Back to Journals » International Journal of Nanomedicine » Volume 13
Exosome-encapsulated antibiotic against intracellular infections of methicillin-resistant Staphylococcus aureus
Authors Yang X , Shi G , Guo J, Wang C, He Y
Received 6 July 2018
Accepted for publication 1 October 2018
Published 29 November 2018 Volume 2018:13 Pages 8095—8104
DOI https://doi.org/10.2147/IJN.S179380
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lei Yang
Xiaohong Yang, Gongming Shi, Jian Guo, Chenhui Wang, Yun He
Chongqing Key Laboratory of Natural Product Synthesis and Drug Research, School of Pharmaceutical Sciences, Chongqing University, Chongqing 401331, People’s Republic of China
Background: Staphylococcus aureus survival inside phagocytes is considered to provide a reservoir of bacteria that are relatively protected from antibiotics, thus enabling long-term colonization of the host and explaining clinical failures and relapses after antibiotic therapy.
Purpose: The objective of this study was to develop a nanovesicle using exosomes loaded with linezolid to overcome intracellular infections by pathogenic bacteria.
Methods: Exosomes were collected from the culture supernatants of RAW 264.7 cells. Their size distribution and zeta potential were characterized by dynamic light scattering, their morphology was characterized by transmission electron microscopy, and their protein content (CD63 and Flotillin 1) was assessed by Western blotting. Linezolid was incorporated into exosomes by co-incubation at 37°C and it’s accumulation in RAW264.7 cells and release in vitro were determined by high performance liquid chromatography. The intracellular bactericidal effect was evaluated in methicillin-resistant S. aureus (MRSA)-infected macrophages in vitro and MRSA peritonitis model in vivo.
Results: We prepared a nanoformulation of the antibiotic linezolid using exosomes harvested from mouse RAW264.7 macrophages. The exosomal formulation of linezolid was more effective against intracellular MRSA infections in vitro and in vivo than the free linezolid. Our data also showed no signs of cytotoxicity in macrophages.
Conclusion: Exosomes provide an effective alternative for intracellular antibiotic delivery of antibiotic that is efficacious, cost-effective, and safe. This regimen can be viewed as a potential antimicrobial agent for use against intracellular infections.
Keywords: exosomes, antibiotic, delivery, intracellular infection, MRSA
Introduction
Staphylococcus aureus is the leading cause of human bacterial infections globally, such as sepsis, infective endocarditis, osteomyelitis, and necrotizing pneumonia, as well as skin and soft tissue infections. Methicillin-resistant S. aureus (MRSA) was first detected in hospitals in 1961 and has rapidly spread worldwide over the last several decades. MRSA is well known to be the major cause of nosocomial and community infections, which has led to difficulties in treating S. aureus infections. Moreover, S. aureus can invade and survive inside mammalian cells including the phagocytic cells. It has been well documented that the surviving S. aureus inside blood-borne phagocytes can get transferred to tissues and invade various nonphagocytic cell types. The intracellular localization of these bacteria creates a niche wherein bacteria can persist, causing cell alterations and possibly be selected for resistance if exposed to subtherapeutic concentrations of antibiotics.7 Therefore, in addition to treating extracellular S. aureus, antibiotic treatments should be optimized against intracellular S. aureus.
However, currently, the treatment of intracellular bacterial infections remains a major challenge because the majority of existing antibiotics are inefficient to kill intracellular S. aureus both in vitro and in vivo. For example, linezolid (LZD), gentamycin, and β-lactams exhibit no or only modest accumulation in eukaryotic cells,8 while macrolides and fluoroquinolones show poor retention inside the cells,9 which results in a drastic reduction in their effectiveness against intracellular pathogens, leading to antibiotic failure in vitro and in vivo.8,9 Therefore, development of an effective carrier for the intracellular delivery of antibiotics is urgently needed.
