):331–348. doi:10.1007/s10549-022-06569-5
12. Qin Y, Liu HJ, Li M, et al. Salidroside improves the hypoxic tumor microenvironment and reverses the drug resistance of platinum drugs via HIF-1α signaling pathway. EBioMedicine. 2018;38:25–36. doi:10.1016/j.ebiom.2018.10.069
13. Cheng S, Huang Y, Lou C, et al. FSTL1 enhances chemoresistance and maintains stemness in breast cancer cells via integrin β3/Wnt signaling under miR-137 regulation. Cancer Biol Ther. 2019;20(3):328–337. doi:10.1080/15384047.2018.1529101
14. Li H, Chen L, Li JJ, et al. miR-519a enhances chemosensitivity and promotes autophagy in glioblastoma by targeting STAT3/Bcl2 signaling pathway. J Hematol Oncol. 2018;11(1):70. doi:10.1186/s13045-018-0618-0
15. Chen HA, Li CC, Lin YJ, et al. Hsa-miR-107 regulates chemosensitivity and inhibits tumor growth in hepatocellular carcinoma cells. Aging. 2021;13(8):12046–12057. doi:10.18632/aging.202908
16. Li P, Lu X, Wang Y, et al. MiR-181b suppresses proliferation of and reduces chemoresistance to temozolomide in U87 glioma stem cells. J Biomed Res. 2010;24(6):436–443. doi:10.1016/S1674-8301(10)60058-9
17. Chen W, Wu M, Cui ST, et al. CircRNA Circ-ITCH inhibits the proliferation and invasion of glioma cells through targeting the miR-106a-5p/SASH1 axis. Cell Transplant. 2021;30:963689720983785. doi:10.1177/0963689720983785
18. Liu J, Huang Y, Wang H, Wu D. MiR-106a-5p promotes 5-FU resistance and the metastasis of colorectal cancer by targeting TGFβR2. Int J Clin Exp Pathol. 2018;11(12):5622–5634.
19. Alharbi M, Lai A, Sharma S, et al. Extracellular vesicle transmission of chemoresistance to ovarian cancer cells is associated with hypoxia-induced expression of glycolytic pathway proteins, and prediction of epithelial ovarian cancer disease recurrence. Cancers. 2021;13:14. doi:10.3390/cancers13143388
20. Sayyed AA, Gondaliya P, Mali M, et al. MiR-155 inhibitor-laden exosomes reverse resistance to cisplatin in a 3D tumor spheroid and xenograft model of oral cancer. Mol Pharm. 2021;18(8):3010–3025. doi:10.1021/acs.molpharmaceut.1c00213
21. Ipas H, Guttin A, Issartel JP. Exosomal MicroRNAs in Tumoral U87 MG versus normal astrocyte cells. Microrna. 2015;4(2):131–145. doi:10.2174/2211536604666150820115707
22. Zhuang L, Zhang B, Liu X, et al. Exosomal miR-21-5p derived from cisplatin-resistant SKOV3 ovarian cancer cells promotes glycolysis and inhibits chemosensitivity of its progenitor SKOV3 cells by targeting PDHA1. Cell Biol Int. 2021;45(10):2140–2149. doi:10.1002/cbin.11671
23. Liu Y, Tan J, Ou S, Chen J, Chen L. Adipose-derived exosomes deliver miR-23a/b to regulate tumor growth in hepatocellular cancer by targeting the VHL/HIF axis. J Physiol Biochem. 2019;75(3):391–401. doi:10.1007/s13105-019-00692-6
24. Zhang S, Guan N, Mao X, et al. Exosomal circRNA_104948 enhances the progression of glioma by regulating miR-29b-3p and DNMT3B/MTSS1 Signaling. J Environ Pathol Toxicol Oncol. 2022;41(2):47–59. doi:10.1615/JEnvironPatholToxicolOncol.2021039775
25. Chen M, Cheng Y, Yuan Z, et al. NCK1-AS1 Increases Drug Resistance of Glioma Cells to Temozolomide by Modulating miR-137/TRIM24. Cancer Biother Radiopharm. 2020;35(2):101–108. doi:10.1089/cbr.2019.3054
26. Vengoji R, Macha MA, Nimmakayala RK, et al. Afatinib and Temozolomide combination inhibits tumorigenesis by targeting EGFRvIII-cMet signaling in glioblastoma cells. J Exp Clin Cancer Res. 2019;38(1):266. doi:10.1186/s13046-019-1264-2
