Back to Journals » OncoTargets and Therapy » Volume 12
Exosomes derived from HCC cells with different invasion characteristics mediated EMT through TGF-β/Smad signaling pathway
Authors Qu Z, Feng J, Pan H, Jiang Y, Duan Y, Fa Z
Received 3 April 2019
Accepted for publication 27 July 2019
Published 23 August 2019 Volume 2019:12 Pages 6897—6905
DOI https://doi.org/10.2147/OTT.S209413
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sanjay Singh
Zhen Qu,1,* Jiawei Feng,1,* Hua Pan,2 Yong Jiang,1 Yunfei Duan,1 Zhenzhong Fa3
1Department of Hepatopancreatobiliary Surgery, The First People’s Hospital of Changzhou, the Third Hospital Affiliated to Soochow University, Changzhou, Jiangsu, People’s Republic of China; 2Department of Hepatobiliary Surgery, The People’s Hospital of Liyang, Liyang, Jiangsu, People’s Republic of China; 3Department of Hepatobiliary Surgery, Changzhou Wujin People’s Hospital, Wujin Hospital Affiliated to Jiangsu University, Changzhou, Jiangsu, People’s Republic of China
Correspondence: Yunfei Duan
Department of Hepatopancreatobiliary Surgery, The First People’s Hospital of Changzhou, The Third Hospital Affiliated to Soochow University, Changzhou, Jiangsu, People’s Republic of China
Email [email protected]
Zhenzhong Fa
Department of Hepatobiliary Surgery, Changzhou Wujin People’s Hospital, Wujin Hospital Affiliated to Jiangsu University, Changzhou, Jiangsu, People’s Republic of China
Email [email protected]
*These authors contributed equally to this work
Background: Exosomes are nano-sized biological vesicles released by many kinds of cells, which play an important role in tumor metastasis through transporting cytokines, RNAs and proteins. However, the molecular mechanisms of exosomes in hepatocellular carcinoma (HCC) metastasis are not completely understood.
Materials and methods: Exosomes derived from hepatoma cell lines with different invasion characteristics. Exosome characteristics, cell migration and invasion, and effects on major signal transduction pathways deregulated in cancer cells were analyzed by transmission electron microscopy (TEM), wound-healing assay, Trans-well invasion assay and Western blot-based assays, respectively. Moreover, exosomes effects on tumor metastasis in vivo were investigated by subcutaneous transplantation tumor model of athymic nude mice.
Results: Exosomes derived from hepatoma cells can promote the migration and invasion of recipient cells, induce the decrease of E-cadherin expression, increase the expression of Vimentin and promote epithelial-mesenchymal transition (EMT) in cells. Moreover, highly invasive hepatoma-cells-derived exosomes effects are stronger than low-invasive hepatoma cells and normal liver cells exosomes. Mechanistic studies showed that the biological alterations in recipient HCC cells treated with MHCC97H and MHCC97L-derived exosomes were caused by inducing EMT via TGF-β/Smad signaling pathway. In vivo experiments also suggest that highly invasive hepatoma cells derived exosomes are more likely to promote lung metastasis of HCC in nude mice.
Conclusion: Our results reveal the important role of tumor-derived exosomes in the migration and invasion of recipient cells and exosomes may be the novel therapeutic and prognostic targets for HCC patients.