Exosomes are membrane-encased vesicles of 40–200 nm that are secreted by cells via fusion of multivesicular bodies with cell plasma membranes.10 Exosomes have recently emerged as a promising drug delivery system with high biocompatibility, high efficacy of delivery, and low immunogenicity.11,12 Several groups have already used exosomes to deliver drugs for cancer therapy in animal models.13–16 Based on these findings, we hypothesized that antibiotic-loaded exosomes could improve the intracellular accumulation of antibiotics and enhance their bactericidal effect.
In this study, we examined the possibility of using exosomes as antibiotic carriers for treatment of an intracellular infection. We hypothesized that exosomes produced by macrophages could deliver an antibiotic into cells. Our investigations revealed robust accumulation and nearly complete colocalization of exosomes with lysosomes, and a greater therapeutic efficacy of exosomal antibiotic in vitro and in vivo compared to that of free antibiotic (LZD). Thus, exosome-based formulations may represent the next generation of intracellular antibiotic delivery systems, given their low immunogenic profile and low cytotoxicity.
Materials and methods
Materials
LZD was obtained from Beijing Solarbio Science & Technology Co. Ltd (Beijing, People’s Republic of China). The CD63 and flotillin 1 antibodies were purchased from Abcam Biotechnology (Cambridge, MA, USA). The β-actin antibody and goat-anti-rabbit horseradish-peroxidase (HRP)-conjugated secondary antibody were purchased from Beijing Biosynthesis Biotechnology Co. Ltd (Beijing, People’s Republic of China). The MRSA strain WHO-2 (WHO-2) was kindly provided by Rongxin Qin (Army Medical University, Chongqing, People’s Republic of China).
Cell culture
RAW264.7 cells were purchased from Shanghai Cell Bank of the Chinese Academy of Sciences (Shanghai, People’s Republic of China). Cells were maintained in DMEM (HyClone) supplemented with 10% FBS (v/v) and 1% penicillin and streptomycin (v/v) at 37°C in a 5% CO2 humidified atmosphere. The conditioned medium for exosome collection was DMEM plus 1% penicillin–streptomycin and 10% FBS pre-centrifuged at 120,000×g for 140 minutes to remove serum exosomes.
Isolation of exosomes
To isolate exosomes, cells were cultured with exosome-depleted serum. We collected the conditioned medium to isolate exosomes using the ExoQuick-TC™ Kit (System BioSciences, Mountain View, CA, USA). The protein concentration of exosomes was measured by a bicinchoninic acid kit (Sangon Biotech, Shanghai, People’s Republic of China), which was used to represent the concentration of exosomes.
Preparation of LZD-loaded exosomes
The exosomal formulation of LZD (ExoLZD) was prepared by simple mixing of the LZD solution (dimethyl sulfoxide [DMSO]) with the exosome dispersion, while keeping the final solvent concentration ≤10% (v/v). The selection of the DMSO concentration was based on our previous observation that this concentration did not significantly affect the quality attributes of the exosomes. After mixing the LZD solution with exosomes, the mixture was incubated at 37°C for 1 hour and then centrifuged at a low speed of 10,000×g for 10 minutes to remove the unbound drug. The ExoLZD mixture was passed through a 0.22 μm syringe filter for sterilization and stored at −80°C until use.
The amount of LZD loaded into exosomes was measured by high performance liquid chromatography (HPLC). Briefly, an equal volume of acetonitrile (ACN) was added to the ExoLZD solution in a microcentrifuge tube and the mixture was vortexed, sonicated, and then centrifuged at 12,280×g for 10 minutes. Following centrifugation, the supernatant was removed and filtered through a Corning Regenerated Cellulose 0.2 μm syringe filter and transferred into HPLC autosampler vials. Ten microliter aliquots were injected into the HPLC system (Agilent 1260; Agilent Technologies, Santa Clara, CA, USA). All data were acquired using a C18 column (Extend-C18, 250×4.6 mm, 5 μm, 100 Å; Agilent Technologies) with the mobile phase H2O:ACN (80:20, v/v) at a flow rate of 1 mL/min at 30°C. Absorbance was measured at 251 nm to monitor the elution of LZD. The standard curve for LZD was obtained from 0.5 to 40 μg/mL concentration range. For data acquisition and analysis, OpenLAB CDS ChemStation Edition software was used.