27. Qian D, Xie Y, Huang M, Gu J. Tumor-derived exosomes in hypoxic microenvironment: release mechanism, biological function and clinical application. J Cancer. 2022;13(5):1685–1694. doi:10.7150/jca.69278
28. Onishi H, Nakamura K, Yanai K, et al. Cancer therapy that targets the Hedgehog signaling pathway considering the cancer microenvironment (Review). Oncol Rep. 2022;47(5). doi:10.3892/or.2022.8304
29. Zeng Z, Zhao Y, Chen Q, et al. Hypoxic exosomal HIF-1alpha-stabilizing circZNF91 promotes chemoresistance of normoxic pancreatic cancer cells via enhancing glycolysis. Oncogene. 2021;40(36):5505–5517. doi:10.1038/s41388-021-01960-w
30. Dong C, Liu X, Wang H, et al. Hypoxic non-small-cell lung cancer cell-derived exosomal miR-21 promotes resistance of normoxic cell to cisplatin. Onco Targets Ther. 2019;12:1947–1956. doi:10.2147/OTT.S186922
31. Zhu X, Shen H, Yin X, et al. Macrophages derived exosomes deliver miR-223 to epithelial ovarian cancer cells to elicit a chemoresistant phenotype. J Exp Clin Cancer Res. 2019;38(1):81. doi:10.1186/s13046-019-1095-1
32. Qian M, Chen Z, Guo X, et al. Exosomes derived from hypoxic glioma deliver miR-1246 and miR-10b-5p to normoxic glioma cells to promote migration and invasion. Lab Invest. 2021;101(5):612–624. doi:10.1038/s41374-020-00522-0
33. Yue X, Lan F, Xia T. Hypoxic glioma cell-secreted exosomal miR-301a Activates Wnt/β-catenin signaling and promotes radiation resistance by targeting TCEAL7. Mol Ther. 2019;27(11):1939–1949. doi:10.1016/j.ymthe.2019.07.011
34. Jeon H, Seo SM, Kim TW, et al. Circulating Exosomal miR-1290 for diagnosis of epithelial ovarian cancer. Curr Issues Mol Biol. 2022;44(1):288–300. doi:10.3390/cimb44010021
35. Wang P, Li GY, Zhou L, et al. Exosomes from M2 macrophages promoted glycolysis in FaDu cells by inhibiting PDLIM2 expression to stabilize PFKL. Neoplasma. 2022. doi:10.4149/neo_2022_220426N455
36. Guo W, Yao X, Lan S, et al. Metabolomics and integrated network pharmacology analysis reveal SNKAF decoction suppresses cell proliferation and induced cell apoptisis in hepatocellular carcinoma via PI3K/Akt/P53/FoxO signaling axis. Chin Med. 2022;17(1):76. doi:10.1186/s13020-022-00628-1
37. Wu W, Lu P, Huang Y, et al. Emodin regulates the autophagy via the miR-371a-5p/PTEN axis to inhibit hepatic malignancy. Biochem Biophys Res Commun. 2022;619:1–8. doi:10.1016/j.bbrc.2022.06.006
38. Conciatori F, Salvati E, Ciuffreda L, et al. Fibroblast-Induced Paradoxical PI3K pathway activation in PTEN-competent colorectal cancer: implications for therapeutic PI3K/mTOR Inhibition. Front Oncol. 2022;12:862806. doi:10.3389/fonc.2022.862806
39. Sahoo SS, Ramanand SG, Gao Y, et al. FOXA2 suppresses endometrial carcinogenesis and epithelial-mesenchymal transition by regulating enhancer activity. J Clin Invest. 2022;132(12). doi:10.1172/JCI157574
40. Zhu M, Zhang N, He S, Lu X. Exosomal miR-106a derived from gastric cancer promotes peritoneal metastasis via direct regulation of Smad7. Cell Cycle. 2020;19(10):1200–1221. doi:10.1080/15384101.2020.1749467
41. Troncone E, Marafini I, Stolfi C, Monteleone G. Involvement of Smad7 in Inflammatory Diseases of the Gut and Colon Cancer. Int J Mol Sci. 2021;22(8):3922. doi:10.3390/ijms22083922
42. Tang J, Li X, Cheng T, Wu J. miR-21-5p/SMAD7 axis promotes the progress of lung cancer. Thorac Cancer. 2021;12(17):2307–2313. doi:10.1111/1759-7714.14060
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.