Keywords: HCC, exosomal, TGF-β, EMT, tumor metastasis
Introduction
Surgical resection is still the primary treatment method for HCC patients at present. However, postoperative recurrence and metastasis of hepatocellular carcinoma (HCC) are the main factors influencing the prognosis of patients.1 The early tumor recurrence is frequently caused by tumor invasion and metastasis. Thus, new treatment targets to control HCC metastasis and recurrence are urgently needed. Tumor metastasis is a multi-step process,2 and the acquisition of mesenchymal changes in epithelial tumor cells, which is also called epithelial-mesenchymal transition (EMT), is the first step in the process of tumor metastasis. Interfering with the process of EMT may decrease tumor metastasis and invasion, and improve the prognosis of HCC patients. More and more studies have found that tumor cells can secrete and release a large number of extracellular vesicles (including exosomes).3,4 Tumor-cells-derived exosomes could regulate the tumor microenvironment by transporting a variety of biologically active molecules (cytokines, proteins, mRNA, miRNAs, etc.), thereby affecting tumor progression, metastasis and drug resistance.5
In recent years, studies have found that exosomes can act as regulators of tumor microenvironment to influence tumor cell invasion and EMT.6 The proteins in exosomes can change with the phenotypic change of lung cancer cells; co-culture of exosomes derived from cells induced by TGF-β with epithelial phenotypic cells revealed changes in the morphological and phenotypic marker proteins of recipient cells.7 Moreover, Tian et al found that the acidic microenvironment can trigger the activation of HIF-1α and HIF-2α and stimulate exosomal miR-21 and miR-10b expression substantially promoting HCC cell proliferation, migration and invasion.8 The highly malignant tumor cells may transport exosomes to tumor cells with low migration capacity, and promote cell proliferation, invasion and metastasis in vitro.9 Lu Chen et al also found that exosomes from high-metastatic MHCC97H cells could induce HCC cells to undergo EMT through different pathways.10 Exosomes derived from advanced lung cancer patient’s serum can induce the migration, invasion and enhancement of non-tumor recipient cells by up-regulating the expression of vimentin and mediating the EMT process.11 The above results indicate that exosomes are released from tumor cells into the extracellular microenvironment and participate in the regulation of target cell progression and metastasis by transporting bioactive molecules. However, the role of HCC-cell-derived exosomes in the invasion and metastasis of HCC in vitro and in vivo remains unclear.
In this study, we carried a systematic study of the role of exosomes in HCC invasion and metastasis. We used different HCC cell lines for purifying exosomes, and we detected whether these exosomes contained different levels of TGF-β, which likely differentially affect HCC metastasis. Animal experiments indicated the influence of exosomes on HCC metastasis. Our study revealed for the first time that exosomes derived from highly metastatic MHCC97H cells could be taken up by low metastatic HCC cells; furthermore, HCC-derived exosomes could mediate EMT and promote HCC cells invasion through TGF-β/Smad signaling pathway, and the highly invasive MHCC97H-derived exosomes were more effective. These findings may contribute to the identification of new therapeutic targets and prognostic prediction marker.
Materials and methods
Cells and reagents
LO2, MHCC97L, MHCC97H and HepG2 cell lines were obtained from the Liver Cancer Institute, Fudan University (Shanghai, China). MHCC97L, MHCC97H and HepG2 were maintained in Dulbecco’s Modified Eagle Medium (DMEM, WISENT, CA, USA) containing 10% fetal bovine serum (FBS) (ExCell Bio, China). LO2 cell line was maintained in 1640 containing 10% FBS. All cells were incubated in 5% CO2 at 37°C. This study was conducted in accordance with Declaration of Helsinki and was approved by the Ethics Committee of the Third Hospital Affiliated to Soochow University.
Isolation of exosomes and transmission electron microscopy
Different cell lines were cultured in media with 10% exosome-free FBS (by ultracentrifugation overnight). After 48 hrs, cell culture media were collected, and exosomes were isolated from the supernatant by differential centrifugation using a OptimaL-80 XP ultracentrifuge (Beckman Coulter, USA) as previously described.12 Briefly, the medium was collected and centrifuged at 1000 g for 15 mins, at 3000 g for 30 mins, at 10,000 g for 60 mins, at 100,000 g for 4 hrs. All centrifugal steps were performed at 4°C. Isolated exosome samples were resuspended in 300 μL PBS. The exosomes were quantified by BCA protein assay. A total of 10 μg exosomes were used for each in vitro experiment.