Formulation characterization
Exosomes were characterized by dynamic light scattering (DLS; Nano ZS90; Malvern Instruments, Malvern, UK), transmission electron microscopy (TEM; HT7700; Hitachi Ltd., Tokyo, Japan) and Western blot analysis, as described previously.13
Accumulation of exosomes and ExoLZD in RAW264.7 cells
Exosomes were prelabeled with the fluorescent dye 3,3′-dioctadecyloxacarbocyanine perchlorate (DiO) (Beyotime, Jiangsu, People’s Republic of China), and the extra dye was removed by MW 3000 Exosome Spin Columns (Thermo Fisher Scientific, Waltham, MA, USA). RAW264.7 cells were grown to 50% confluence in 24-well chamber slides and incubated with DiO-labeled exosomes (200 μg/mL) for 0.5, 4, and 24 hours. The cells were then washed two times with PBS solution. Subsequently, the cells were fixed with 4% paraformaldehyde (PFA, v/v) for 20 minutes and washed three times with PBS solution. Nuclei were stained using DAPI (Beyotime). Finally, the cells were rinsed with cold PBS and viewed under a confocal laser scanning microscope (Leica TCS SP8; Leica Microsystems, Wetzlar, Germany).
In vitro cytotoxicity assay
The cytotoxicity of exosomes and ExoLZD was evaluated by the tetrazolium-based colorimetric (MTT) assay. Cells were seeded in 96-well plates at a density of 5×103 per well and incubated for 72 hours with either vehicle (DMSO) or desired concentrations of exosomes or ExoLZD at 37°C in a 5% CO2 incubator. Then, the medium was replaced with 100 μL of fresh medium without serum and 10 μL of MTT (5 mg/mL; Sangon Biotech) solution and incubated for an additional 4 hours at 37°C in a 5% CO2 incubator. Subsequently, the medium was removed and 100 μL of DMSO was added. The absorbance at 490 nm was measured using a microplate reader (Bio-Rad Laboratories Inc., Hercules, CA, USA). Cell viability was calculated as the ratio of the absorbance of the treated cells to that of the control group cells.
Intracellular survival of MRSA inside macrophages treated with ExoLZD in vitro
RAW264.7 cells were plated at a density of 4×105 cells/well and infected with MRSA WHO-2 at a ratio of 10–20 bacteria per macrophage as previously described.17 The infected macrophages were incubated with LZD or ExoLZD (20 μg/mL equiv. LZD) for 2, 4, and 24 hours. At each time point, the medium was decanted and the cells were quickly washed twice with PBS. Then, the cells were lysed with Hanks Buffered Saline Solution (HBSS) supplemented with 0.1% BSA (w/v) and 0.1% Triton-X (v/v), and serial dilutions of the lysate were made in a PBS solution containing 0.05% Tween-20 (v/v). The number of surviving intracellular bacteria was determined by plating on tryptic soy agar plates with 5% defibrinated sheep blood (v/v). In this experiment, macrophage cultures were maintained in growth media supplemented with 50 μg/mL gentamycin to inhibit the growth of extracellular bacteria.
To further confirm the antimicrobial activity, the LIVE/DEAD BacLight Bacterial Viability kit (Molecular Probes) was used. RAW264.7 cells were infected with MRSA WHO-2 as explained above for intracellular colony-forming unit (CFU) determination and then treated with LZD or ExoLZD (20 μg/mL equiv. LZD) for 4 hours. Then, the infected cells were washed three times with PBS, fixed with 4% PFA, and permeabilized with 0.2% Triton-X100. Subsequently, the cells were mixed with the LIVE/DEAD BacLight bacterial viability kit for 15 minutes in the dark. After washing with PBS, the RAW264.7 cells were viewed under a confocal laser scanning microscope (Leica TCS SP8; Leica Microsystems).
Quantification of released antibiotic inside the macrophages
RAW264.7 cells were infected in 24-well tissue culture dishes as described above for the determination of intracellular MRSA survival. Infected cells were incubated with LZD or ExoLZD (200 μg/mL equiv. LZD) for 2 and 24 hours. At each time point, the supernatant and cellular fractions were collected, and ACN was then added to a final concentration of 75% (v/v) and the mixture incubated for 30 minutes. The cellular and supernatant extracts were lyophilized by evaporation under a Termovap Sample Concentrator (Shanghai Joyn, Shanghai, People’s Republic of China), reconstituted in 100 μL of 50% ACN, filtered using a 0.2 μm syringe filter, and analyzed by HPLC.
In vitro release of LZD
The in vitro release profile of LZD from ExoLZD was determined in four different media: 1) PBS at pH 7.4, 2) sodium citrate 0.1 M citrated PBS (CPBS) at pH 4.5, 3) PBS at pH 7.4 with 1 mg of RAW264.7 cell lysates, and 4) CPBS at pH 4.5 with 1 mg of RAW264.7 cell lysates. The media were incubated at 37°C with continuous shaking at 80 rpm in a shaker bath (WE-1 Shaking water bath; Tianjin Honor Inc.). At different time intervals, 200 μL aliquots of the samples were collected and analyzed by HPLC.
Localization of exosomes and ExoLZD in MRSA-infected RAW264.7 cells
For the intracellular localization studies, exosomes were prelabeled with DiO as explained above for the assessment of exosomes and ExoLZD accumulation in RAW264.7 cells. RAW264.7 cells were infected in 24-well chamber slides as described above for intracellular CFU determination. The infected cells were incubated with DiO-labeled exosomes or ExoLZD (Exo=200 μg/mL; LZD=20 μg/mL) for 0.5, 4, and 24 hours. At the desired time point, the cells were washed twice with PBS solution and then incubated with 100 nM LysoTracker Red (Beyotime) for 30 minutes. Subsequently, the cells were washed with PBS three times and fixed with 4% PFA for 20 minutes. Finally, the cells were rinsed with cold PBS and viewed under a confocal laser scanning microscope (Leica TCS SP8; Leica Microsystems).
Assessment of ExoLZD activity in vivo
Female Kun Ming mice (weight: 20–22 g) were purchased from Chongqing Byrness Weil Biotechnology Co. Ltd (Chongqing, People’s Republic of China) and were used for all studies. Each animal was housed under standard conditions (21°C±1°C, 50%–10% relative humidity, 12 hours light/dark cycle) and had free access to food and water. All animal studies were approved by the Laboratory Animal Welfare and Ethics Committee of the Third Military Medical University, and the animal handling procedures followed the guidelines set by the Animal Care Committee, Third Military Medical University.
To confirm the antibacterial effect in mice, an MRSA infected peritonitis model was used and performed with minor modification as described previously.18,19 Mice were infected with 5×107 CFU of MRSA WHO-2 by intraperitoneal injection. After infection for 2 hours, antibiotic treatments were administered subcutaneously. At 4 or 24 hours after drug treatment, the peritoneal wash was harvested by injecting 2 mL HBSS intraperitoneally, massaging the abdomen and opening the peritoneum to collect peritoneal fluid. The collected peritoneal fluid from one mouse was diluted 1:1 with HBSS. The total CFU count of the diluted sample was determined before any further procedures were performed. The diluted sample was then divided into two equal fractions of ~1.5 mL each (fractions A and B). For extracellular CFU quantification, fraction A was centrifuged at 300×g at room temperature for 10 minutes and the extracellular CFU in the supernatant was quantified. For intracellular CFU quantification, lysostaphin (Sangon Biotech) was added to fraction B to a final concentration of 15 μg/mL and the fraction was incubated for 15 minutes at room temperature. These conditions ensured complete killing of the extracellular bacteria contaminating the preparation. Lysostaphin was removed by washing the sample four times with HBSS by centrifugation (300×g) for 5 minutes at room temperature, and the fraction was prepared for CFU quantification as described previously.