Transmission electron microscopy and size distribution analysis
The extracted pellets were observed under transmission electron microscopy (TEM) as previously described.13 A drop of purified exosomes (approximately 10 μL) was fixed with 1% glutaraldehyde for 10 mins, washed, and contrasted in 2% uranyl acetate. Images were obtained by TEM (JEM-2100, Jeol, Japan), and the size distribution of the isolated exosomes was analyzed by a Zetasizer Nano ZS90 instrument (Malvern, UK) according to the manufacturer’s instructions. All samples were measured with parameters of 44.5 mm and 0.64 V voltage using NP100 membranes. Samples were calibrated by CPC100 standard particles diluted 1000-fold under identical settings.
Wound-healing assay and trans-well invasion assay
Cell migration ability was measured by wound-healing assay. Full confluent cells were seeded into 24-wells plate; acellular area was created by scraping using a pipette tip. Wound closure was measured at 24 and 48 hrs interval.
Trans-well invasion assay was performed using matrigel invasion chamber (BD Biosciences, Bedford, MA). Briefly, cells were harvested and resuspended in serum-free medium. For migration assay, 1×105 cells were added into the upper chamber, DMEM medium containing 10% FBS were added into the bottom chamber and served as a chemoattractant. After incubation for 24 hrs at 37°C in 5% CO2, remaining cells in the upper chamber were wiped out with cotton swap. The cells that migrated or invaded to the lower surface of the membrane were fixed with 100% methanol, stained with hematoxylin and counted under a microscope (Olympus Corporation, Japan).
Western blot assay
To determine the level of indicated proteins, exosomes and hepatoma cells were lysed with RIPA peptide lysis buffer (Beyotime Biotechnology, China) containing 1% protease inhibitors (Pierce). Equal amounts of proteins were loaded, the PVDF membranes with transferred proteins were incubated with primary antibodies at 4°C overnight and HRP-conjugated secondary antibodies at room temperature for 2 hrs. The signal was developed by the enhanced chemiluminescence (ECL) reagent (Millipore, Bedford, MA, USA) and visualized by FluorChem FC2 Imaging System (Alpha Innotech, San Leandro, CA, USA). The antibodies for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), CD9, CD63, E-cadherin, Vimentin, p-Smad2 and p-Smad3 were obtained from Cell Signaling Technology (Beverly, MA, USA). The TGF-β inhibitor LY2109761 (S-2704) was purchased from Selleck (USA).
Enzyme-linked immunosorbent assay (ELISA)
The concentration of TGF-β in the cellular supernatant was detected using a human TGF-β ELISA kit (ExCell, Shanghai, China) following the manufacturer’s instructions. Briefly, after the cells were treated with HCC-cell-derived exosomes for 48 hrs, the media was collected and centrifuged at 5000 rpm for 5 mins. Total media with 10% FBS was used as the control.
Hematoxylin-eosin (H&E) stain
All of the animals were sacrificed at the indicated time, and the mice lung tissues were collected for histological examination. The lung tissues were fixed in 4% paraformaldehyde and embedded in paraffin. The slices (5 μm thickness) were stained with hematoxylin and eosin (H&E). The cancer embolus area in the lung was observed under an Olympus BX51 microscope.
Animal model
All animal procedures were performed according to national guidelines and approved by the Animal Care Ethics Committee of the Third Hospital Affiliated to Soochow University. Twenty male BALB/c nu/nu mice (4–6 weeks old, Laboratory Animal Center of Shanghai, Academy of Science) were procured. All mice received subcutaneous injections of HCC cells in the right armpit (1×107 cells in 200 μL of PBS per mouse). Twenty mice were randomly divided into four groups (the control group, LO2-exosome group, MHCC97L-exosome group and MHCC97H-exosom group) when the tumors reached a volume of 50–100 mm3 (15 days after subcutaneous injections of tumor cells); exosomes (100 μg of total protein) were injected into the implanted tumors every 2 days for 7 times, respectively; the control group was injected with equal volume PBS. The mice were examined every 2 days, and all mice were sacrificed by cervical dislocation under general anesthesia with chloral hydrate (5%, 100 μL/10 g).
Statistical analyses
All results are described as the mean ± s.d. The assay data were analyzed using Student’s t-tests. All differences were considered significant at the level of P<0.05. All statistical analyses were performed using SPSS for Windows release 22.0 (SPSS Inc., Chicago, IL, USA).
Results
Exosome extraction and morphological identification
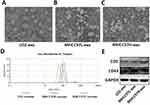
According to previous reports, we used sequential ultracentrifugation to isolate exosomes from the cell culture supernatants of normal liver cells (LO2) and two HCC cell lines (MHCC97H and MHCC97L), and MHCC97H cells have high metastatic potential among them. In addition, morphology and characteristic of exosomes were studied by TEM, particle size analysis and marker protein detection. Different cell-derived exosomes were found to be vesicular, approximately 50–150 nm in diameter (Figure 1A–C), and the nanoparticle tracking analysis by Zetasizer Nano ZS90 revealed the exosomes size distribution (Figure 1D). Detection of two commonly used exosomal marker proteins, CD9 and CD63, confirmed the identity of the exosomes (Figure 1E).
The exosomes derived from HCC cells promote cell migration
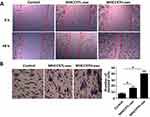
To investigate the effect of exosomes derived from HCC cells on the migration and invasion of recipient cells, we used the scratch healing assay and found that exosomes derived from MHCC97L and MHCC97H cells promoted the migration of recipient cells; besides, the highly invasive MHCC97H-derived exosomes were more effective (Figure 2A). In addition, transwell results showed that the number of positively stained cells in the MHCC97H-derived exosomal group increased significantly (Figure 2B). Overall, these data suggested that HCC-cell-derived exosomes could promote HepG2 cells migration and invasion, and the highly invasive MHCC97H-derived exosomes were more effective.
TGF-β pathway activated by exosomes derived from HCC cells
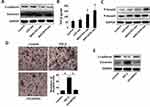
After observing that exosomes derived from HCC cells can promote the migration and invasion of recipient HCC cells, we next examined the EMT marker protein in HepG2 cells after treatment with MHCC97L- and MHCC97H-derived exosomes by Western blot. The results showed that incubation with MHCC97L- and MHCC97H-derived exosomes suppressed the expression of the epithelial marker E-cadherin and promoted the expression of mesenchymal marker Vimentin (Figure 3A).
TGF-β is one of the most important cytokines in the EMT process of tumors. To identify the signaling pathway involved in exosomes mediated EMT, we used ELISA to detect the level of TGF-β changes in the supernatant of the recipient cells. We found that after incubation with MHCC97L- and MHCC97H-derived exosomes, the level of TGF-β in the culture supernatant of HepG2 cells was significantly elevated (Figure 3B). In addition, we detected the expression levels of phosphorylated active forms of Smad2 and Smad3 by Western blot. The results showed that phosphorylation of Smad2 and Smad3 were significantly elevated in recipient HepG2 cells incubated with MHCC97L- and MHCC97H-derived exosomes (Figure 3C). In summary, these results reveal that HCC-cell-derived exosomes could induce HepG2 cells to undergo EMT through the TGF-β/Smad signaling pathway.
In order to clarify the role of endogenous cytokine TGF-β in the exosomes, we treated MHCC97L cells with TGF-β and TGF-β inhibitor LY2109761 for 72 hrs to obtain exosomes released in different states of MHCC97L cells. The transwell assay showed that the number of positive cells penetrating the pores of the MHCC97L cells treated with the TGF-β group was significantly higher than that of the control group and the LY2109761 group (Figure 3D).
We detected the changes of phenotypic marker proteins after treatment of recipient cells with exosomes from different sources by Western blot. The results showed that MHCC97L exosomes induced by TGF-β decreased the expression of E-cadherin and increased the expression of Vimentin compared to the control group. However, the effect of MHCC97L exosomes treated with LY2109761 was significantly reduced (Figure 3E).