Statistical analysis
Data were expressed as the mean±SD, and P<0.05 was considered statistically significant. Student’s t-test was used to compare the means between two groups. Data analysis was performed using SPSS (V19.0; IBM Corporation, Armonk, NY, USA).
Results
Preparation, purification, and characterization of ExoLZD
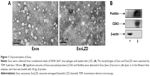
We collected exosomes from the culture supernatants of RAW264.7 cells. Their size distribution and zeta potential were characterized by DLS (Table S1), their morphology was characterized by TEM (Figure 1A), and their protein content was assessed by Western blotting (Figure 1B). Similar to previously published results,13 the exosomes were heterogeneous in size with an intensity-weighted z-average diameter of 70.42±1.52 nm as determined by DLS. Exosomes were negatively charged (zeta potential −7.18±0.51 mV) in PBS. TEM showed a spherical morphology, as previously reported. As revealed by Western blotting, the exosomes were more enriched with CD63 and flotillin, two exosomal markers, than the cell lysate.
LZD was incorporated into exosomes by incubation at 37°C. The obtained ExoLZD formulations were purified from the nonincorporated drug by low-speed centrifugation and analyzed by HPLC to determine the loading capacity. The loading capacity of the ExoLZD formulation was 5.06%±0.45%. Interestingly, DLS studies revealed that the size of the ExoLZD nanoformulations increased slightly (Table S1). In addition, the loading procedures did not significantly alter the zeta potential of the nanocarriers, suggesting that there were no major alterations to the lipid content of the exosomal membranes.
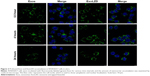
Uptake of ExoLZD by RAW264.7 cells
The internalization of ExoLZD by RAW264.7 cells was investigated by confocal laser scanning microscopy. As shown in Figure 2, both exosomes and ExoLZD were efficiently and rapidly taken up by RAW264.7 macrophages in 24 hours. Interestingly, the cells displayed similar intracellular fluorescence when treated with either exosomes or ExoLZD, which indicated that the drug payload did not affect the ability to deliver exosomes to macrophages.
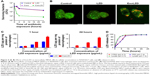
Cytotoxicity of exosome-delivered antibiotics
The cytotoxic effects of the exosomes-delivered antibiotic LZD were examined in RAW264.7 cells (Figure 3). Empty exosomes (10–200 μg/mL as quantified by the amount of total protein) had no effect on cell viability compared to the media control. Similarly, ExoLZD (LZD=10–200 μg/mL; exosomes=200 μg/mL) also had no effect on cell death compared to the media control. These results indicated that the exosomes themselves and ExoLZD had no significant inhibitory effect on cells viability.
Intracellular bactericidal effect of ExoLZD
MRSA-infected macrophages were treated with free LZD (20 μg/mL) or ExoLZD at the same concentrations for 24 hours. At 2, 4, and 24 hours after treatment of the MRSA-infected macrophages, free LZD failed to kill the MRSA strain WHO-2 in vitro, as the bacteria were sequestered inside the macrophages exposed to clinically achievable concentrations of the antibiotic (Figure 4A), consistent with previous studies. Interestingly, the CFU counts obtained at 2, 4, and 24 hours after treatment with ExoLZD were ~0.6, 1.6, and 1.5 log units lower than those obtained after treatment with the free drug, respectively (P<0.001).
To further confirm the antimicrobial activity, additional confocal microscopy analyses were performed on infected cells treated with ExoLZD or free LZD. Using the LIVE/DEAD BacLight bacterial viability assay, it was possible to distinguish between dead bacteria (labeled in red) and live bacteria (labeled in green). As shown in Figure 4B, most of the intracellular bacteria remained alive when incubated with free LZD, while treatment with ExoLZD succeeded in killing most intracellular bacteria, as soon as 4 hours after treatment.