The exosomes derived from HCC cells promote the invasion and metastasis of HCC in vivo
To better understand the role of HCC-cells-derived exosomes in the lung metastasis, we established the model of liver orthotopic tumor transplantation in nude mice and treated it with HCC-cells-derived exosomes (100 μg of total protein) by tail vein injections. Mice lung tissue HE stain results showed that the numbers of tumor thrombus positive in MHCC97L- and MHCC97H-derived exosomes group were higher than that in PBS-injected and LO2-derived exosomes group, and the highest metastasis rate was found in the highly invasive exosomes group (Figure 4).
Discussion
HCC progression and metastasis are the result of a variety of molecular mechanisms. It is not entirely clear how the primary tumors interact with local and distant cell information during HCC progression and metastasis. Exosomes are used as carriers of many kinds of bioactive molecules. There is more and more evidence that exosomes play a multiple regulatory role in tumor progression, invasion, angiogenesis and metastasis by transporting functional molecules to tumor microenvironment and even distant cells.14,15 For example, tumor-cell-derived exosomes can activate recipient cells by transporting fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF) and epidermal growth factor (EGF).16 Previous studies have found that tumor-derived exosomes are chemoattractant to endothelial cells.17 We have found that exosomes derived from HCC cells can influence cell growth and migration by delivering transforming growth factor β (TGF-β) to recipient cells.
The loss of E-cadherin, a key protein of intercellular adhesion, is one of the important reasons why EMT affects tumor metastasis by promoting the loss of intercellular adhesion and the ability of cells to migrate and invade.18 In this study, we observed that the expression of E-cadherin decreased after co-culture of recipient HCC cells with exosomes derived from MHCC97L and MHCC97H cells, while the expression of mesenchymal phenotypic protein Vimentin increased, suggesting that recipient HCC cells undergo EMT. Exosomes derived from HCC cells may promote the progression and metastasis of HCC by driving cell phenotypic changes through autocrine or paracrine modes. Similar to our results, it is reported that exosomes derived from melanoma cells can also promote the phenotypic change of bone marrow progenitor cells to pre-metastasis.19 Tumor-derived exosomes can stimulate phenotypic transformation of smooth muscle progenitor cells to tumor-promoting cells.20 Phenotypic changes in myofibroblasts induced by exosomes derived from breast and prostate cancer.21 The above results suggest that many different types of cells in tumor microenvironment can be influenced by tumor-cell-derived exosomes, thus promoting cell phenotypic changes, which may be one of the mechanisms of enhanced transformation or migration and invasion of different types of recipient cells to tumor.
It has been reported that various signaling pathways may be activated to affect EMT of melanoma (e.g. MAPK, NF-KB, P13K/AKT, etc.).22–24 Soluble cytokine TGF-β family is one of the most important factors in EMT inducer. Activation of the TGF-β receptor and the downstream Smad pathway can activate transcription factors including the Snail family, the ZEB family and the Twist family, induce cell reprogramming, ultimately enable epithelial phenotype primary tumor cells to acquire interstitial cell characteristics and acquire the ability to invade the extracellular matrix, which become the trigger point for tumor metastasis.25 We have found that the exosomes derived from HCC cells promote the invasion and migration of HCC cells by affecting their TGF-β signaling pathways. Moreover, the exosomes derived from HCC cells with high invasive properties are more effective. In conclusion, the above results suggest that the exosomes derived from tumor cells can transfer similar carcinogenic molecules to other tumor cells or normal recipient cells in a manner of autocrine or paracrine, and promote the recipient cells to acquire tumor invasion characteristics. When gene mutation occurs in some cells, the exocrine secreted by the cells transports the mutation information to other recipient cells through fusion membrane, which causes activation of protooncogene, expression of anti-apoptosis gene and enhancement of anchor-independent growth ability of the recipient cells.
In addition to that effect of the above biological factors on EMT in tumors, some miRNAs may also participate in the process of EMT in tumors. It is reported that inhibition of miR-191 expression may block process of EMT in tumors, decreasing ability of invasion and migration of tumor cells.26 MiR-23a can regulate EMT in tumors by targeting E-cadherin.27 Et-7a has also been shown to be involved in tumor migration, invasion and EMT processes by targeting specific proteins such as LIN28B and HMGA2.28 Our team will explore the role and mechanism of some key miRNAs in EMT induced by exosomes derived from HCC cells with different invasive properties, and further clarify the role of exosomes in the progression and metastasis of HCC.
Conclusion
In conclusion, we have found that exosomes derived from HCC cells can alter the molecular composition of tumor microenvironment through autocrine or paracrine transport of some bioactive factors and regulate the occurrence of tumor EMT. Accordingly, it is reasonable to predict that inhibitors of exosome production or release, as well as targeted drugs of biomolecules such as TGF-β, may be helpful in improving the prognosis of patients with HCC.
Acknowledgment
This study was supported by the National Natural Science Foundation of China (Nos. 81172143, 11334004, and 81421091), Scientific and Technological Projects for Young Talents, Changzhou Health and Family Planning Commission (QN201809).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kudo M. Adjuvant therapy after curative treatment for hepatocellular carcinoma. Oncology. 2011;81 Suppl 1:50–55. doi:10.1159/000333259
2. Vanharanta S, Massague J. Origins of metastatic traits. Cancer Cell. 2013;24(4):410–421. doi:10.1016/j.ccr.2013.09.007
3. Ji H, Greening DW, Barnes TW, et al. Proteome profiling of exosomes derived from human primary and metastatic colorectal cancer cells reveal differential expression of key metastatic factors and signal transduction components. Proteomics. 2013;13(10–11):1672–1686. doi:10.1002/pmic.201200562
4. H G Z, Grizzle WE. Exosomes: a novel pathway of local and distant intercellular communication that facilitates the growth and metastasis of neoplastic lesions. Am J Pathol. 2014;184(1):28–41. doi:10.1016/j.ajpath.2013.09.027
5. Thompson CA, Purushothaman A, Ramani VC, Vlodavsky I, Sanderson RD. Heparanase regulates secretion, composition, and function of tumor cell-derived exosomes. J Biol Chem. 2013;288(14):10093–10099. doi:10.1074/jbc.C112.444562
6. Vella LJ. The emerging role of exosomes in epithelial-mesenchymal-transition in cancer. Front Oncol. 2014;4:361. doi:10.3389/fonc.2014.00361
7. Kim J, Kim TY, Lee MS, Mun JY, Ihm C, Kim SA. Exosome cargo reflects TGF-beta1-mediated epithelial-to-mesenchymal transition (EMT) status in A549 human lung adenocarcinoma cells. Biochem Biophys Res Commun. 2016;478(2):643–648. doi:10.1016/j.bbrc.2016.07.124
8. Tian XP, Wang CY, Jin XH, et al. Acidic microenvironment up-regulates exosomal miR-21 and miR-10b in early-stage hepatocellular carcinoma to promote cancer cell proliferation and metastasis. Theranostics. 2019;9(7):1965–1979. doi:10.7150/thno.30958
9. Martins VR, Dias MS, Hainaut P. Tumor-cell-derived microvesicles as carriers of molecular information in cancer. Curr Opin Oncol. 2013;25(1):66–75. doi:10.1097/CCO.0b013e32835b7c81
10. Chen L, Guo P, He Y, et al. HCC-derived exosomes elicit HCC progression and recurrence by epithelial-mesenchymal transition through MAPK/ERK signalling pathway. Cell Death Dis. 2018;9(5):513. doi:10.1038/s41419-018-0534-9
11. Rahman MA, Barger JF, Lovat F, et al. Lung cancer exosomes as drivers of epithelial mesenchymal transition. Oncotarget. 2016;7(34):54852–54866. doi:10.18632/oncotarget.10243
12. Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi:10.1038/ncb1596
13. Lasser C, Eldh M, Lotvall J. Isolation and characterization of RNA-containing exosomes. J Vis Exp. 2012;9:e3037.
14. Rappa G, Mercapide J, Anzanello F, et al. Biochemical and biological characterization of exosomes containing prominin-1/CD133. Mol Cancer. 2013;12:62.