Cellular accumulation of LZD in RAW264.7 cells
The intracellular LZD concentration was assessed in all lysates of MRSA-infected RAW264.7 cells by HPLC. As shown in Figure 4C, uptake of the drug was dose dependent in all studies; a higher concentration of initial LZD, in either the free LZD solution or the exosome formulation, resulted in more intracellular LZD. However, the intracellular concentration of LZD following 1-hour incubation with the free LZD solution was greater than after 24 hours of incubation. This trend was also observed in macrophages treated with exosome formulations. Interestingly, at both 1 and 24 hours, more intracellular LZD was observed in macrophages treated with the exosome formulations than in macrophages in the free LZD group. This was especially true at 24 hours when the concentration of intracellular LZD in macrophages treated with ExoLZD was approximately five times higher than that in the free LZD group. These data confirm the prior observations made by others that LZD accumulates in only modest amounts in eukaryotic cells. The increased intracellular accumulation of LZD delivered via exosomes may have marked bactericidal effects on intracellular MRSA present within infected phagocytes.
In vitro release of LZD
The in vitro release profile of LZD was assessed in PBS (pH 7.4) or CPBS (pH 4.5) and compared with that in RAW264.7 cell lysates at predetermined time intervals. As shown in Figure 4D, the presence of cell extracts triggered fast LZD release, and LZD was released in a time-dependent manner irrespective of the release media. Only ~25% of LZD was released with ExoLZD treatment in either CPBS (pH 4.5) or PBS (pH 7.4) media at 2 hours, and the amount of LZD released increased to ~40% after 4 hours. The release kinetics was similar in the two media preparations, which suggests a minimal effect of pH. In the presence of cell lysates, ExoLZD released >50% of the drug at 2 hours, and then the amount of drug released increased to ~80% at 4 hours. ExoLZD exerted an obvious bactericidal effect on intracellular MRSA, likely due to the efficient release of this antibiotic.
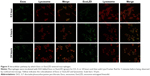
Intracellular localization of ExoLZD vs bacteria in RAW264.7 cells
To investigate the intracellular distribution, exosomes or ExoLZD labeled with DiO were incubated with RAW264.7 macrophages. At 0.5, 4, or 24 hours, cells were treated with LysoTracker, a marker of late endosomal and lysosomal vesicles. Colocalization of exosomes or ExoLZD with the fluorescent lysosomal marker was observed (Figure 5), which was similar to the results of a previous report. More importantly, the exosomes remained in the lysosomes for 24 hours.
Antibacterial effect of ExoLZD in mice
Intra- and extracellular time-kill studies (24 hours exposure) with LZD and ExoLZD were performed in vivo. Mice were infected with MRSA WHO-2 by peritoneal injection for 2 hours and then treated with a single subcutaneous dose of LZD (17 mg/kg of body weight) or ExoLZD (LZD=17 mg/kg) (n=5). The peritoneal fluid was sampled just before (time 0) and at 4 and 24 hours after treatment onset and immediately processed for the intra- and extracellular separation assay and CFU determination. The results (Figure 6) show that compared to free LZD, our ExoLZD significantly inhibited MRSA infection in vivo at both 4 and 24 hours with one injection (P<0.01). These exciting results confirmed that LZD delivered by exosome had high antibacterial activity both in vitro and in vivo.
Discussion
The high prevalence of MRSA strains is a challenge in the treatment of S. aureus infections, and S. aureus is one of the most frequently occurring antibiotic-resistant threats because of its ability to form nongrowing, dormant, “persister” subpopulations.21,22 It has been reported that treatment of patients with invasive MRSA infections with conventional antibiotics has a failure rate of up to 50% and measurable outgrowth of antibiotic-resistant strains is not observable in most cases.17 Although considered an extracellular pathogen, S. aureus can escape from the endosomes/phagosomes of phagocytes and proliferate within the cytoplasm, which makes treatment of S. aureus infection difficult.6,7 The majority of existing antibiotics are inefficient at killing intracellular S. aureus both in vitro and in vivo.8 LZD is usually recommended for the treatment of difficult-to-treat staphylococcal infections, but it fails to kill intracellular MRSA inside the macrophages exposed to clinically achievable concentrations.20 In this study, we found that administration of LZD in the exosome-encapsulated form considerably enhanced the killing of MRSA in macrophages in vitro and in vivo.
LZD accumulates in only modest amounts in eukaryotic cells, which explains the poor activity of this antibiotic against intracellular bacteria.20 Interestingly, exosomes delivered a sufficient concentration to the cells for bacterial killing, while this concentration showed less cytotoxicity to macrophages. In addition, more exosome-encapsulated LZD accumulated in RAW264.7 macrophages than free LZD within 24 hours. It is speculated that the bactericidal effects of ExoLZD against intracellular MRSA could be due to the fact that LZD may reach intracellular concentrations that far exceed the MICs for a prolonged time.
On the other hand, the LZD-loaded exosomes exhibited good colocalization to the lysosome, where S. aureus is expected to reside.9 Thus, treatment of intracellular infections by targeting a subcellular compartment is feasible since the colocalization of ExoLZD with bacteria in the same intracellular compartments should enhance the local concentration of the delivered antibiotic and improve the drug’s efficiency. Exosome uptake is clathrin independent, having an endocytic profile indicative of macropinocytosis with eventual delivery to the lysosomes.3,23 It has been documented that phagocytosis of S. aureus might be attributed to lysosome–phagosome fusion and a portion of the bacteria escape from the endosomes/phagosomes of phagocytes and proliferate within the cytoplasm.9 A plausible mechanism of action of this drug after being released into the acidic environment of the lysosomes is as follows: 1) a portion of the LZD kills bacteria in the lysosomes and 2) some LZD crosses the intracellular endolysosomal membranes into the cytoplasm and thereby exerts a bactericidal effect.
Conclusion
This work demonstrates that exosomes are exceptionally effective carriers for therapeutic antibiotics. Exosomes loaded with LZD efficiently accumulated in macrophages and exerted a potent intracellular bactericidal effect. Exosomes could potentially load with both hydrophilic and lipophilic drugs, since they resemble liposomes with a bilipid membrane and an aqueous core. The use of an exosomes-based formulation could be a novel platform for delivering potent antibacterial drugs to treat intracellular infections. However, further studies are necessary to understand the exact mechanism of drug loading and release by exosomes in macrophages. Detailed pharmacodynamics and pharmacokinetic studies in different in vivo models are needed to fully assess the potential of ExoLZD for clinical applications. Additional researches are also warranted to translate our results from animal models into clinical applications in humans.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (numbers 21572027, 81703424, and 81741169). We thank Zhipeng Wang for providing technical assistance with HPLC analysis and Yanfei Qiu for providing technical assistance with the animal studies. We also thank Dr Ping Hu for the helpful discussions on experimental designs and Dr Rongxin Qin for kindly providing the methicillin-resistant Staphylococcus aureus WHO-2 strain.