15. Liu Y, Zhu XJ, Zeng C, et al. Microvesicles secreted from human multiple myeloma cells promote angiogenesis. Acta Pharmacol Sin. 2014;35(2):230–238. doi:10.1038/aps.2013.141
16. Cho JA, Park H, Lim EH, et al. Exosomes from ovarian cancer cells induce adipose tissue-derived mesenchymal stem cells to acquire the physical and functional characteristics of tumor-supporting myofibroblasts. Gynecol Oncol. 2011;123(2):379–386. doi:10.1016/j.ygyno.2011.08.005
17. Taverna S, Flugy A, Saieva L, et al. Role of exosomes released by chronic myelogenous leukemia cells in angiogenesis. Int J Cancer. 2012;130(9):2033–2043. doi:10.1002/ijc.26217
18. Keshamouni VG, Michailidis G, Grasso CS, et al. Differential protein expression profiling by iTRAQ-2DLC-MS/MS of lung cancer cells undergoing epithelial-mesenchymal transition reveals a migratory/invasive phenotype. J Proteome Res. 2006;5(5):1143–1154. doi:10.1021/pr050455t
19. Peinado H, Alec KM, Lavotshkin S, et al. Corrigendum: melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2016;22(12):1502. doi:10.1038/nm1216-1502b
20. Atay S, Banskota S, Crow J, et al. Oncogenic KIT-containing exosomes increase gastrointestinal stromal tumor cell invasion. Proc Natl Acad Sci U S A. 2014;111(2):711–716. doi:10.1073/pnas.1310501111
21. Webber J, Steadman R, Mason MD, Tabi Z, Clayton A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 2010;70(23):9621–9630. doi:10.1158/0008-5472.CAN-10-1722
22. Meier F, Schittek B, Busch S, et al. The RAS/RAF/MEK/ERK and PI3K/AKT signaling pathways present molecular targets for the effective treatment of advanced melanoma. Front Biosci. 2005;10:2986–3001. doi:10.2741/1755
23. Weiss MB, Abel EV, Mayberry MM, et al. TWIST1 is an ERK1/2 effector that promotes invasion and regulates MMP-1 expression in human melanoma cells. Cancer Res. 2012;72(24):6382–6392. doi:10.1158/0008-5472.CAN-12-1033
24. Lin K, Baritaki S, Militello L, Malaponte G, Bevelacqua Y, Bonavida B. The role of B-RAF mutations in melanoma and the induction of EMT via dysregulation of the NF-kappaB/Snail/RKIP/PTEN circuit. Genes Cancer. 2010;1(5):409–420. doi:10.1177/1947601910373795
25. Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172(7):973–981. doi:10.1083/jcb.200601018
26. Xu W, Ji J, Xu Y, et al. MicroRNA-191, by promoting the EMT and increasing CSC-like properties, is involved in neoplastic and metastatic properties of transformed human bronchial epithelial cells. Mol Carcinog. 2015;54 Suppl 1:E148–E161. doi:10.1002/mc.22221
27. Zheng H, Li W, Wang Y, et al. miR-23a inhibits E-cadherin expression and is regulated by AP-1 and NFAT4 complex during Fas-induced EMT in gastrointestinal cancer. Carcinogenesis. 2014;35(1):173–183. doi:10.1093/carcin/bgt274
28. Wu A, Wu K, Li J, et al. Let-7a inhibits migration, invasion and epithelial-mesenchymal transition by targeting HMGA2 in nasopharyngeal carcinoma. J Transl Med. 2015;13:105. doi:10.1186/s12967-015-0541-x
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.