Author contributions
XY, JG, CW, and YH conceived the entire study and designed the experiments; XY carried out the experiments and wrote the manuscript; GS participated in the in vivo experiments and assisted in designing the in vivo studies and interpreting the results; XY and GS analyzed the data; and XY, JG, CW, and YH reviewed the manuscript. All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
DeLeo FR, Otto M, Kreiswirth BN, Chambers HF. Community-associated meticillin-resistant Staphylococcus aureus. Lancet. 2010;375(9725):1557–1568. | ||
Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015;28(3):603–661. | ||
Grundmann H, Aires-de-Sousa M, Boyce J, Tiemersma E. Emergence and resurgence of meticillin-resistant Staphylococcus aureus as a public-health threat. Lancet. 2006;368(9538):874–885. | ||
Levy SB, Marshall B. Antibacterial resistance worldwide: causes, challenges and responses. Nat Med. 2004;10(12 Suppl):S122–S129. | ||
Almeida RA, Matthews KR, Cifrian E, Guidry AJ, Oliver SP. Staphylococcus aureus invasion of bovine mammary epithelial cells. J Dairy Sci. 1996;79(6):1021–1026. | ||
Kubica M, Guzik K, Koziel J, et al. A potential new pathway for Staphylococcus aureus dissemination: the silent survival of S. aureus phagocytosed by human monocyte-derived macrophages. PLoS One. 2008;3(1):e1409. | ||
Fraunholz M, Sinha B. Intracellular Staphylococcus aureus: live-in and let die. Front Cell Infect Microbiol. 2012;2:43. | ||
Barcia-Macay M, Seral C, Mingeot-Leclercq MP, Tulkens PM, van Bambeke F. Pharmacodynamic evaluation of the intracellular activities of antibiotics against Staphylococcus aureus in a model of THP-1 macrophages. Antimicrob Agents Chemother. 2006;50(3):841–851. | ||
Carryn S, Chanteux H, Seral C, Mingeot-Leclercq MP, Van Bambeke F, Tulkens PM. Intracellular pharmacodynamics of antibiotics. Infect Dis Clin North Am. 2003;17(3):615–634. | ||
Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–579. | ||
Vader P, Mol EA, Pasterkamp G, Schiffelers RM. Extracellular vesicles for drug delivery. Adv Drug Deliv Rev. 2016;106(Pt A):148–156. | ||
Jiang XC, Gao JQ. Exosomes as novel bio-carriers for gene and drug delivery. Int J Pharm. 2017;521(1–2):167–175. | ||
Kim MS, Haney MJ, Zhao Y, et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine. 2016;12(3):655–664. | ||
Yuan D, Zhao Y, Banks WA, et al. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials. 2017;142:1–12. | ||
Haney MJ, Klyachko NL, Zhao Y, et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release. 2015;207:18–30. | ||
Tian T, Zhang HX, He CP, et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials. 2018;150:137–149. | ||
Lehar SM, Pillow T, Xu M, et al. Novel antibody-antibiotic conjugate eliminates intracellular S. aureus. Nature. 2015;527(7578):323–328. | ||
Sandberg A, Lemaire S, Van Bambeke F, et al. Intra- and extracellular activities of dicloxacillin and linezolid against a clinical Staphylococcus aureus strain with a small-colony-variant phenotype in an in vitro model of THP-1 macrophages and an in vivo mouse peritonitis model. Antimicrob Agents Chemother. 2011;55(4):1443–1452. | ||
Shi G, Chen X, Wang H, Wang S, Guo X, Zhang X. Activity of sitafloxacin against extracellular and intracellular Staphylococcus aureus in vitro and in vivo: comparison with levofloxacin and moxifloxacin. J Antibiot (Tokyo). 2012;65(5):229–236. | ||
Lemaire S, Van Bambeke F, Appelbaum PC, Tulkens PM. Cellular pharmacokinetics and intracellular activity of torezolid (TR-700): studies with human macrophage (THP-1) and endothelial (HUVEC) cell lines. J Antimicrob Chemother. 2009;64(5):1035–1043. | ||
Kim MS, Haney MJ, Zhao Y, et al. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: in vitro and in vivo evaluations. Nanomedicine. 2018;14(1):195–204. | ||
Klevens RM, Morrison MA, Nadle J, et al; Active Bacterial Core surveillance (ABCs) MRSA Investigators. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298(15):1763–1771. | ||
McKelvey KJ, Powell KL, Ashton AW, Morris JM, McCracken SA. Exosomes: mechanisms of uptake. J Circ Biomark. 2015;4:7. |
Supplementary material
  | Table S1 Physicochemical characteristics of Exo and ExoLZD |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.